Abstract
OBJECTIVE
To investigate whether diabetes and regular hemodialysis are associated with false elevation of ankle systolic blood pressure and ankle-brachial systolic pressure index (ABI) because of their arterial calcification in patients with critical limb ischemia (CLI).
RESEARCH DESIGN AND METHODS
We recruited 269 Japanese patients who underwent endovascular therapy for CLI. Ankle systolic blood pressure and ABI were assessed before endovascular therapy. Arterial stenosis and calcification were evaluated angiographically. We investigated the associations among clinical comorbidities, arterial calcification, and measurements of ankle systolic blood pressure and ABI.
RESULTS
Ankle systolic blood pressure was 85 ± 56 mmHg, and ABI was 0.59 ± 0.37. Arterial calcification was observed in 69% of the patients. The prevalence of diabetes and regular hemodialysis was 71 and 47%. Diabetes and regular hemodialysis were both significantly associated with the presence of arterial calcification; their adjusted odds ratios were 2.33 (P = 0.01) and 7.40 (P < 0.01), respectively. However, there was no significant difference in ankle systolic blood pressure or ABI level between those with and without these comorbidities. Furthermore, the presence of arterial calcification was not associated with ankle systolic blood pressure or ABI level, whereas arterial stenoses of all segments in the lower body had independent associations with reduced ankle systolic blood pressure and ABI level.
CONCLUSIONS
Diabetes and regular hemodialysis were significantly associated with arterial calcification, but not with elevated measurements of ankle systolic blood pressure or ABI, in CLI patients.
Ankle systolic blood pressure and its ratio to brachial systolic blood pressure, that is, ankle-brachial systolic pressure index (ABI), reflect arterial hemodynamics in lower extremity. A reduced value of these measurements is caused by hemodynamically significant arterial stenosis and therefore indicates the presence of peripheral arterial disease (PAD). ABI ≤0.90 in symptomatic individuals, for example, is approximately 95% sensitive in detecting arteriogram-positive PAD and almost 100% specific in identifying healthy individuals (1). Because of their predictive capability and noninvasiveness, these measurements are recommended for screening and determining PAD in many clinical settings (2). Furthermore, ABI is also known as an independent prognostic risk factor for fatal and nonfatal cardiovascular disease and all-cause mortality in a general population (3–5), as well as in diabetic patients (6) and in patients with end-stage kidney disease receiving hemodialysis (7). Their usefulness in clinical practice is now widely recognized.
On the other hand, it has often been pointed out that patients with diabetes and renal failure would have their ankle systolic blood pressure and ABI falsely elevated because of their noncompressible vessels caused by vascular calcification (2,8). As previous studies showed, elevated ABI indicates a clinically important characteristic and has attracted increasing attention in clinical practice (9–11).
No clinical data are so far available, however, about whether these falsely elevated measurements could be similarly observed in patients with critical limb ischemia (CLI). CLI is a manifestation of PAD that describes patients with chronic ischemic rest pain or with ischemic skin lesions, either ulcers or gangrene. It is associated with an extremely poor prognosis for both survival and limb salvage; its prompt diagnosis and following revascularization are therefore important. Although TransAtlantic Inter-Society Consensus (TASC) (12), a worldwide clinical guideline for PAD, and its revised consensus TASC II (1) acknowledge the validity of using ankle systolic blood pressure and ABI in the detection of CLI, little is known about the distribution of these measurements and the influence of comorbidities on these measurements in CLI patients. A previous clinical study disclosed in patient characteristics that about half of the recruited CLI patients with ischemic skin lesions had ankle systolic blood pressure higher than 70 mmHg (13). Elevation of these measurements might be more common in CLI patients than expected.
We hypothesized that in CLI patients, diabetes and end-stage renal disease were associated with arterial calcification and consequently with falsely elevated ankle systolic blood pressure and ABI. To verify the hypothesis, we investigated in the current study whether these clinical comorbidities had significant associations with ankle systolic blood pressure and ABI, as well as arterial calcification, in CLI patients.
RESEARCH DESIGN AND METHODS
Study population and definitions
We recruited 269 consecutive Japanese patients with CLI who underwent angiographical examinations in the lower body including tibial segments as well as the measurement of ankle systolic blood pressure and ABI and were treated with subsequent endovascular therapy in Kansai Rosai Hospital, Hyogo, Japan. The hospital was positioned as the regional core hospital supporting community medicine in Hyogo Prefecture, and patients with chronic ischemic rest pain and/or foot ulcer or gangrene were referred to the cardiovascular division of the hospital.
