Abstract
Background
The Chronic Kidney Disease Epidemiology Collaboration equation for estimation of glomerular filtration rate (eGFRCKD-EPI) improves GFR estimation compared with the Modification of Diet in Renal Disease Study equation (eGFRMDRD) but its association with mortality in a nationally representative population sample in the US has not been studied.
Methods
We examined the association between eGFR and mortality among 16,010 participants of the Third National Health and Nutrition Examination Survey (NHANES III). Primary predictors were eGFRCKD-EPI and eGFRMDRD. Outcomes of interest were all-cause and cardiovascular disease (CVD) mortality. Improvement in risk categorization with eGFRCKD-EPI was evaluated using adjusted relative hazard (HR) and Net Reclassification Improvement (NRI).
Results
Overall, 26.9% of the population was reclassified to higher eGFR categories and 2.2% to lower eGFR categories by eGFRCKD-EPI, reducing the proportion of prevalent CKD classified as stage 3–5 from 45.6% to 28.8%. There were 3,620 deaths (1,540 from CVD) during 215,082 person-years of follow-up (median, 14.3 years). Among those with eGFRMDRD 30–59 ml/min/1.73 m2, 19.4% were reclassified to eGFRCKD-EPI 60–89 ml/min/1.73 m2 and these individuals had a lower risk of all-cause mortality (adjusted HR, 0.53; 95% CI, 0.34-0.84) and CVD mortality (adjusted HR, 0.51; 95% CI, 0.27-0.96) compared with those not reclassified. Among those with eGFRMDRD >60 ml/min/1.73 m2, 0.5% were reclassified to lower eGFRCKD-EPI and these individuals had a higher risk of all-cause (adjusted HR, 1.31; 95% CI, 1.01-1.69) and CVD (adjusted HR, 1.42; 95% CI, 1.01-1.99) mortality compared with those not reclassified. Risk prediction improved with eGFRCKD-EPI; NRI was 0.21 for all-cause mortality (p < 0.001) and 0.22 for CVD mortality (p < 0.001).
Conclusions
eGFRCKD-EPI categories improve mortality risk stratification of individuals in the US population. If eGFRCKD-EPI replaces eGFRMDRD in the US, it will likely improve risk stratification.
Keywords: Glomerular filtration rate, Chronic kidney disease, Epidemiology, Outcomes
Background
Decreased kidney function is an independent risk factor for mortality. While measured glomerular filtration rate (GFR) remains the gold standard for assessing decreased kidney function, in routine clinical practice, GFR is usually estimated from serum creatinine by the Modification of Diet in Renal Disease (MDRD) Study equation (eGFRMDRD). Understanding the association of eGFR categories with clinical outcomes is an important aspect of the chronic kidney disease (CKD) staging system. Accurate classification of individuals with CKD can inform healthcare utilization and therapeutic decision making by reducing false positive diagnoses of CKD while correctly classifying those with CKD to appropriate risk categories [1].
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for estimation of GFR from serum creatinine (eGFRCKD-EPI) improves GFR estimation compared with the MDRD Study equation [2]. eGFRCKD-EPI, as compared to eGFRMDRD, results in a lower prevalence of decreased eGFR [2]. Recent publications have demonstrated that use of the CKD-EPI equation results in reclassification of individuals previously classified using eGFRMDRD to different eGFRCKD-EPI categories with more appropriate risk stratification [3-6]. However, the effect of this reclassification from eGFRMDRD categories by CKD-EPI equation on long-term risk prediction in a nationally representative sample of the US population has not been described. The objective of this study was to evaluate the effect of reclassification using eGFRCKD-EPI on the estimated risk of all-cause and cardiovascular disease (CVD) mortality in the US population using data from the Third National Health and Nutrition Examination Survey (NHANES III) and relevant subgroups.
Methods
Study sample
NHANES III is a cross-sectional, multistage, stratified, clustered probability sample of the noninstitutionalized US civilian population conducted during 1988–1994 by the National Center for Health Statistics (NCHS), a branch of the Center for Disease Control and Prevention [7]. In NHANES III, certain subgroups of the population were oversampled including Mexican Americans, non-Hispanic blacks and elderly persons to ensure adequate sample sizes of these groups. We limited the study population to 16,010 persons aged 17 years or older who were examined at the mobile examination center (MEC), were not missing serum creatinine data or covariates of interest, and had available mortality follow-up. Mortality follow-up was available for 99.9% of the eligible participants. The protocols for conduct of NHANES were approved by the NCHS institutional review board and informed consent was obtained from all participants. Procedures were followed in accordance with ethical standards of the Johns Hopkins School of Public Health Office of Human Subjects Research and Institutional Review Board.
Measurements
NHANES III procedures have been previously described [7]. Briefly, standardized questionnaires were administered at home and physical examination and laboratory tests specimen collection was performed at the MEC. Self-reported race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Mexican-American or other. Smoking was defined as either active cigarette smoking, having smoked >100 cigarettes in life, or never having smoked. Participants were considered to have diabetes mellitus if they reported being told by a doctor that they had diabetes at a time other than pregnancy or if they were taking insulin or oral hypoglycemic agents. Cardiovascular disease (CVD) was considered to be present at baseline if the participant reported being informed by a doctor of prior heart attack, congestive heart failure or stroke. Antihypertensive medication use was based on self-report. Blood pressure (BP) was measured using standard techniques and reported as the average of all systolic and diastolic readings. Participants were advised to fast prior to specimen collection but fasting was not required. Overall, 87% of the participants had fasted for ≥ 6 hours prior to blood draw.
