Abstract
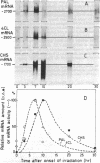
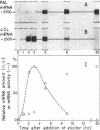
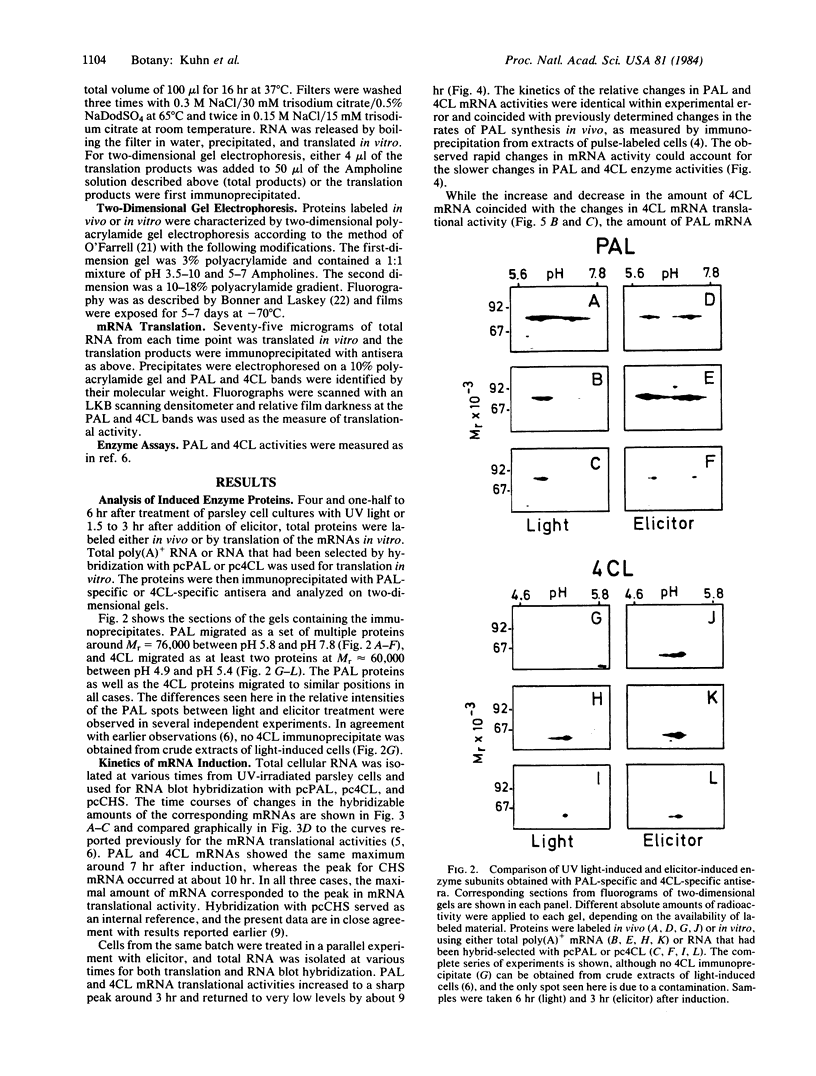
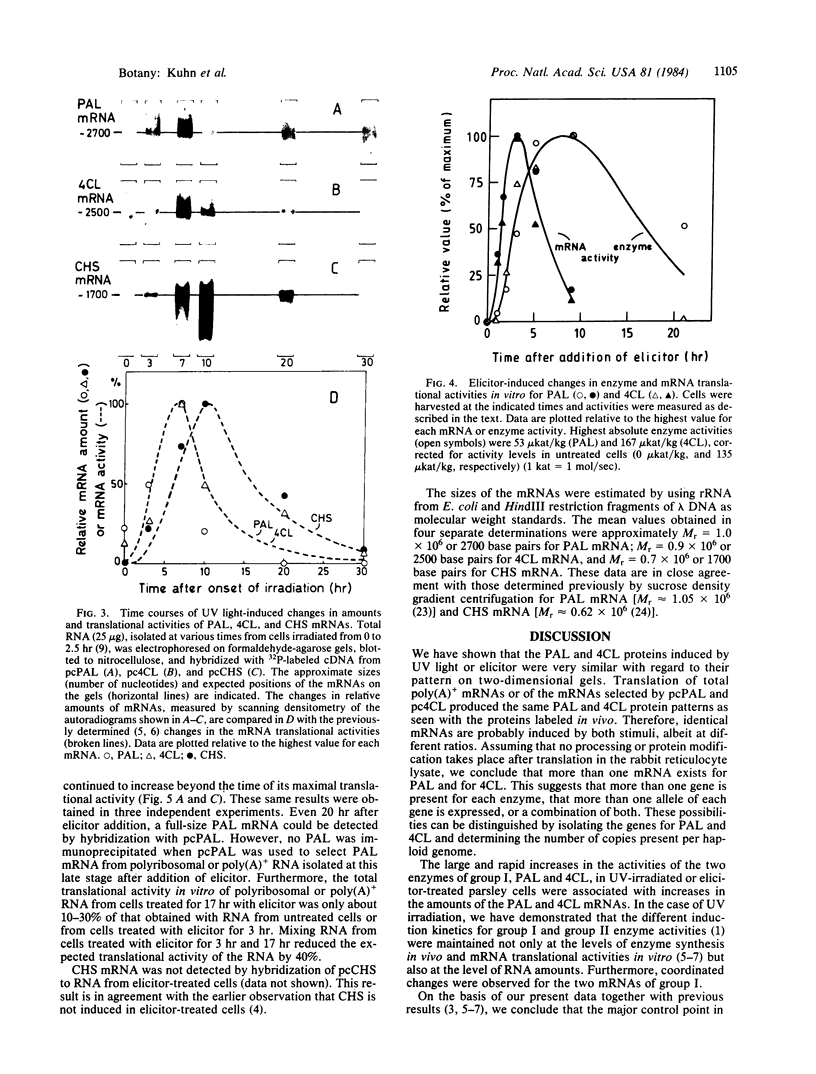
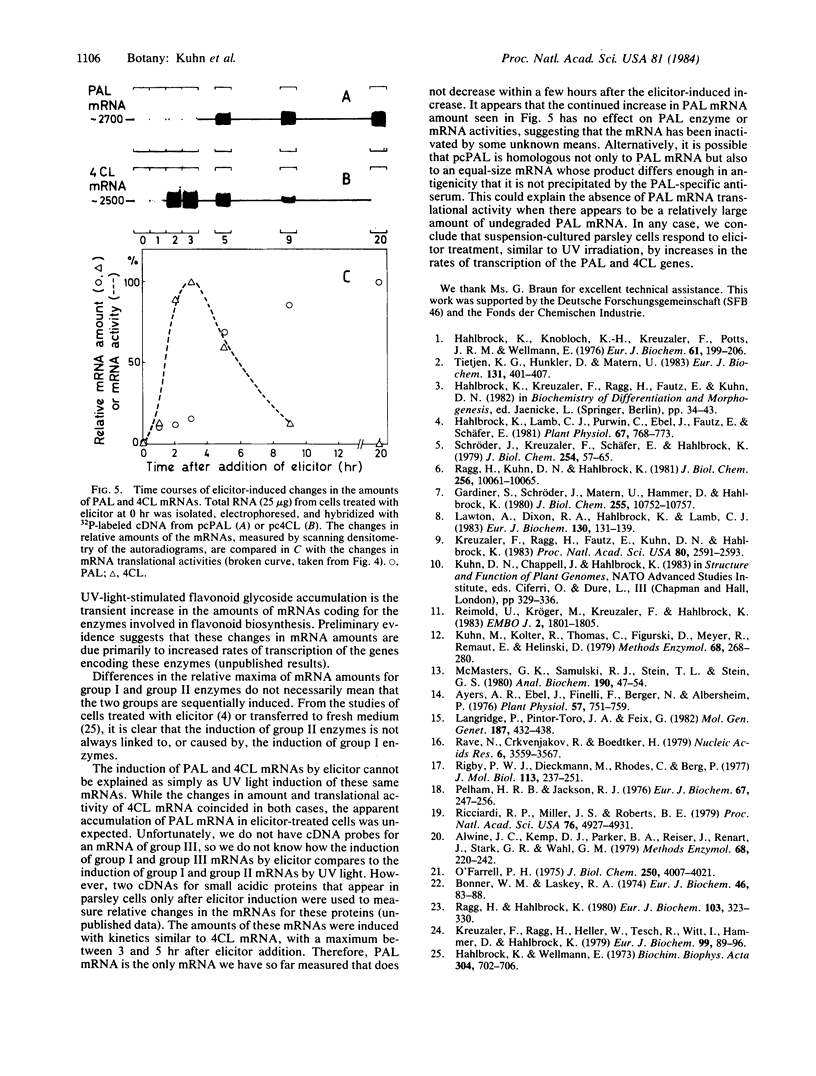
The mRNAs encoding two enzymes of phenylpropanoid metabolism, phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) and 4-coumarate:CoA ligase (4CL; EC 6.2.1.12), were induced in cultured parsley cells (Petroselinum hortense) either by irradiation with UV light or by treatment with elicitor, a cell-wall fraction of the fungus Phytophthora megasperma f. sp. glycinea. Two-dimensional gel electrophoresis of the encoded PAL and 4CL proteins revealed that the mRNAs induced by either treatment were very similar if not identical. RNA blot hybridization with cDNAs complementary to these mRNAs was used to measure changes in the mRNA amounts at various times after either treatment. Total cellular PAL and 4CL mRNA amounts increased coordinately after UV irradiation to a maximum at 7 hr and then decreased to uninduced levels by 30 hr with the same kinetics as observed previously for the changes in the translational activities. Treatment with the fungal elicitor also caused coordinated, but more rapid, changes in PAL and 4CL mRNA translational activities, with a sharp peak occurring 3 hr after the addition of elicitor. Corresponding changes in mRNA amounts were observed only for 4CL, whereas the amount of PAL mRNA continued to increase at least up to 20 hr after elicitor addition. Our results suggest that parsley cells respond to UV irradiation or addition of fungal elicitor by increased rates of transcription of genes involved in the synthesis of compounds related to UV or disease resistance, respectively.
