Abstract
OBJECTIVE
To explore associations between diabetes etiology (type 1 diabetes mellitus [T1DM] vs. T2DM) and glycemic control in the prediction of 5-year periodontal status change.
RESEARCH DESIGN AND METHODS
The Study of Health in Pomerania (SHIP) is a population-based stratified sample of German men and women. Healthy participants and those determined to have T2DM arose from the SHIP cohort, and T1DM participants were recruited from diabetes clinics in the catchment area that gave rise to SHIP. Dentate participants (n = 2,626; 53% women; 20–81 years of age) were included. Diabetes was determined via physician diagnosis and/or HbA1c ≥6.5% (uncontrolled diabetes >7.0%). Examiners blinded to diabetes status performed random half-mouth periodontal examinations, assessing probing depth (PD) and attachment loss (AL) (four sites/tooth) at baseline and follow-up. Participants were categorized into six groups as follows: 1) diabetes free (n = 2,280), 2) incident T2DM (n = 79), 3) controlled T2DM (n = 80), 4) uncontrolled T2DM (n = 72), 5) controlled T1DM (n = 43), and 6) uncontrolled T1DM (n = 72). In multivariable regressions, mean PD change (ΔMPD), mean AL change (ΔMAL), or incident tooth-loss values were regressed across the aforementioned diabetes categories.
RESULTS
Mean (SD) ΔMPD and ΔMAL values among all participants were −0.08 ± 0.5 mm and 0.08 ± 1.03 mm, respectively, and 34% lost one or more teeth. Relative to diabetes-free participants, those with uncontrolled T2DM experienced greater ΔMPD ± SE (P < 0.05), whereas participants with either uncontrolled T1DM or uncontrolled T2DM realized greater ΔMAL (P < 0.05). Uncontrolled T1DM and T2DM were both associated with an increased risk of future tooth loss (P < 0.05).
CONCLUSIONS
Diabetes control, but not etiology, was associated with future tooth loss and accelerated AL progression.
Periodontal disease is a common condition (1) characterized in its early stages by gingival inflammation. If left untreated, progressive collagen and bone loss can weaken the tooth’s anchoring in the alveolar housing and result in tooth loss. Several periodontal disease risk factors have been identified previously (2,3), including diabetes, which has long been viewed as a strong causal risk factor for periodontal pathology (4–10).
The biological plausibility has been well documented, and the best evidence suggests the potential influence of diabetes on periodontal disease is likely explained by 1) a hyperinflammatory response to infection, 2) uncoupling of bone destruction and repair, and/or 3) the effects of advanced glycation end products (10,11). Numerous reports have repeatedly documented elevated levels of prevalent periodontal disease among individuals with diabetes when compared with healthy participants. However, most studies have been cross-sectional, precluding the ability to firmly demonstrate temporality of associations. Of the few longitudinal studies published to date, many have been from selected patient populations with low sample sizes and limited confounder adjustment, and these studies often do not specify the type of diabetes included in the study (8). No study to date has examined the influence of both type 1 diabetes mellitus (T1DM) and T2DM together alongside healthy controls in a prospective cohort study.
The goal of the current study was to examine whether diabetes status and glycemic control were associated with 5-year progression of clinical periodontal disease among a representative sample of community-based participants enrolled in the Study of Health in Pomerania (SHIP) as well as among a cohort of T1DM patients identified via a diabetes clinic and diabetologists in the same catchment area as SHIP.
RESEARCH DESIGN AND METHODS
SHIP is a population-based prospective cohort in East Germany involving the cities of Greifswald, Stralsund, and Anklam and 29 surrounding villages; the 1995 population in this catchment area was 212,157. German subjects with main residency in the area were randomly drawn, proportional to each community population and stratified by age and sex. A representative sample of 7,008 adults 20–79 years of age was invited to participate. This two-stage cluster sampling method was adopted from the MONICA Project (Augsburg, Germany) and yielded 12 five-year age strata (20–79 years) for both sexes. After removing 746 individuals (126 died, 615 moved away, and 5 had severe medical problems), 6,262 inhabitants were invited. The final sample included 4,308 individuals, yielding a 68.8% response (12,13). Between the baseline and the 5-year follow-up examinations, there were 130 passive nonrespondents (due to migration) and 231 deceased subjects. Of the remaining 3,949 eligible people, 649 were active nonrespondents and 3,300 subjects were reexamined, resulting in an 83.6% follow-up response (14).
Enrollment of participants with T1DM
The T1DM cohort (at baseline, 233 subjects 20–81 years of age) was recruited from the Centre of Cardiology and Diabetes, Karlsburg, and diabetologic practices in the study region. Diagnosis of T1DM in these subjects was confirmed by the physician. These subjects lived in the same catchment area as the subjects recruited for SHIP. Baseline data collection for T1DM subjects was performed between December 1997 and December 2000. Data collection protocols among participants with T1DM were performed using the same methods as for the SHIP population. This sampling strategy was performed to ensure that adequate numbers of participants with T1DM were included in the study.