Serum creatinine was measured using a kinetic rate Jaffe method. Serum creatinine measurements were recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, Ohio) as described previously [standard creatinine = (0.960 x serum creatinine) – 0.184] [8]. We calculated eGFR using the isotope dilution mass spectrometry (IDMS)-traceable 4-variable MDRD Study equation and the CKD-EPI equation [2,9]. There was no difference in eGFRCKD-EPI based on fasting status (0.98 ml/min/1.73 m2 lower in those fasting ≥6 hours; p = 0.14). We categorized eGFR into the following clinically relevant categories: ≥120, 90–119, 60–89, 30–59 and <30 ml/min/1.73 m2. Within each category of eGFRMDRD, individuals were reclassified into three groups based on eGFRCKD-EPI: a) higher eGFRCKD-EPI category; (b) same eGFR category by both eGFRMDRD and eGFRCKD-EPI, and; (c) lower eGFRCKD-EPI category.
C-reactive protein (CRP) was measured by latex-enhanced nephelometry (Dade Behring). CRP level was categorized as either undetectable by the assay (<0.22 mg/dL), minimal (0.22-0.99 mg/dL) or elevated (≥1.0 mg/dL). Urinary albumin level was measured by solid-phase fluorescence immunoassay, and urinary creatinine level was measured by the modified kinetic method of Jaffe using a Beckman Coulter Synchron AS/Astra Analyzer (Beckman Coulter, Inc., Fullerton, California). Albuminuria was expressed as urinary albumin-to-creatinine ratio (ACR) and categorized into 3 categories; <30 mg/g, 30–299 mg/g and ≥300 mg/g.
Causes of death
Causes of death were obtained using the NHANES III Linked Mortality Public-use File [10]. This file contains mortality follow-up data on NHANES III participants obtained via National Death Index (NDI) linkage through December 31, 2006. Mortality ascertainment is performed using probabilistic matching between NHANES III and NDI using previously validated methods [10]. Cause of death coding for all U.S. deaths occurring prior to 1999 followed the 9th revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-9) guidelines, while all deaths after 1998 followed the 10th revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-10) guidelines. All deaths occurring prior to 1999 were recoded from ICD-9 to comparable ICD-10 (I) based cause of death groups [11]. We defined cardiovascular disease mortality using the following cause of death groups: 056 (hypertensive heart disease [I11]), 057 (hypertensive heart and renal disease [I13]), 058–063 (ischemic heart diseases [I20-I25]), 067 (heart failure [I50]), 068 (valvular heart diseases and cardiomyopathy [I26-I28, I34-I38, I42-I49, I51]), 069 (essential hypertension and hypertensive renal disease [I10, I12]), 070 (cerebrovascular disease [I60-I69]), and 071 (atherosclerosis [I70]).
Statistical analysis
All analyses were performed using 6-year MEC sampling weights provided by the National Center for Health Statistics that account for the complex survey design of NHANES III as well as probabilities for non-response. Analyses were performed using survey (svy) commands Stata 10.1 and 11.2. (Stata Corp, http://www.stata.com) Baseline characteristics were compared across eGFR categories and reclassification status of the participants. Survival analysis techniques were used to analyze the risk of all-cause and CVD mortality. Individuals who were alive on December 31, 2006 were censored in the analyses. Modified Cox proportional hazards regression was used to model the risk of death across eGFR and reclassification categories. Hazard ratios (HR) were calculated to assess risk of death after adjustment for age, race/ethnicity, sex, body mass index, systolic and diastolic BP, antihypertensive medication use, history of diabetes and CVD, smoking status, serum total cholesterol, ACR categories and CRP categories. Poisson regression was used to calculate and display incidence rates with eGFR modeled as a restricted cubic spline with knots at 30, 45, 60, 90 and 120 ml/min/1.73 m2.
Reclassification
To assess reclassification we calculated net reclassification improvement (NRI) [12]. Net reclassification improvement (NRI) is a statistic that allows calculation of the effect of reclassification of individuals from one disease category to the other. It is a difference of two ratios; clinically correct reclassification minus clinically incorrect classification. The range of this difference is from −1 to +1 with a negative number reflecting incorrect reclassification and a positive number indication correct reclassification.
For NRI calculations we excluded individuals with eGFR ≥120 ml/min/1.73 m2 by either equation as the high eGFR in this group reflects low serum creatinine likely from malnutrition, intercurrent illness and low muscle mass [3]. To calculate NRI, we first created cross-tabulation of participants in eGFRMDRD and eGFRCKD-EPI categories stratified by the outcome status (alive or dead). We then calculated the proportion of individuals in each category of eGFRMDRD that are reclassified by eGFRCKD-EPI. Clinically correct reclassification was defined as: proportion of participants reclassified to higher eGFR category by eGFRCKD-EPI among those who are alive + the proportion of participants reclassified to lower eGFR category by eGFRCKD-EPI among those who died. Clinically incorrect reclassification was defined as: proportion of participants reclassified to higher eGFR category by eGFRCKD-EPI among those who died + the proportion of participants reclassified to lower eGFR category by eGFRCKD-EPI among those who are alive. NRI = clinically correct reclassification – clinically incorrect reclassification. Statistical significance for NRI was calculated using bootstrapping with replacement. To account for the confounding effect of age, sex and race on outcomes, we also calculated stratum-specific NRI in these subgroups.
