Abstract
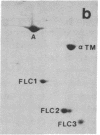
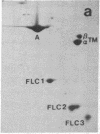
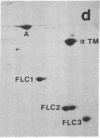
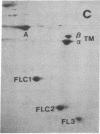
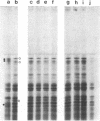
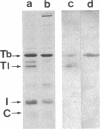
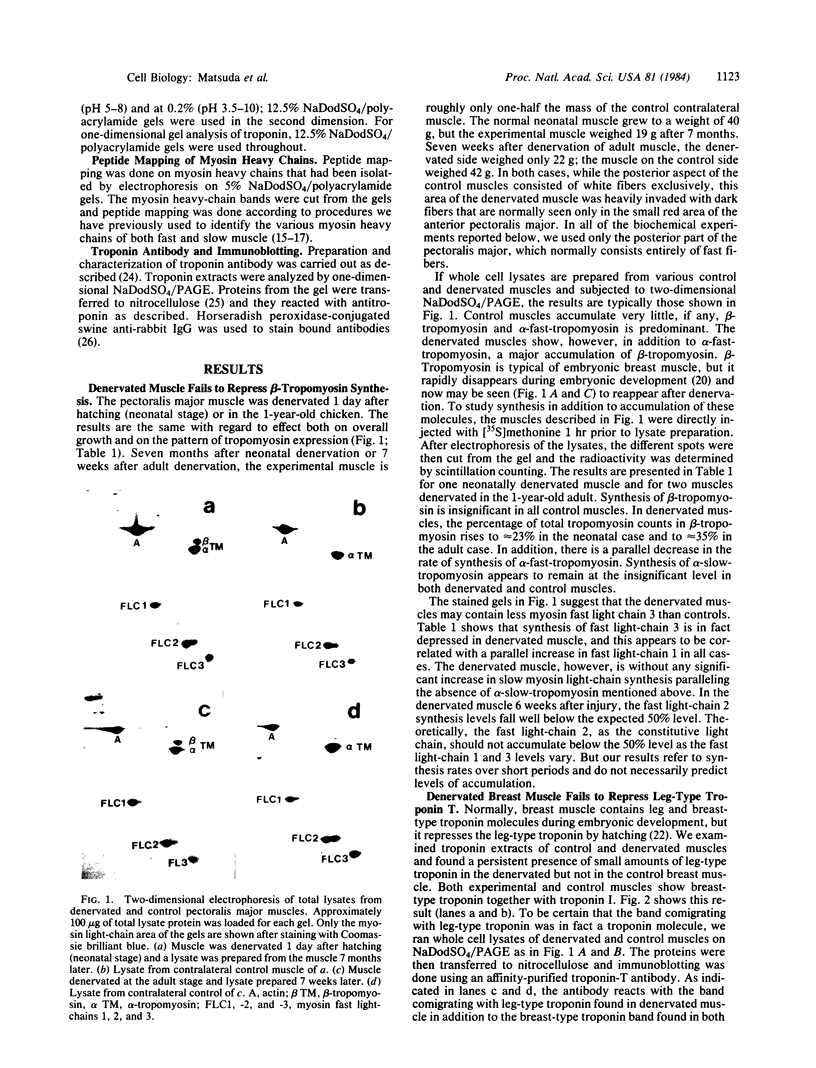
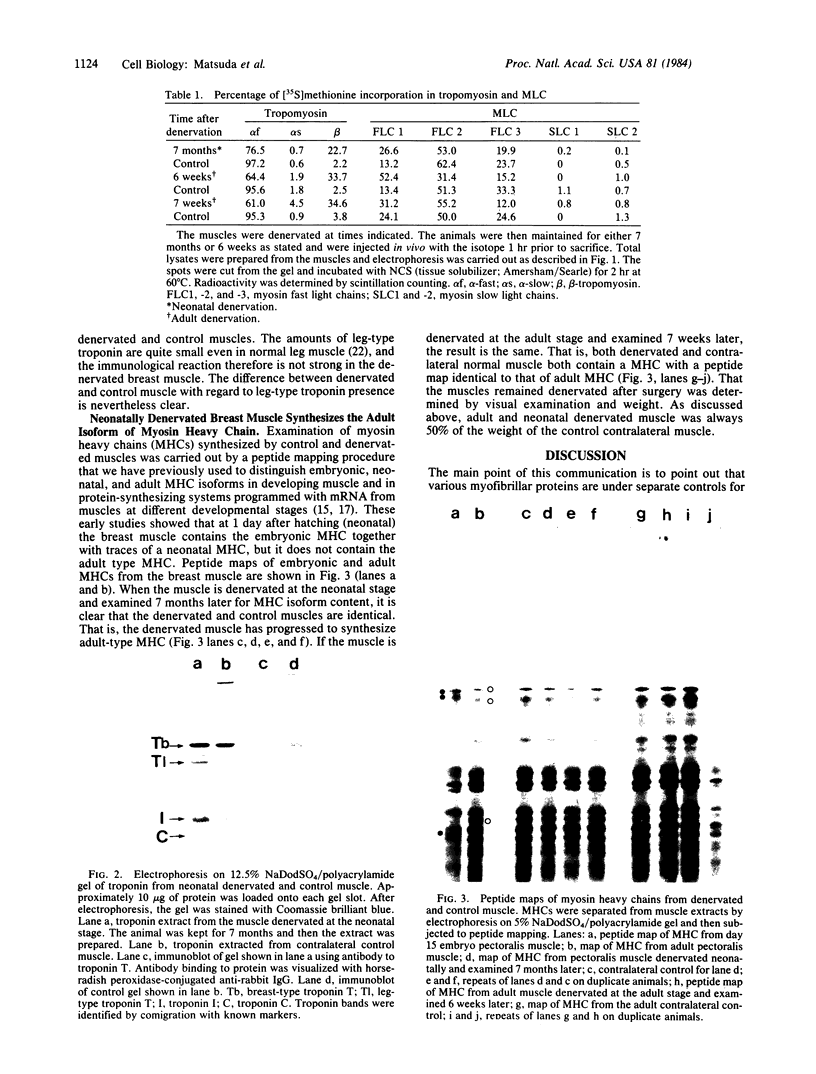
Synthesis patterns of myosin heavy- and light-chain isoforms, tropomyosin and troponin, have been studied in chicken fast muscle denervated at both neonatal and adult stages. Denervated neonatal muscle does not synthesize the adult myosin heavy-chain isoform at the time of denervation, but it does synthesize the adult isoform several months after denervation. Thus, innervation does not appear to be necessary for the normal sequential replacement of embryonic and neonatal myosin heavy chain by the adult variant. Nerve is required, however, for the regulation of tropomyosin and troponin expression. Normally the pectoralis major muscle represses synthesis of both beta-tropomyosin and leg-type troponin T during late embryonic development. After denervation, however, the muscle relaxes its ongoing repression of these proteins and significant amounts of both beta-tropomyosin and leg-type troponin T are synthesized by the muscle. Denervation also results in an altered pattern of myosin light-chain synthesis so that the ratio of fast light-chain 3/fast light-chain 1 decreases. Similar results are found in muscle denervated at the adult stage. In denervated muscle, therefore, synthesis of these myofibrillar proteins is not coordinated: ongoing isoform shifts proceed to express an adult pattern of myosin heavy chain while tropomyosin, troponin, and myosin light-chain patterns appear to revert to embryonic configurations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Perry S. V., Syska H., Brown M. D., Vrbova G. Cross innervation and the regulatory protein system of rabbit soleus muscle. Nature. 1975 Oct 16;257(5527):602–604. doi: 10.1038/257602a0. [DOI] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Differentiation of fast and slow muscles in the cat hind limb. J Physiol. 1960 Feb;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandman E., Matsuda R., Micou-Eastwood J., Strohman R. In vitro translation of RNA from embryonic and from adult chicken pectoralis muscle produces different myosin heavy chains. FEBS Lett. 1981 Dec 28;136(2):301–305. doi: 10.1016/0014-5793(81)80640-0. [DOI] [PubMed] [Google Scholar]