Keywords: two-dimensional gels, cloned cDNAs, RNA blot hybridization, coordinated gene expression, Petroselinum hortense
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Schröder J., Matern U., Hammer D., Hahlbrock K. mRNA-dependent regulation of UDP-apiose synthase activity in irradiated plant cells. J Biol Chem. 1980 Nov 25;255(22):10752–10757. [PubMed] [Google Scholar]
- Hahlbrock K., Knobloch K. H., Kreuzaler F., Potts J. R., Wellmann E. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1976 Jan 2;61(1):199–206. doi: 10.1111/j.1432-1033.1976.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Lamb C. J., Purwin C., Ebel J., Fautz E., Schäfer E. Rapid Response of Suspension-cultured Parsley Cells to the Elicitor from Phytophthora megasperma var. sojae: INDUCTION OF THE ENZYMES OF GENERAL PHENYLPROPANOID METABOLISM. Plant Physiol. 1981 Apr;67(4):768–773. doi: 10.1104/pp.67.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K., Wellmann E. Light-independent induction of enzymes related to phenylpropanoid metabolism in cell suspension cultures from parsley. Biochim Biophys Acta. 1973 May 28;304(3):702–706. doi: 10.1016/0304-4165(73)90215-8. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Fautz E., Kuhn D. N., Hahlbrock K. UV-induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc Natl Acad Sci U S A. 1983 May;80(9):2591–2593. doi: 10.1073/pnas.80.9.