Results
Baseline characteristics
The baseline characteristics of the participants stratified by reclassification status by eGFRCKD-EPI are presented in Table1. Overall, the number of participants (population %) reclassified were as follows: 3,464 (26.9%) of the participants were reclassified to a higher eGFR category, 559 (2.2%) were reclassified to a lower eGFR category and 11,987 (70.8%) were not reclassified. There was only 1 participant with eGFRMDRD <30 ml/min/1.73 m2 who was reclassified upward (data not presented in Table1). Individuals reclassified to higher eGFRCKD-EPI categories were more likely to be younger, female, had lower prevalence of diabetes and CVD, and had lower BP, cholesterol, CRP and less albuminuria. These differences were much more pronounced at lower eGFR categories (<60 ml/min/1.73 m2). Among participants with eGFRMDRD <120 ml/min/1.73 m2, no participants below 65 years were reclassified to a lower eGFRCKD-EPI category.
Table 1.
Baseline characteristics of 16,010 NHANES III (1988–1994) participants stratified by eGFRMDRDand eGFRCKD-EPI
|
EGFRMDRD |
≥120 |
90-119 |
60-89 |
30-59 |
<30 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
(No. of participants)a |
(2,729) |
(6,604) |
(5,606) |
(1,015) |
(56) |
|||||||
| EGFRCKD-EPI | ≥120 | 90-119 | ≥120 | 90-119 | 60-89 | 90-119 | 60-89 | 30-59 | 60-89 | 30-59 | <30 | <30 |
| Number of participants a (%) b |
2,416 |
312 d |
1,473 |
4,969 |
162 |
1,870 |
3,664 |
72 |
120 |
883 |
12 |
55 e |
| (88.3) |
(11.7) |
(20.2) |
(78.4) |
(1.4) |
(42.9) |
(56.6) |
(0.5) |
(19.4) |
(79.5) |
(1.1) |
(99.5) |
|
|
Characteristicc |
|
|
|
|
|
|
|
|
|
|
|
|
| Serum Creatinine (mg/dl) |
0.64 |
0.59 |
0.76 |
0.79 |
0.70 |
0.87 |
0.94 |
1.07 |
1.07 |
1.26 |
1.97 |
2.76 |
| (0.004) |
(0.01) |
(0.003) |
(0.002) |
(0.01) |
(0.004) |
(0.004) |
(0.03) |
(0.02) |
(0.02) |
(0.11) |
(0.20) |
|
| EGFRMDRD (ml/min/1.73 m2) |
138 |
131 |
112 |
100 |
98 |
85 |
74 |
61 |
58 |
49 |
31 |
23 |
| (0.63) |
(0.72) |
(0.17) |
(0.18) |
(0.28) |
(0.09) |
(0.13) |
(0.09) |
(0.13) |
(0.27) |
(0.32) |
(0.88) |
|
| EGFRCKD-EPI (ml/min/1.73 m2) |
133 |
112 |
124 |
109 |
87 |
96 |
79 |
59 |
63 |
50 |
29 |
22 |
| (0.36) |
(0.51) |
(0.14) |
(0.12) |
(0.27) |
(0.18) |
(0.20) |
(0.13) |
(0.20) |
(0.30) |
(0.24) |
(0.92) |
|
| Age (years) |
26 (0.2) |
53 (0.8) |
28 (0.3) |
39 (0.2) |
79 (0.5) |
40 (0.5) |
59 (0.6) |
83 (1.0) |
56 (1.11) |
72 (0.74) |
81 (2.12) |
72 (1.75) |
| Age < 65 years, N (%) a b |
2,415 |
247 |
1,473 |
4,614 |
0 |
1,754 |
1,717 |
0 |
100 |
115 |
0 |
12 |
| (99.9) |
(85.8) |
(100) |
(96.4) |
|
(95.8) |
(61.3) |
|
(83.8) |
(18.0) |
|
(16.9) |
|
| Male (%) |
40 |
55 |
44 |
53 |
56 |
46 |
49 |
60 |
24 |
40 |
72 |
35 |
| Race/Ethnicity (%) |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-Hispanic White |
52 |
67 |
65 |
74 |
85 |
86 |
86 |
86 |
90 |
86 |
90 |
73 |
| Non-Hispanic Black |
25 |
9 |
19 |
11 |
7 |
4 |
7 |
13 |
1 |
8 |
9 |
18 |
| Mexican American |
13 |
10 |
8 |
6 |
2 |
4 |
2 |
1 |
2 |
1 |
1 |
2 |
| Other |
11 |
13 |
9 |
10 |
7 |
6 |
6 |
1 |
6 |
5 |
0 |
8 |
| Ever or Current Smoker (%) |
45 |
68 |
46 |
56 |
47 |
51 |
54 |
43 |
57 |
55 |
84 |
56 |
| Diabetes (%) |
3 |
13 |
<1 |
3 |
11 |
3 |
8 |
6 |
14 |
15 |
27 |
26 |
| Prior CVD (%) |
<1 |
<1 |
<1 |
3 |
16 |
3 |
10 |
22 |
13 |
26 |
73 |
35 |
| Body Mass Index (kg/m2) |
25 |
27 |
25 |
26 |
25 |
26 |
27 |
25 |
29 |
28 |
25 |
26 |
| (0.23) |
(0.75) |
(0.20) |
(0.14) |
(0.26) |
(0.20) |
(0.15) |
(0.45) |
(0.68) |
(0.24) |
(1.02) |
(1.18) |
|
| Systolic Blood Pressure, mm Hg |
112 |
130 |
112 |
119 |
145 |
118 |