- Bandman E., Matsuda R., Strohman R. C. Developmental appearance of myosin heavy and light chain isoforms in vivo and in vitro in chicken skeletal muscle. Dev Biol. 1982 Oct;93(2):508–518. doi: 10.1016/0012-1606(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Butler-Browne G. S., Bugaisky L. B., Cuénoud S., Schwartz K., Whalen R. G. Denervation of newborn rat muscle does not block the appearance of adult fast myosin heavy chain. Nature. 1982 Oct 28;299(5886):830–833. doi: 10.1038/299830a0. [DOI] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro U., Dalla Libera L., Catani C. Myosin light and heavy chains in muscle regenerating in absence of the nerve: transient appearance of the embryonic light chain. Exp Neurol. 1983 Jan;79(1):106–117. doi: 10.1016/0014-4886(83)90382-5. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Changes in the forms of the components of the troponin complex during regeneration of injured skeletal muscle. Muscle Nerve. 1982 Jan;5(1):39–47. doi: 10.1002/mus.880050108. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Heilig A., Pette D. Changes in transcriptional activity of chronically stimulated fast twitch muscle. FEBS Lett. 1983 Jan 24;151(2):211–214. doi: 10.1016/0014-5793(83)80071-4. [DOI] [PubMed] [Google Scholar]
- Hoh J. F. Developmental changes in chicken skeletal myosin isoenzymes. FEBS Lett. 1979 Feb 15;98(2):267–270. doi: 10.1016/0014-5793(79)80197-0. [DOI] [PubMed] [Google Scholar]
- Hoh J. F. Neural regulation of mammalian fast and slow muscle myosins: an electrophoretic analysis. Biochemistry. 1975 Feb 25;14(4):742–747. doi: 10.1021/bi00675a015. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Nonaka I., Sugita H., Mikawa T. Effect of denervation of neonatal rat sciatic nerve on the differentiation of myosin in a single muscle fiber. Exp Neurol. 1981 Aug;73(2):487–495. doi: 10.1016/0014-4886(81)90282-x. [DOI] [PubMed] [Google Scholar]
- Margreth A., Dalla Libera L., Salviati G., Ischia N. Spinal transection and the postnatal differentiation of slow myosin isoenzymes. Muscle Nerve. 1980 Nov-Dec;3(6):483–486. doi: 10.1002/mus.880030604. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Umegane T. The primary structure of L-1 light chain of chicken fast skeletal muscle myosin and its genetic implication. FEBS Lett. 1981 Apr 6;126(1):111–113. doi: 10.1016/0014-5793(81)81045-9. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Bandman E., Strohman R. C. Regional differences in the expression of myosin light chains and tropomyosin subunits during development of chicken breast muscle. Dev Biol. 1983 Feb;95(2):484–491. doi: 10.1016/0012-1606(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Bandman E., Strohman R. C. The two myosin isoenzymes of chicken anterior latissimus dorsi muscle contain different myosin heavy chains encoded by separate mRNAs. Differentiation. 1982;23(1):36–42. doi: 10.1111/j.1432-0436.1982.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Obinata T., Shimada Y. Types of troponin components during development of chicken skeletal muscle. Dev Biol. 1981 Feb;82(1):11–19. doi: 10.1016/0012-1606(81)90424-3. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Spector D. H., Strohman R. C. Regenerating adult chicken skeletal muscle and satellite cell cultures express embryonic patterns of myosin and tropomyosin isoforms. Dev Biol. 1983 Dec;100(2):478–488. doi: 10.1016/0012-1606(83)90240-3. [DOI] [PubMed] [Google Scholar]