The study was approved by the University of Greifswald’s Institutional Review Board. All participants provided written informed consent.
Oral examination
Calibrated licensed dentists performed the oral examinations, including a full-mouth tooth count. The periodontal probe PCP 11 (Hu-Friedy, Chicago, IL), in SHIP-0, and the periodontal probe PCP 2 (Hu-Friedy), in SHIP-1, were used to assess periodontal probing depth (PD) and clinical attachment loss (AL) for examined teeth. Periodontal measurements were taken at four sites per tooth (mesiobuccal, midbuccal, distobuccal, and midlingual), using the half-mouth method on the right or left side in alternate subjects. On the same teeth, coronal caries was scored visually and with the periodontal probe. Carious and filled teeth were registered by surface, and decayed filled-tooth index was calculated according to World Health Organization criteria. Yearly, calibration exercises (15) yielded an intraclass correlation of 0.82–0.91 per examiner and an interrater correlation of 0.84 relative to AL. The use of different periodontal probes at baseline and follow-up can reduce precision of periodontal measures. Thus, we converted periodontal site measurements for SHIP-1 (PCP 2) according to correction values retrieved from a crossover study (16).
Diabetes classification
Among SHIP participants, diabetes was defined by self-reported physician’s diagnosis, use of antidiabetic drugs (Anatomical Therapeutic Chemical codes A10AB, A10BB, A10BC, A10BD, A10BF, A10BG, A10BH, and A10BX), or HbA1c ≥6.5%. Among SHIP participants identified as having diabetes, eight were reclassified as having T1DM based on age of onset (diagnosis before 30 years of age) or timing of insulin therapy initiation relative to diagnosis (<1 year from diagnosis). The remaining participants meeting the aforementioned case definition were defined as having T2DM. Uncontrolled diabetes was defined by HbA1c >7.0% (17).
HbA1c was measured from nonfasting blood samples at one laboratory that participated in the official Germany INSTAND (formerly Institut für Standardisierung und Dokumentation in medizinischen Laboratorium; currently, Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien) round-robin tests for quality assurance in analytical laboratories, at least semiannually, and internal quality controls were measured daily. At both visits, HbA1c was measured by cation-exchange chromatography (high-performance liquid chromatography) with spectrophotometric detection (Diamat Analyzer; Bio-Rad, Munich, Germany) and a coefficient of variation of 1.5%.
Risk factor assessment
Participants were queried by computer-aided face-to-face interviews on sociodemographic characteristics, medical histories, and common diabetes medications (i.e., Anatomical Therapeutic Chemical codes A10AB, A10BB, A10BC, A10BD, A10BF, A10BG, A10BH, and A10BX). A self-administered questionnaire assessed region (rural vs. urban) and education level (<10, 10, or >10 years of schooling). Smoking behavior was assessed with a validated questionnaire and categorized as never/occasional, former, or current smoker (18).
Height and weight were determined using calibrated scales. Waist circumference was measured at the narrowest place between the last rib and the highest part of the abdomen. Hip circumference accorded to the greatest circumference between the highest point of the iliac crest and the crotch.
High-sensitivity C-reactive protein (hsCRP) was determined in serum by particle-enhanced immunonephelometry (hsCRP Kit; Dade Behring, Inc.) with a test sensitivity of 0.2 mg/L. Triglycerides were determined enzymatically using reagents from Roche Diagnostics (Hitachi 717; Roche Diagnostics, Mannheim, Germany). Plasma fibrinogen concentrations were assayed according to Clauss using an Electra 1600 Analyzer (Instrumentation Laboratory, Barcelona, Spain). White blood cell (WBC) count was measured by the impedance measurement method using the Coulter principle (Coulter MaxM; Coulter Electronics, Miami, FL). Urinary excretion of deoxypyridinoline (DPD) was determined by a chemiluminescence enzyme immunoassay (Fa. DPC Biermann, Bad Nauheim, Germany). All values were related to the creatinine concentration in urine. Creatinine was determined by standard laboratory methods. DPD results were not performed among participants with T1DM in this study.
Among the 3,446 participants who were either diabetes free or had T1DM or T2DM at baseline, we excluded participants from the current analysis if they did not receive a periodontal exam during the follow-up visit (n = 769) or if there was missing data on variables considered essential; the analysis included HbA1c, age, sex, waist-to-hip ratio, education level, and smoking status (n = 51), leaving 2,626 participants for analysis.