131 |
143 |
130 |
143 |
150 |
145 |
| (0.46) |
(1.90) |
(0.34) |
(0.31) |
(1.86) |
(0.45) |
(0.59) |
(1.99) |
(2.22) |
(1.03) |
(6.01) |
(3.66) |
|
| Diastolic Blood Pressure, mm Hg |
68 |
78 |
69 |
74 |
73 |
75 |
76 |
72 |
76 |
75 |
71 |
73 |
| (0.41) |
(0.95) |
(0.35) |
(0.28) |
(1.05) |
(0.32) |
(0.25) |
(1.14) |
(0.85) |
(0.49) |
(2.11) |
(2.98) |
|
| Antihypertensive medication use (%) |
4 |
26 |
2 |
10 |
32 |
10 |
27 |
44 |
36 |
60 |
75 |
74 |
| Total Cholesterol (mg/dl) |
183 |
217 |
181 |
198 |
208 |
204 |
217 |
213 |
230 |
232 |
209 |
230 |
| (1.69) |
(4.92) |
(1.68) |
(1.14) |
(3.82) |
(1.57) |
(1.21) |
(5.49) |
(4.65) |
(2.81) |
(10.31) |
(14.24) |
|
| CRP (mg/dl; %) |
|
|
|
|
|
|
|
|
|
|
|
|
| <0.22 |
73 |
58 |
80 |
75 |
71 |
76 |
67 |
62 |
64 |
52 |
29 |
42 |
| 0.22-0.99 |
19 |
29 |
14 |
19 |
22 |
19 |
25 |
28 |
23 |
32 |
12 |
40 |
| ≥1.00 |
9 |
13 |
6 |
6 |
7 |
5 |
8 |
10 |
13 |
16 |
59 |
18 |
| ACR (mg/g; %) |
|
|
|
|
|
|
|
|
|
|
|
|
| <30 |
93 |
84 |
93 |
94 |
74 |
95 |
89 |
78 |
83 |
70 |
9 |
34 |
| 30-300 |
6 |
11 |
7 |
5 |
25 |
5 |
10 |
18 |
15 |
23 |
68 |
20 |
| ≥300 | <1 | 5 | <1 | <1 | 17 | 1 | 1 | 4 | 3 | 7 | 23 | 46 |
Abbreviations: CVD, Cardiovascular Disease; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease Study; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CRP, C-reactive protein; ACR, albumin-to-creatinine ratio.
Note: Conversion factors for units: low- and high-density lipoprotein cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129; creatinine in mg/dL to μmol/L, ×88.4; eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667; albumin-creatinine ratio in mg/g to mg/mmol, divide by 8.84.
a Participant numbers are unweighted N.
b Percent represents the population percent representative of the non-institutionalized US population; total may not equal 100% due to rounding.
c Values presented are population % for categorical variables and mean (linearized standard error) for continuous variables.
d One 86 year old Mexican-American female with serum creatinine = 0.488 mg/dL had eGFRMDRD = 120 ml/min/1.73 m2 and eGFRCKD-EPI 89 ml/min/1.73 m2 (data not shown).
e One 64 year old Mexican-American female with serum creatinine = 1.736 had eGFRMDRD = 29.5 ml/min/1.73 m2 and eGFRCKD-EPI = 30.6 ml/min/1.73 m2 (data not shown).
Estimated GFR and the risk of death
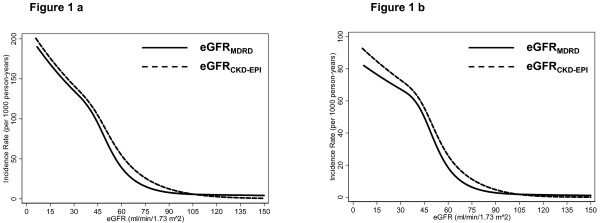
There were 3,620 deaths over 215,082 person-years of follow-up (median, 14.3 years). The weighted unadjusted incidence rate for all-cause mortality was higher for eGFRCKD-EPI compared with eGFRMDRD below 90 ml/min/1.73 m2 (Figure1a). Within categories of eGFR, the hazards for all-cause mortality adjusted for demographic characteristics, comorbidities, CRP and ACR were higher for eGFRCKD-EPI categories compared with eGFRMDRD categories (Table2). With both eGFR equations, there was U-shaped association with mortality with higher risk of death for eGFR above 120 ml/min/1.73 m2 and below 60 ml/min/1.73 m2 compared with eGFR 90–119 ml/min/1.73 m2. There were few individuals with eGFR <30 ml/min/1.73 m2 (eGFRMDRD, n = 56; eGFRCKD-EPI, n = 67) and few deaths in individuals with eGFR ≥120 ml/min/1.73 m2 (eGFRMDRD, n = 228; eGFRCKD-EPI, n = 179). Overall, the trends for association between eGFR categories and CVD mortality (n = 1,540) were very similar to all-cause mortality (Figure1b and Table2).
Figure 1 .
Unadjusted Incidence Rates of Mortality with eGFRMDRDand eGFRCKD-EPIin the US Population: NHANES III (1988–1994).a: All-Cause Mortality. b: Cardiovascular Disease Mortality.
Table 2.