- Metafora S., Felsani A., Cotrufo R., Tajana G. F., Del Rio A., De Prisco P. P., Rutigliano B., Esposito V. Neural control of gene expression in the skeletal muscle fibre: changes in the muscular mRNA population following denervation. Proc R Soc Lond B Biol Sci. 1980 Aug 13;209(1175):257–273. doi: 10.1098/rspb.1980.0094. [DOI] [PubMed] [Google Scholar]
- Montarras D., Fiszman M. Y., Gros F. Changes in tropomyosin during development of chick embryonic skeletal muscles in vivo and during differentiation of chick muscle cells in vitro. J Biol Chem. 1982 Jan 10;257(1):545–548. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Obinata T., Shimada Y., Matsuda R. Troponin in embryonic chick skeletal muscle cells in vitro. An immunoelectron microscope study. J Cell Biol. 1979 Apr;81(1):59–66. doi: 10.1083/jcb.81.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Direct detection of antigens in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1977 Apr;78(2):483–490. doi: 10.1016/0003-2697(77)90108-7. [DOI] [PubMed] [Google Scholar]
- Robert B., Weydert A., Caravatti M., Minty A., Cohen A., Daubas P., Gros F., Buckingham M. cDNA recombinant plasmid complementary to mRNAs for light chains 1 and 3 of mouse skeletal muscle myosin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2437–2441. doi: 10.1073/pnas.79.8.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. K., Mabuchi K., Sarkar S., Mis C., Sreter F. A. Changes in tropomyosin subunit pattern in chronic electrically stimulated rabbit fast muscles. Biochem Biophys Res Commun. 1979 Jul 12;89(1):181–187. doi: 10.1016/0006-291x(79)90961-6. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Pepe F. A., Holtzer H. Myosin types during the development of embryonic chicken fast and slow muscles. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4524–4527. doi: 10.1073/pnas.74.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streter F. A., Gergely J., Salmons S., Romanul F. Synthesis by fast muscle of myosin light chains characteristic of slow muscle in response to long-term stimulation. Nat New Biol. 1973 Jan 3;241(105):17–19. doi: 10.1038/newbio241017a0. [DOI] [PubMed] [Google Scholar]
- Toyota N., Shimada Y. Isoform variants of troponin in skeletal and cardiac muscle cells cultured with and without nerves. Cell. 1983 May;33(1):297–304. doi: 10.1016/0092-8674(83)90358-6. [DOI] [PubMed] [Google Scholar]
- Umeda P. K., Sinha A. M., Jakovcic S., Merten S., Hsu H. J., Subramanian K. N., Zak R., Rabinowitz M. Molecular cloning of two fast myosin heavy chain cDNAs from chicken embryo skeletal muscle. Proc Natl Acad Sci U S A. 1981 May;78(5):2843–2847. doi: 10.1073/pnas.78.5.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]