Statistical analyses
Analyses were performed using SAS for Windows version 9.2. Multivariable linear regression analyses modeled diabetes status as an independent variable and change in periodontal status during 5 years of follow-up as the dependent variable. Diabetes status was defined as 1) diabetes free (n = 2,280), 2) incident T2DM (n = 79), 3) controlled T2DM (n = 80), 4) uncontrolled T2DM (n = 72), 5) controlled T1DM (n = 43), or 6) uncontrolled T1DM (n = 72). In sensitivity analyses, continuous HbA1c (as opposed to diabetes type and control) was modeled as an independent variable. Periodontal disease change was defined by change in mean PD (ΔMPD), mean AL (ΔMAL), or mean percent of sites with ≥5 mm; change was calculated as year 5 minus baseline values so that negative values indicate improvement and positive values indicate progression of periodontal disease. Selected multivariable models included baseline periodontal status as a covariable for the prediction of 5-year periodontal change. These models are included for the readers’ information, although we caution that these models are highly susceptible to biased findings, as previously discussed (19). A multivariable, modified Poisson regression method (20) was used to examine the risk of incident tooth loss associated with baseline diabetes status. Multivariable linear regression also considered ΔMPD, ΔMAL, and number of lost teeth during follow-up as outcomes.
RESULTS
Participants were Caucasian, 46 ± 14 years of age (mean ± SD), and 52% female. Baseline diabetes status was associated with many sociodemographic, behavior/lifestyle, and medical variables, as summarized in Supplementary Table 1. At baseline, the mean tooth count was 22 ± 6, and MPD and MAL values were 2.4 ± 0.6 and 2.4 ± 1.6 mm, respectively. The prevalence of any site with AL ≥5 mm was 56%, and the mean percent of sites/mouth with ≥5 mm AL was 14 ± 23%. Mean 5-year tooth loss was 0.8 ± 1.7, and 34% of participants lost one or more teeth. Mean 5-year ΔMPD and ΔMAL were −0.08 ± 0.51 and 0.08 ± 1.03 mm, respectively. Baseline AL was strongly correlated with tooth loss (r = 0.40; P < 0.0001), but change in AL was unrelated to tooth loss (r = 0.04; P = 0.07).
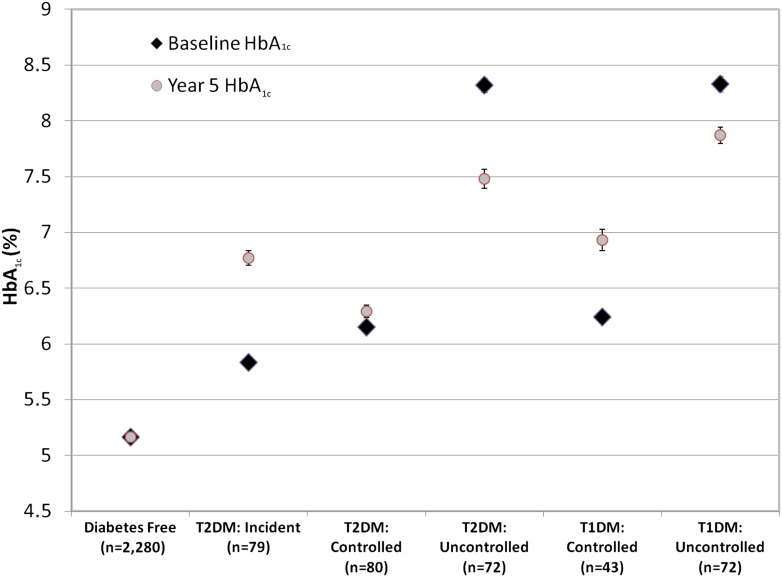
The proportions of individuals with T1DM and T2DM at baseline were 4.4 and 5.8%, respectively; the relatively high proportion of T1DM is due to the enrollment strategy, which oversampled T1DM to obtain adequate sample size. Among participants with T2DM at baseline, the prevalence of undiagnosed diabetes was 26%. Baseline HbA1c was 5.4 ± 0.9%, and change in HbA1c was 0.01 ± 0.7%. Figure 1 shows the baseline and year-5 HbA1c values according to diabetes status. Among participants with controlled T2DM, 37% were receiving pharmacological treatment as compared with 62% of participants with uncontrolled T2DM. The distribution of different drug classifications used among participants with controlled and uncontrolled T2DM is summarized in Supplementary Fig. 1A and B.
Figure 1.
Mean ± SD values of baseline and 5-year follow-up HbA1c by diabetes status in SHIP. (A high-quality color representation of this figure is available in the online issue.)