Adjustedahazard ratio (95% Confidence interval) of all-Cause and cardiovascular disease mortality, by eGFR categories among 16,010 participants of NHANES III (1988–1994) with follow-up till December 31, 2006
| |
|
Categories of eGFR (ml/min/1.73 m2) |
||||
|---|---|---|---|---|---|---|
| |
|
≥ 120 |
90-119 |
60-89 |
30-59 |
< 30 |
| All-Cause Mortality | ||||||
|
EGFRCKD-EPI |
Participants, N |
3,889 |
7,151 |
3,947 |
956 |
67 |
| |
Deaths, N |
179 |
921 |
1,686 |
775 |
59 |
| |
HR |
2.05 |
Reference |
0.97 |
1.39 |
1.38 |
| |
(95% CI) |
(1.55-2.71) |
|
(0.86-1.10) |
(1.17-1.65) |
(0.88-2.16) |
|
EGFRMDRD |
Participants, N |
2,729 |
6,604 |
5,606 |
1,015 |
56 |
| |
Deaths, N |
228 |
805 |
1,788 |
750 |
49 |
| |
HR |
1.70 |
Reference |
0.94 |
1.31 |
1.96 |
| |
(95% CI) |
(1.36-2.14) |
|
(0.84-1.05) |
(1.11-1.56) |
(1.11-3.44) |
|
CVD Mortality |
|
|
|
|
|
|
|
EGFRCKD-EPI |
Participants, N |
3,889 |
7,151 |
3,947 |
956 |
67 |
| |
Deaths, N |
41 |
303 |
755 |
412 |
29 |
| |
HR |
2.70 |
Reference |
1.05 |
1.49 |
1.64 |
| |
(95% CI) |
(1.54-4.73) |
|
(0.87-1.26) |
(1.16-1.92) |
(1.02-2.65) |
|
EGFRMDRD |
Participants, N |
2,729 |
6,604 |
5,606 |
1,015 |
56 |
| |
Deaths, N |
64 |
278 |
774 |
401 |
23 |
| |
HR |
1.53 |
Reference |
0.95 |
1.32 |
2.17 |
| (95% CI) | (0.96-2.45) | (0.79-1.13) | (0.99-1.78) | (1.18-3.98) | ||
Abbreviations: eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease Study; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, Cardiovascular Disease.
a Adjusted for age, sex, race/ethnicity, prior CVD, diabetes, smoking, systolic and diastolic blood pressure, use of antihypertensive medications, body mass index, cholesterol, C-reactive protein category and albumin-to-creatinine ratio category.
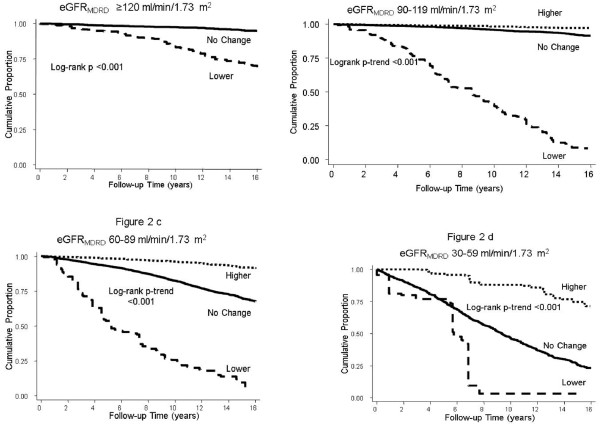
Reclassification by eGFRCKD-EPI and the risk of death
Figure2 displays the unadjusted cumulative incidence of death with reclassification by eGFRCKD-EPI. Those classified upwards to a higher eGFRCKD-EPI category had lower cumulative incidence of mortality while those reclassified downward to lower eGFRCKD-EPI category had higher cumulative incidence of death compared with those not reclassified. Overall, compared with no reclassification, the hazard ratio (HR) of all-cause mortality with reclassification to a higher eGFRCKD-EPI category was 0.34 (95% CI, 0.28-0.41) and with reclassification to a lower eGFRCKD-EPI category was 3.56 (95% CI, 3.04-4.16). After adjustment for age, sex and race/ethnicity, the HR was 0.91 (95% CI, 0.75-1.10) and 1.28 (95% CI, 1.11-1.48) for reclassification to a higher and lower eGFRCKD-EPI category, respectively.
Figure 2 .
Cumulative Incidence of All-Cause Mortality with Reclassification by eGFRCKD-EPIwithin eGFRMDRDCategories in the US Population: NHANES III (1988–1994).a: Reclassification within eGFRMDRD Category ≥120 ml/min/1.73 m2. b: Reclassification within eGFRMDRD Category 90–119 ml/min/1.73 m2. c: Reclassification within eGFRMDRD Category 60–89 ml/min/1.73 m2. d: Reclassification within eGFRMDRD Category 30–59 ml/min/1.73 m2.
Table3 displays the adjusted HR for all-cause and CVD mortality with reclassification by eGFRCKD-EPI within categories of eGFRMDRD. Among those classified as eGFRMDRD 30–59 ml/min/1.73 m2, reclassification to a higher eGFRCKD-EPI category was associated with a 47% lower hazard of death compared with those not reclassified (HR adjusted for demographic characteristics, comorbidities, CRP and albuminuria, 0.53; 95% CI, 0.34-0.84). There were very few individuals (n = 12) with eGFRMDRD 30–59 ml/min/1.73 m2 who were reclassified to a lower eGFRCKD-EPI category.
Table 3.