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Heller W., Tesch R., Witt I., Hammer D., Hahlbrock K. Flavanone synthase from Petroselinum hortense. Molecular weight, subunit composition, size of messenger RNA, and absence of pantetheinyl residue. Eur J Biochem. 1979 Aug 15;99(1):89–96. doi: 10.1111/j.1432-1033.1979.tb13235.x. [DOI] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Hahlbrock K., Lamb C. J. Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur J Biochem. 1983 Jan 17;130(1):131–139. [PubMed] [Google Scholar]
- McMaster G. K., Samulski R. J., Stein J. L., Stein G. S. Rapid purification of covalently closed circular DNAs of bacterial plasmids and animal tumor viruses. Anal Biochem. 1980 Nov 15;109(1):47–54. doi: 10.1016/0003-2697(80)90008-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ragg H., Hahlbrock K. Messenger RNA coding for phenylalanine ammonia-lyase. Characterization and partial purification from cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1980 Jan;103(2):323–330. doi: 10.1111/j.1432-1033.1980.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Ragg H., Kuhn D. N., Hahlbrock K. Coordinated regulation of 4-coumarate:CoA ligase and phenylalanine ammonia-lyase mRNAs in cultured plant cells. J Biol Chem. 1981 Oct 10;256(19):10061–10065. [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold U., Kröger M., Kreuzaler F., Hahlbrock K. Coding and 3' non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J. 1983;2(10):1801–1805. doi: 10.1002/j.1460-2075.1983.tb01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]