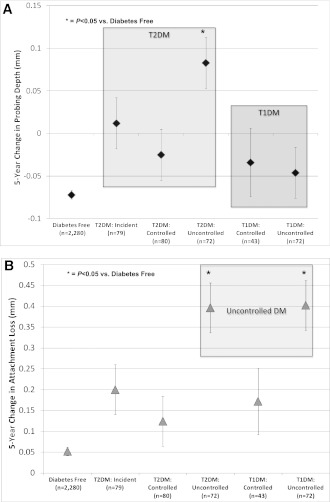
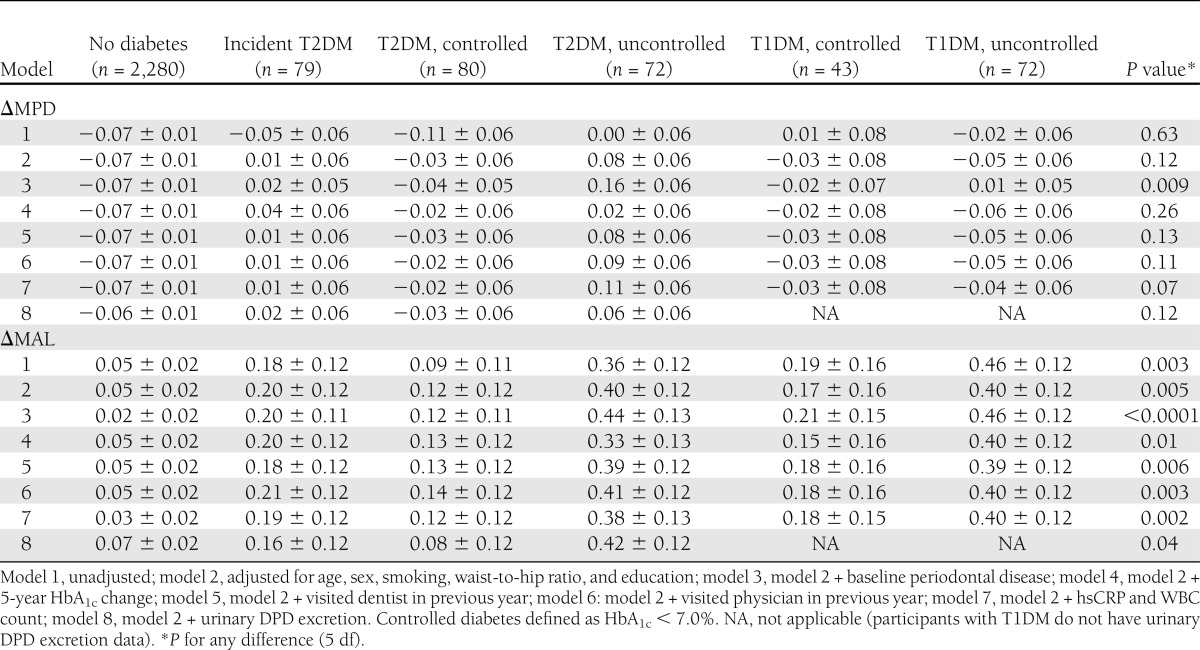
Both uncontrolled T2DM and T1DM were statistically significantly associated with progression of AL relative to diabetes-free participants, whereas controlled diabetes was not associated with AL progression (Fig. 2). When compared with diabetes free participants, those with either incident T2DM or uncontrolled T2DM experienced less favorable PD changes, although these findings were only statistically significant for uncontrolled T2DM (Fig. 2). PD changes among participants with T1DM did not differ from diabetes-free participants (Fig. 2). Tooth loss adjustments did not meaningfully impact these periodontal disease change patterns. As shown in Table 1, adjustment for age, sex, smoking status, obesity, and educational level attenuated findings, whereas additional adjustments had little influence on the association between diabetes status and periodontal disease change. Findings were similar when considering change in the percent of sites with ≥5 mm AL. Relative to diabetes-free participants, both uncontrolled T1DM and T2DM were associated with a statistically significant 4% greater increase in the percent of 5-mm sites/mouth during 5 years of follow-up, whereas no statistically significant changes were observed among individuals with controlled diabetes.
Figure 2.
Five-year change in either MPD (P for any difference = 0.09) (A) or MAL (P for any difference = 0.09) (B) according to diabetes status. Adjusted for age, sex, smoking, waist-to-hip ratio, and education. Error bars represent SEs. DM, diabetes mellitus.
Table 1.
Adjusted mean ± SE absolute periodontal disease change by diabetes status: SHIP, 1997–2006
In multivariable linear regression analyses considering continuous HbA1c (as opposed to diabetes type and control) as the independent variable, a 1-point increase in baseline HbA1c was associated with a 0.04-mm increase in ΔMPD (P = 0.003) and a 0.07-mm increase in ΔMAL (P = 0.0008).