Reclassification and adjusted hazard ratiosaof all-cause and cardiovascular disease mortality by eGFR categories determined using the MDRD and the CKD-EPI study equation: NHANES III (1988–1994) - follow-up till December 31, 2006
| EGFRCKD-EPICategories | |||||
|---|---|---|---|---|---|
|
EGFRMDRDCategories |
>120 |
90-119 |
60-89 |
30-59 |
<30 |
|
>120 |
|
|
|
|
|
| Reclassified, N b (%)c |
2,416 (88.3) |
312 (11.7) |
|
|
|
|
All-Cause Mortality |
|
|
|
|
|
| Deaths, N b |
126 |
101 |
|
|
|
| HR (95% CI) |
REFERENCE |
0.57 (0.26-1.26) |
|
|
|
|
CVD Mortality |
|
|
|
|
|
| Deaths b |
28 |
36 |
|
|
|
| HR (95% CI) |
REFERENCE |
0.37 (0.15-0.94) |
|
|
|
|
90-119 |
|
|
|
|
|
| Reclassified, N b (%)c |
1,473 (20.2) |
4,969 (78.4) |
162 (1.4) |
|
|
|
All-Cause Mortality |
|
|
|
|
|
| Deaths, N b |
53 |
604 |
148 |
|
|
| HR (95% CI) |
1.42 (0.85-2.37) |
REFERENCE |
1.39 (0.94-2.07) |
|
|
|
CVD Mortality |
|
|
|
|
|
| Deaths b |
13 |
193 |
72 |
|
|
| HR (95% CI) |
2.95 (1.03-8.42) |
REFERENCE |
1.79 (0.94-3.39) |
|
|
|
60-89 |
|
|
|
|
|
| Reclassified, N b (%)c |
|
1,870 (42.9) |
3,664 (56.6) |
72 (0.5) |
|
|
All-Cause Mortality |
|
|
|
|
|
| Deaths, N b |
|
216 |
1,506 |
66 |
|
| HR (95% CI) |
|
1.13 (0.91-1.42) |
REFERENCE |
1.31 (1.01-1.69) |
|
|
CVD Mortality |
|
|
|
|
|
| Deaths b |
|
74 |
669 |
31 |
|
| HR (95% CI) |
|
1.02 (0.69-1.50) |
REFERENCE |
1.42 (1.01-1.99) |
|
|
30-59 |
|
|
|
|
|
| Reclassified, N b (%)c |
|
|
120 (19.4) |
883 (79.5) |
12 (1.1) d |
|
All-Cause Mortality |
|
|
|
|
|
| Deaths, N b |
|
|
31 |
708 |
11 |
| HR (95% CI) |
|
|
0.53 (0.34-0.84) |
REFERENCE |
N/A |
|
CVD Mortality |
|
|
|
|
|
| Deaths b |
|
|
14 |
381 |
6 |
| HR (95% CI) | 0.51(0.27-0.96) | REFERENCE | N/A | ||
Abbreviations: eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease Study; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HR, Hazard Rate; CVD, cardiovascular disease; N/A, not applicable.
a Adjusted for age, sex, race/ethnicity, prior CVD, systolic or diastolic blood pressure, use of antihypertensive medications, diabetes status, smoking status, body mass index, cholesterol, CRP category and albumin-to-creatinine ratio category.
b Numbers represent crude (un-weighted) participant number.
c Percent represents the population percent representative of the non-institutionalized US population; total may not equal 100% due to rounding.
d HR not presented as there are less than 30 people in the cell.
Those who were reclassified upwards to eGFRCKD-EPI ≥60 ml/min/1.73 m2 from eGFRMDRD 30–59 ml/min/1.73 m2 had similar risk of all-cause mortality as those with eGFR ≥60 ml/min/1.73 m2 by both equations (adjusted HR, 1.05; 95% CI, 0.62-1.77). Reclassification from eGFRMDRD category 60–89 ml/min/1.73 m2 to a lower eGFRCKD-EPI category (30–59 ml/min/1.73 m2) was associated with a higher risk of death (adjusted HR, 1.31; 95% CI, 1.01-1.69) compared with those not reclassified. There were no significant differences noted in all-cause mortality among individuals reclassified in eGFRCKD-EPI categories 90–119 and ≥120 ml/min/1.73 m2. Very similar trends were seen with CVD mortality.
Net reclassification improvement by eGFRCKD-EPI
To evaluate the effect of reclassification on mortality, we restricted our analysis to individuals with eGFRMDRD and eGFRCKD-EPI <120 ml/min/1.73 m2. Table4 presents the NRI for all-cause and CVD mortality. The overall NRI for eGFRCKD-EPI for all-cause mortality was 0.21 (p < 0.001) and for CVD mortality was 0.22 (p < 0.001). In age stratified analyses, the NRIs was lower as expected but remained substantial for participant age >65 years (0.14 for all-cause and 0.09 for CVD, p < 0.001). The NRI was also significant stratified by sex and most ethnicity groups as well as stratified by sex and limited to older participants (Additional file 1 Table S1, NRI 0.09 for men and 0.15 for women age >65 years).
Table 4.