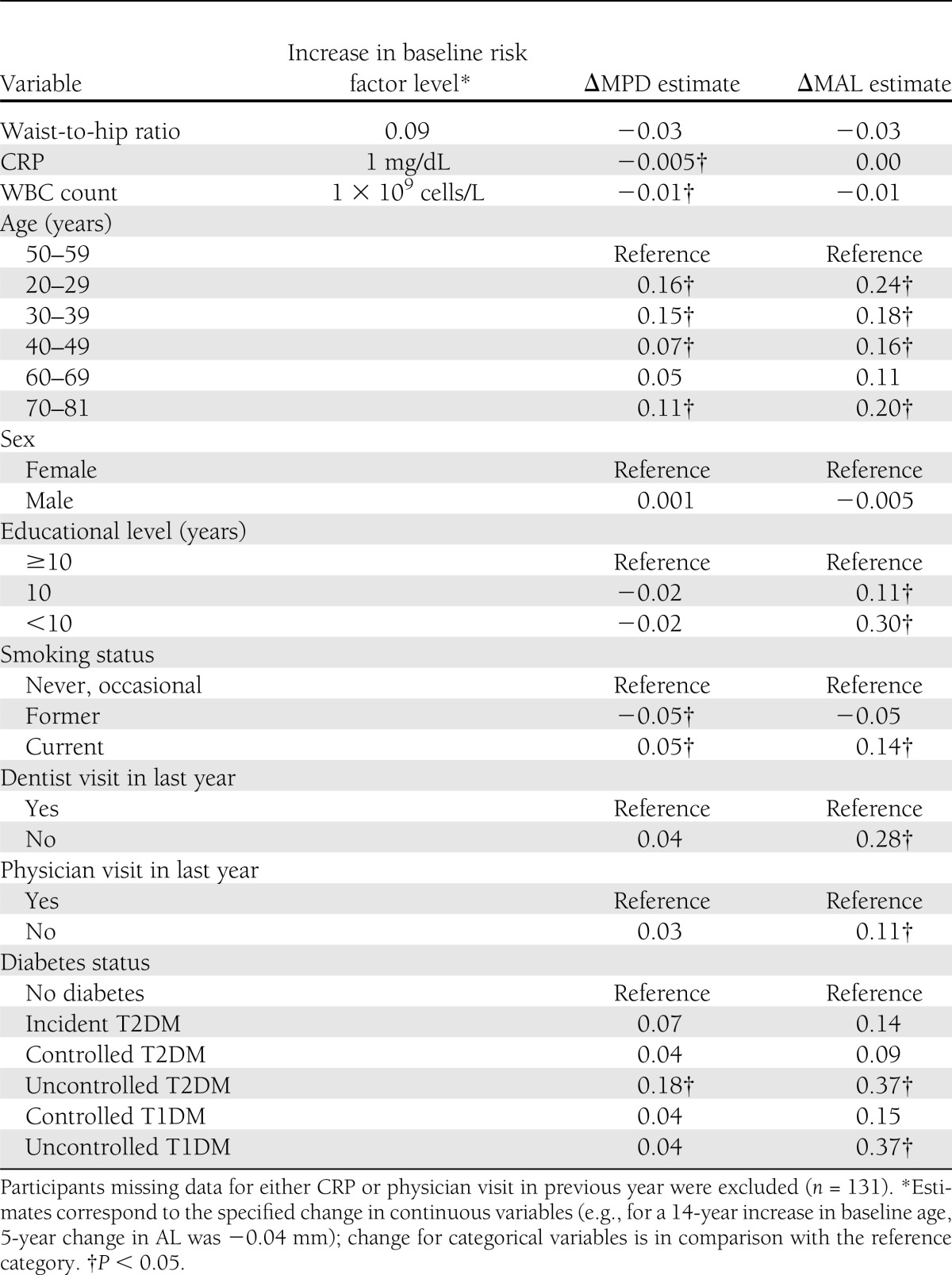
In multivariable models, CRP, WBC, and waist-to-hip ratio were associated with statistically significant improvements in MPD (Table 2). Higher education, visits to health care professionals, and never having been a smoker were associated with AL during follow-up (Table 2).
Table 2.
Five-year ΔMPD and ΔMAL estimates derived from multivariable regression modeling: SHIP, 1997–2006
Diabetes status was related to prospective tooth loss, and the association was primarily driven by tooth-loss patterns among participants with uncontrolled diabetes (T2DM or T1DM). Relative to diabetes-free participants, the risk ratios (RRs) (95% CI) for any tooth loss among uncontrolled T2DM or T1DM were 1.36 (1.11–1.67) and 1.93 (1.55–2.39), respectively, and RRs among controlled T2DM or T1DM were 1.01 (0.79–1.28) and 1.08 (0.67–1.71). Additional adjustment for baseline AL, but not 5-year AL, changes attenuated tooth-loss findings; RRs for any tooth loss among uncontrolled T2DM or T1DM in models adjusted for baseline AL were 1.21 (0.98–1.49) and 1.66 (1.27–2.15). Findings were stronger when predicting the risk of losing more than one tooth (Supplementary Fig. 2), particularly among T1DM. The periodontal status of teeth that were lost during the 5-year follow-up was substantially worse as compared with retained teeth (Supplementary Table 2).
In addition to diabetes status, higher age, lower education, current (but not former) smoking, elevated CRP (data not shown), and baseline AL were also tooth-loss predictors. A 1-mm increase in baseline AL was associated with a 25% increased risk of any tooth loss: RR 1.25 (1.19–1.28). Change in AL was not associated with incident tooth loss; RR for 1 mm of prospective AL 1.03 (0.98–1.08).
CONCLUSIONS
We have found uncontrolled T1DM and T2DM to be consistently related to increased rates of AL progression and tooth loss, whereas only uncontrolled T2DM was associated with PD changes.
To our knowledge, these are the first population-based, longitudinal data that can comprehensively and simultaneously examine the influence of both diabetes etiology and diabetes control on periodontal disease progression rates. Previous studies on the topic, although seminal, were limited by a lack of suitable control groups (i.e., diabetes-free participants) (6) or by the inclusion of only T1DM (7,21) or T2DM (4,9,22) but not both, precluding a side-by-side comparison of periodontal disease progression among participants free of diabetes or with T1DM or T2DM. Side-by-side comparisons are valuable given the different etiologies underlying T1DM versus T2DM and the potential for these underlying differences in pathobiology, as opposed to diabetes-related consequences such as hyperglycemia, to explain adverse periodontal outcomes. Moreover, previous research has often been limited to small, clinically based samples with limited external generalizability as well as limited multivariable modeling to remove confounding. Collectively, these limitations are particularly important for the question under study because it is not ethical or feasible to conduct randomized controlled trials to examine the influence of diabetes etiology or HbA1c control (i.e., these exposures cannot be randomized) on periodontal pathology. Therefore, we must rely on data from high-quality, longitudinal, observational studies. This information is important for dentists and physicians to best determine how carefully patients with uncontrolled diabetes should be monitored to prevent progression of periodontal disease and tooth loss.
The finding that only uncontrolled T1DM and T2DM were statistically significantly associated with progression of periodontal disease is consistent with previous studies examining glycemic control and progression of periodontal disease. Our current findings add importantly to previous literature by utilizing a more contemporary HbA1c cut point of 7.0% to define controlled versus uncontrolled diabetes (17); previous studies have used an HbA1c cut point of 9.0% (9,22). In doing so, the current findings from SHIP suggest that maintaining HbA1c as low as 7.0% is potentially important for reducing the risk for adverse periodontal outcomes.
The underlying biological mechanisms that might explain elevated risk of periodontal disease progression among individuals with diabetes have been extensively studied in animal models. Research to date implicates one or a combination of the following three mechanisms as recently summarized (10): 1) a hyperinflammatory response to infection, 2) uncoupling of bone destruction and repair, and 3) the role of the receptor for advanced glycation end products. Although our data cannot address the role of the receptor for advanced glycation end products, we can make an inference in relation to the first two possibilities of a hyperinflammatory response to infection and/or bone metabolism. In regard to bone metabolism, multivariable models including urinary DPD levels had no impact on the current findings, which was not unexpected because DPD levels were similar among diabetes-free participants and those with both controlled and uncontrolled diabetes. SHIP participants with diabetes demonstrated elevated levels of both hsCRP and WBC, and this result was more pronounced among participants with uncontrolled diabetes. Despite these elevations in systemic inflammatory biomarkers, statistical adjustment for both hsCRP and WBC did not attenuate the association between diabetes status and periodontal disease change. This suggests that if a hyperinflammatory phenotype is a mechanistic intermediate linking accelerated periodontal destruction and diabetes, it is likely to be a localized inflammatory response restricted to the gingival tissues. Alternatively, measures of generalized systemic inflammation, such as CRP and WBC, might not be appropriate epidemiological surrogates to adequately characterize the “diabetic hyperinflammatory phenotype” involved in periodontal destruction.
Uncontrolled diabetes was associated with mean full-mouth AL increases of ∼0.35 mm during 5 years of follow-up. By extrapolation, this would suggest the potential for ∼1 mm of AL per 15 years of life lived with uncontrolled diabetes. AL rates of this nature are likely to be clinically relevant, although it is difficult to draw strong conclusions regarding the clinical significance of our findings because measures of MAL are substantially diluted by the majority of measured sites that had little or no disease and experienced no progression. Moreover, teeth that experience substantial longitudinal progression in disease are more likely to be lost during follow-up and unavailable for longitudinal analysis, which likely biases findings toward the null. It is also possible that previous reports concerning the contribution of diabetes to periodontal disease progression might have been overstated and our current analysis is a more realistic estimate of the true association. This is quite possible given the fact that these results arise from a population-based sample and that the analysis considers longitudinal periodontal disease progression (as opposed to cross-sectional differences in disease) and controls for a broad range of putative confounders.