Net reclassification improvement by the CKD-EPI equation among participants with eGFR <120 ml/min/1.73 m2by both equations stratified by age, sex and race
| |
Reclassification, number (Population %)a |
Deaths, |
NRI |
|
|---|---|---|---|---|
| All-cause (CVD) | All-cause | CVD | ||
|
Overall |
11,808 (24.1%) |
3,339 (1,463) |
0.2073*** |
0.2216*** |
|
By Age Categories | ||||
| 17-44 |
4,822 (30.3%) |
208 (58) |
0.0216 |
−0.0883 |
| 45-64 |
3,491 (21.8%) |
715 (258) |
−0.0146 |
−0.0006 |
| ≥65 |
3,495 (11.4%) |
2,416 (1,147) |
0.1362*** |
0.0943*** |
|
By Sex | ||||
| Male |
5,839 (22.1%) |
1,844 (796) |
0.2077*** |
0.2277*** |
| Females |
5,969 (26.0%) |
1,495 (667) |
0.2063*** |
0.2157*** |
|
By Race/Ethnicity | ||||
| NH Whites |
5,736 (25.7%) |
1,957 (903) |
0.2258*** |
0.2368*** |
| NH Blacks |
2,701 (12.8%) |
710 (290) |
0.1245*** |
0.1229*** |
| Mex-Am |
2,868 (20.2%) |
594 (239) |
0.0648** |
0.0682* |
| Others | 503 (20.2%) | 78 (31) | 0.1427* | 0.2559*** b |
Abbreviations: eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease Study; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; NRI, Net Reclassification Improvement; NH, Non-Hispanic; Mex-Am, Mexican-American.
a Reclassification to a different eGFR category by CKD-EPI equation. Number represents the number of participants; Population % is representative of the non-institutionalized US population.
b NRI estimates less precise as there are <50 events in this cell.
* p < 0.05; ** p < 0.01; *** p < 0.001.
Discussion
In this study of a representative sample of US adults during 18 years of follow-up, eGFRCKD-EPI improved risk stratification. Among those classified as eGFRMDRD 30–59 ml/min/1.73 m2, 19.4% were reclassified to eGFR >60 ml/min/1.73 m2 by the CKD-EPI equation and this upward reclassification was associated with 47% lower risk of all-cause mortality and 49% lower risk of CVD mortality compared with individuals with eGFR 30–59 ml/min/1.73 m2 by both the MDRD Study and CKD-EPI equations. Among those with eGFRMDRD 60–89 ml/min/1.73 m2, 0.5% were reclassified downwards to eGFR 30–59 ml/min/1.73 m2 by the CKD-EPI equation and this downward classification was associated with 31% higher risk of all-cause mortality and 42% higher risk of CVD mortality. Overall, CKD-EPI equation significantly improved risk prediction for both all-cause and CVD mortality. The better risk categorization by the CKD-EPI equation was observed particularly in those older than 65 years at baseline.
CKD-EPI equation improves GFR estimation compared with the MDRD Study equation and eGFRCKD-EPI is significantly more accurate than eGFRMDRD above and below 60 ml/min/1.73 m2 as well as across racial and ethnic subgroups [2,13]. The properties of the CKD-EPI equation result in higher eGFR in younger individuals, whites and females. In NHANES, we noted that reclassification moved individuals with higher comorbidities to a lower eGFRCKD-EPI category and individuals with lower comorbidities to higher eGFRCKD-EPI categories. In the clinically important CKD stage 3 (eGFRMDRD 30–59 ml/min/1.73 m2), almost 20% of the population was reclassified to a higher eGFRCKD-EPI category. In unadjusted and adjusted analyses, this reclassification was associated with a lower risk of death, suggesting that eGFRCKD-EPI in this range may have clinical significance even without information about other comorbidities. These findings have important implications for both individual clinical risk stratification and screening.
A number of recent studies have demonstrated that the use of eGFRCKD-EPI results in improvement in risk classification of individuals. In the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study, 25% of the participants with eGFRMDRD <60 ml/min/1.73 m2 were reclassified to higher eGFRCKD-EPI category [4]. The risk of death in individuals with eGFRMDRD <60 ml/min/1.73 m2 reclassified to eGFR >60 ml/min/1.73 m2 by the CKD-EPI equation was similar to those with eGFRMDRD ≥60 ml/min/1.73 m2 (HR, 1.01; 95% CI, 0.62-1.97). In the Atherosclerosis Risk in Communities (ARIC) Study, a prospective cohort of 45–64 year old whites and African-Americans from 4 US communities (N = 13,905), reclassification from eGFRMDRD 30–59 ml/min/1.73 m2 to eGFRCKD-EPI 60–89 ml/min/1.73 m2 was associated with a lower risk of all-cause mortality in the unadjusted models but was no longer significant after adjustment for age, sex and race. NRI for all-cause mortality was 0.095 (p < 0.001) [3]. Among participants of Kidney Early Evaluation Program (KEEP), a US screening program for individuals at high risk of chronic kidney disease, 17.5% were reclassified to higher eGFR categories and 2.7% to lower categories. Upward reclassification was associated with lower mortality, downward reclassification was associated with higher mortality and NRI was 0.159 [6]. In a post hoc analysis of the VALIANT trial, eGFRCKD-EPI categories improved risk stratification and the NRI for the composite end point of CVD death, recurrent myocardial infarction, heart failure, or stroke was 0.087 [5]. Our study extends these findings in a study with results generalizable to the US population and quantifies the improvement in risk stratification in African-Americans and Mexican-Americans.