These findings also indicate that uncontrolled diabetes is associated with a 1.3–3.0-fold increase in the risk for prospective tooth loss. Adjustment for baseline AL moderately attenuated the association between uncontrolled diabetes and tooth loss, suggesting that increased AL levels among participants with uncontrolled diabetes explained a portion of the observed increased tooth-loss risk. The substantial elevations in mean AL among teeth that were lost during follow-up (see results) support the role of periodontal pathology in tooth loss. However, no firm conclusions can be drawn about the meditational role of periodontal disease without specific information regarding the indication for tooth extraction or other reasons for tooth loss. Another explanation for the high tooth-loss rate observed among uncontrolled diabetes might be failing endodontic treatment or periapical inflammation (23). Unfortunately, we do not have any information about the endodontic and the periapical situation in our sample.
SHIP data support the hypothesis of a bidirectional relationship between periodontal infection and diabetes, which has been detailed extensively in the literature (8,11,24). Original discussions on the topic were supported by well-articulated epidemiological arguments based on a comprehensive review of the literature showing diabetes–periodontal disease associations to be consistent in multiple contexts (i.e., cross-sectionally and longitudinally, with periodontal disease as both predictor and consequence of poorly controlled diabetes). In support of this work, the current report, in combination with previously published SHIP data, succinctly demonstrates multiple perspectives of the bidirectional hypothesis arising from the same study population. Specifically, SHIP has shown that 1) periodontal disease is associated with elevated HbA1c levels cross-sectionally (25) and predicts prospective HbA1c progression longitudinally among diabetes-free participants (26), thereby bolstering other reports showing periodontal disease to predict diabetes development longitudinally (27); 2) diabetes status is associated with cross-sectional levels of periodontal disease (28); and 3) uncontrolled diabetes is associated with periodontal disease progression (current data).
These findings bolster the notion that tight control of diabetes status may prevent progression of periodontal disease and suggest the value of increased communication between the dental and medical professionals in enhancing overall patient health. Dentists and physicians alike should be aware that diabetes and periodontal diseases are common comorbidities and be able to communicate these risks to patients and make appropriate referrals (i.e., dentists referring to physicians for diabetes screening and physicians to dentists for periodontal disease screening) (29).
Although the use of half-mouth examinations and a lack of radiographic assessments has the potential to underestimate periodontal disease, our use of relative outcomes (e.g., mean AL vs. absolute number of sites per mouth with severe AL) minimizes this problem, as relative measures are less likely to be biased than absolute measures of periodontal disease (30,31). Our ability to explore evidence supporting inflammatory mediators of disease was limited by the fact that we only have two markers of systemic inflammation collected at baseline. Additionally, we could not account for changes in body fat composition during follow-up, and given the previous findings of associations between obesity, inflammation, and periodontal disease (32–34) independent of diabetes status, it is possible that the evolution of body composition might further explain associations between diabetes status and periodontal disease. Future studies that can prospectively collect a broader range of inflammatory biomarkers as well as longitudinal changes in body fat composition will be informative.
We have found uncontrolled T1DM and T2DM to be associated with declining periodontal health during 5 years of longitudinal follow-up in a population-based sample of adult men and women in Germany. These findings were consistent after robust adjustment for periodontal disease risk factors. The data do not provide strong support for the biological hypothesis that a hyperinflammatory response to infection or abnormal bone metabolism mediates the associations observed, although more focused mechanistic studies are necessary to adequately address this possibility. These findings substantially advance previous research in this area as a result of the strong design features, including a large, relatively homogenous population-based sample, longitudinal data collection, robust multivariable statistical models, and the concurrent examination of both T1DM and T2DM alongside diabetes-free participants.
Supplementary Material
Acknowledgments
This research was supported by SHIP, which is part of the Community Medicine Net (http://www.medizin.uni-greifswald.de/cm) of the University of Greifswald, and was funded by grants from the German Federal Ministry of Education and Research (grants 01ZZ96030 and 01ZZ0701), the Ministry for Education, Research, and Cultural Affairs, and the Ministry for Social Affairs of the Federal State of Mecklenburg-West Pomerania. This work was also supported by National Institutes of Health grants R00-DE-018739 (to R.T.D.) and R01-DE-13094 (to M.D.) and a Mayo Chair Endowment (School of Public Health, University of Minnesota) to D.R.J.
GABA International (Switzerland) provided unlimited education funds to support B.H. No other potential conflicts of interest relevant to this article were reported.
R.T.D. and B.H. analyzed the data and wrote the manuscript. M.D. and D.R.J. analyzed the data and reviewed and edited the manuscript. W.K., M.N., and H.V. collected the data and reviewed and edited the manuscript. T.K. collected the data and wrote the manuscript. R.T.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2453/-/DC1.
References
- 1.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000 2010;53:28–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol 1994;65:260–267 [DOI] [PubMed] [Google Scholar]
- 3.Grossi SG, Genco RJ, Machtei EE, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol 1995;66:23–29 [DOI] [PubMed] [Google Scholar]
- 4.Nelson RG, Shlossman M, Budding LM, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care 1990;13:836–840 [DOI] [PubMed] [Google Scholar]
- 5.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993;16:329–334 [PubMed] [Google Scholar]
- 6.Seppälä B, Ainamo J. A site-by-site follow-up study on the effect of controlled versus poorly controlled insulin-dependent diabetes mellitus. J Clin Periodontol 1994;21:161–165 [DOI] [PubMed] [Google Scholar]
- 7.Tervonen T, Karjalainen K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J Clin Periodontol 1997;24:505–510 [DOI] [PubMed] [Google Scholar]
- 8.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 2001;6:99–112 [DOI] [PubMed] [Google Scholar]
- 9.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol 2002;30:182–192 [DOI] [PubMed] [Google Scholar]
- 10.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 2011;7:738–748 [DOI] [PubMed] [Google Scholar]
- 11.Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc 2006;137(Suppl.):26S–31S [DOI] [PubMed] [Google Scholar]
- 12.John U, Greiner B, Hensel E, et al. Study of Health in Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed 2001;46:186–194 [DOI] [PubMed] [Google Scholar]
- 13.Völzke H, Alte D, Schmidt CO, et al. Cohort profile: the Study of Health in Pomerania. Int J Epidemiol 2011;40:294–307 [DOI] [PubMed] [Google Scholar]
- 14.Haring R, Alte D, Völzke H, et al. Extended recruitment efforts minimize attrition but not necessarily bias. J Clin Epidemiol 2009;62:252–260 [DOI] [PubMed] [Google Scholar]
- 15.Hensel E, Gesch D, Biffar R, et al. Study of Health in Pomerania (SHIP): a health survey in an East German region. Objectives and design of the oral health section. Quintessence Int 2003;34:370–378 [PubMed] [Google Scholar]
- 16.Gätke D, Holtfreter B, Biffar R, Kocher T. Five-year change of periodontal diseases in the Study of Health in Pomerania (SHIP). J Clin Periodontol 2012;39:357–367 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S33–S50 [DOI] [PubMed] [Google Scholar]
- 18.Desvarieux M, Schwahn C, Völzke H, et al. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke 2004;35:2029–2035 [DOI] [PubMed] [Google Scholar]
- 19.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 2005;162:267–278 [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 21.Firatli E. The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol 1997;68:136–140 [DOI] [PubMed] [Google Scholar]
- 22.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol 1998;3:30–39 [DOI] [PubMed] [Google Scholar]
- 23.López-López J, Jané-Salas E, Estrugo-Devesa A, Velasco-Ortega E, Martín-González J, Segura-Egea JJ. Periapical and endodontic status of type 2 diabetic patients in Catalonia, Spain: a cross-sectional study. J Endod 2011;37:598–601 [DOI] [PubMed] [Google Scholar]
- 24.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol 1998;3:51–61 [DOI] [PubMed] [Google Scholar]
- 25.Demmer RT, Kocher T, Schwahn C, Völzke H, Jacobs DR, Jr, Desvarieux M. Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dent Oral Epidemiol 2008;36:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the Study of Health in Pomerania (SHIP). Diabetes Care 2010;33:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care 2008;31:1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur G, Holtfreter B, Rathmann W, et al. Association between type 1 and type 2 diabetes with periodontal disease and tooth loss. J Clin Periodontol 2009;36:765–774 [DOI] [PubMed] [Google Scholar]
- 29.Deschner J, Haak T, Jepsen S, et al. Diabetes mellitus and periodontitis. Bidirectional relationship and clinical implications. A consensus document. Internist (Berl) 2011;52:466–477[in German] [DOI] [PubMed] [Google Scholar]
- 30.Susin C, Kingman A, Albandar JM. Effect of partial recording protocols on estimates of prevalence of periodontal disease. J Periodontol 2005;76:262–267 [DOI] [PubMed] [Google Scholar]
- 31.Kingman A, Susin C, Albandar JM. Effect of partial recording protocols on severity estimates of periodontal disease. J Clin Periodontol 2008;35:659–667 [DOI] [PubMed] [Google Scholar]
- 32.Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, Pischon T. Obesity, inflammation, and periodontal disease. J Dent Res 2007;86:400–409 [DOI] [PubMed] [Google Scholar]
- 33.Ylöstalo P, Suominen-Taipale L, Reunanen A, Knuuttila M. Association between body weight and periodontal infection. J Clin Periodontol 2008;35:297–304 [DOI] [PubMed] [Google Scholar]
- 34.Meisel P, Wilke P, Biffar R, Holtfreter B, Wallaschofski H, Kocher T. Total tooth loss and systemic correlates of inflammation: role of obesity. Obesity (Silver Spring) 2012;20:644–650 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.