We used NRI to assess the improvement in risk prediction with CKD-EPI equation compared with the MDRD Study equation. The improvement in risk prediction is expected to be relatively small as both equations use the same variables and have the inherent limitation of serum creatinine as a marker of GFR. Traditional methods for risk prediction, such as area under the receiver-operating-characteristic curve (AUC) require large independent associations, often over 2–3 fold, to result in meaningful improvement in AUC [14-16]. The NRI is particularly well suited when categories are associated with clinical action as is the case for estimated GFR. The NRI was lower in younger age groups despite high reclassification rates, possibly since death rates are low at younger age. Among older age groups (>65 years), NRIs were quite high (0.14 for all-cause and 0.09 for CVD mortality) despite reclassification being lower (11% compared to >30% in younger ages). Notably, the NRIs in the older age group remain significant even after stratification with sex or race/ethnicity. In contrast to ARIC and KEEP, NRIs in NHANES were not positive or statistically significant in those 45–64 years of age. These discrepant findings require further study across a range of populations.
We noted increased risk of all-cause and CVD death among individuals with eGFR ≥120 ml/min/1.73 m2 by either equation compared with eGFR 90–119 ml/min/1.73 m2 after the adjustment for potential confounders (Table2). The HR was higher for eGFRCKD-EPI than for eGFRMDRD in this category. This association has been noticed in previous studies and replicates the well-known U- shaped relationship between serum creatinine and mortality. This U-shape likely includes components of low muscle mass and hyperfiltration which cannot be separated using only serum creatinine as a marker of GFR. They persist despite the inclusion of a spline term for serum creatinine in the CKD-EPI equation which reduces the very high eGFRs calculated using the MDRD Study equation.
The strengths of our study includes its large sample size, prospective design, large sample of racial/ethnic minorities, broad age range of the population, rigorous data collection and extensive information on covariates, prior work to standardize serum creatinine, measurement of ACR and CRP, near-complete mortality follow-up using the NDI and large number of events during the follow-up period. The results of our study are generalizable to the non-institutionalized population of the U.S. Some limitations of our study also deserve mention. GFR was not measured but was estimated using serum creatinine and not all participants were fasting prior to serum creatinine measurement. Nonetheless, serum creatinine and estimated GFR are routinely used measures of kidney function in clinical practice and our data reflect common clinical information. We had relatively few individuals with eGFRMDRD <30 ml/min/1.73 m2. Cause of death was ascertained via NDI linkage of NHANES III data and not independently adjudicated and there is potential of misclassification of CVD mortality. Importantly, we did not have information about kidney failure requiring renal replacement therapy, an important outcome that deserves examination in future studies.
Conclusion
The CKD-EPI equation for estimating GFR predicts risk at least as well as the MDRD Study equation in the general US population and improves risk classification of individuals, particularly among those older than 65 years. Our data, in conjunction with previously reported studies, suggest that adoption of CKD-EPI equation for eGFR reporting may improve clinical risk stratification.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TS, KM, LA and JC developed the study design. TS performed the statistical analyses and KM, BA, ES and YS assisted with statistical analyses and interpretation of data. TS drafted the manuscript and all authors read and approved the manuscript.
Disclosure
Dr. Shafi was supported by K23-DK-083514. Dr. Selvin was supported by K01-DK-076595. Drs. Astor and Coresh are supported by R01-DK-076770. Dr. Stevens is supported by K23-DK-081017.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Table S1. Net Reclassification Improvement by the CKD-EPI Equation among Participants with eGFR <120 ml/min/1.73 m2 by both equations stratified by Sex, Age and Race.
Contributor Information
Tariq Shafi, Email: tshafi@jhmi.edu.
Kunihiro Matsushita, Email: kmatsus5@jhmi.edu.
Elizabeth Selvin, Email: lselvin@jhsph.edu.
Yingying Sang, Email: ysang@jhsph.edu.
Brad C Astor, Email: bcastor@medicine.wisc.edu.
Lesley A Inker, Email: LInker@Tuftsmedicalcenter.org.
Josef Coresh, Email: coresh@jhu.edu.
Acknowledgement
Parts of this work were presented at American Society of Nephrology Annual Meeting, 2010 in Denver, Colorado.
References
- Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55(4):648–659. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55(4):660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162(3):548–554. doi: 10.1016/j.ahj.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Li S, Kurella Tamura M, Chen SC, Vassalotti JA, Norris KC, Whaley-Connell AT, Bakris GL, McCullough PA. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57(3 Suppl 2):S9–S16. doi: 10.1053/j.ajkd.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville; [ http://www.cdc.gov/nchs/nhanes/nh3data.htm]. Last accessed May 16, 2012. [Google Scholar]
- Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Office of Analysis and Epidemiology, Public-use Third National Health and Nutrition Examination Survey Linked Mortality File. , Hyattsville; 2010. [Available at the following address: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage_public_use.htm] [Google Scholar]
- National Center for Health Statistics. Office of Analysis and Epidemiology, The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality follow-up through 2006. Matching Methodology May 2009, Hyattsville; 2006. Available at the following address: http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf. Last accessed May 16, 2012. [Google Scholar]
- Pencina MJ, D'Agostino RB, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ. et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56(3):486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland P, O'Malley PG. When is a new prediction marker useful? A consideration of lipoprotein-associated phospholipase A2 and C-reactive protein for stroke risk. Arch Intern Med. 2005;165(21):2454–2456. doi: 10.1001/archinte.165.21.2454. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- Ware JH. The limitations of risk factors as prognostic tools. N Engl J Med. 2006;355(25):2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Net Reclassification Improvement by the CKD-EPI Equation among Participants with eGFR <120 ml/min/1.73 m2 by both equations stratified by Sex, Age and Race.