All the referred patients were evaluated for limb ischemia by angiography. The diagnosis and management of CLI were compliant with TASC (12) or its revised consensus, TASC II (1). Once patients were diagnosed as CLI, endovascular therapy was used as the first-line procedure for revascularization, within the recommendations in TASC (II). The indication of endovascular therapy was judged by consensus among vascular specialists including vascular surgeons. Patients were excluded if they were considered to be poor candidates for angiography and subsequent revascularization as a result of severe comorbidities including hemodynamic risk or difficulty having supine rest during the intervention or they refused the intervention. Ankle systolic blood pressure and ABI were evaluated in all the recruited patients before endovascular therapy with the use of the automated oscillometric device provided by OMRON COLIN Co., Ltd. (Tokyo, Japan). Its validity has been reported elsewhere (14,15). Arterial stenoses of the lower body and calcification of tibial arteries were assessed angiographically. Calcified lesions were defined quantitatively as obvious densities noted within the apparent vascular wall in the angiogram (16).
Diabetes was determined when patients had been treated for it or when they met the following criteria: fasting plasma glucose level ≥7.0 mmol/L, casual plasma glucose level ≥11.1 mmol/L, or HbA1c level ≥6.5% (17). Dyslipidemia was defined as serum LDL cholesterol ≥2.6 mmol/L or HDL cholesterol <1.0 mmol/L or triglycerides ≥1.7 mmol/L or having been treated for dyslipidemia. We performed the current study in accordance with the declaration of Helsinki, and it was approved by the ethics committee of Kansai Rosai Hospital. We obtained written informed consent from all the recruited patients.
Statistical analyses
Data are given as mean ± SD for continuous variables and as percentages for dichotomous variables if not mentioned otherwise.
We first assessed the distributions of ankle systolic blood pressure and ABI in the recruited CLI patients. We also examined the prevalence of ankle systolic blood pressure ≥250 mmHg and ABI >1.40, suggesting typical noncompressible measurements (1), as well as that of ankle systolic blood pressure ≥70 mmHg and ABI >0.90, suggesting false elevations in CLI patients (1).
We subsequently assessed the following three associations in the recruited CLI patients: 1) the association of clinical characteristics with ankle systolic blood pressure and ABI, 2) the association of clinical characteristics with arterial calcification, and finally 3) the association of angiographic observations, including calcification as well as arterial stenosis, with ankle systolic blood pressure and ABI. Measurements of ankle systolic blood pressure and ABI were assessed as continuous dependent variables. Arterial calcification was treated as a dichotomous variable.
In unadjusted analyses for continuous outcomes, the significant difference between patients with and without each comorbidity was assessed by the use of unpaired t test. Because ankle systolic blood pressure was expected to be strongly influenced by systemic blood pressure, we also demonstrated its difference adjusted for measurements of brachial systolic blood pressure by ANCOVA. The association with dichotomous outcomes was analyzed in a univariate logistic regression model. Explanatory variables were subsequently entered all together into multivariate model to reveal the independent influence on the outcomes.
A P value <0.05 was considered statistically significant. Differences in continuous dependent variables and odds ratios (ORs) of dichotomous dependent variables, as well as their 95% confidence intervals (CI), are reported. Statistical analyses were performed using IBM SPSS Statistics Version 19 (SPSS, an IBM company).
RESULTS
The recruited patients were 71 ± 11 years old, and 184 of 269 (68%) were male. A total of 191 patients (71%) had diabetes, and their median diabetic duration and its quartiles were 20 (10: 27) years. Regular hemodialysis was received by 126 patients (47%), with their median dialysis vintage 5 (2: 10) years. One hundred ninety-three patients (72%) had antihypertensive treatment. The prevalence of dyslipidemia, smoking, and foot ulceration was 77 (n = 206), 67 (n = 181), and 84% (n = 227), respectively. Ankle systolic blood pressure was 85 ± 56 mmHg and ABI was 0.59 ± 0.37, whereas brachial systolic blood pressure was 143 ± 27 mmHg. Ankle systolic blood pressure was significantly associated with brachial systolic blood pressure (Pearson’s r = 0.36, P < 0.01), whereas ABI was not (Pearson’s r = 0.08, P = 0.18). A total of 171 patients (64%) presented ankle systolic blood pressure ≥70 mmHg and 60 patients (22%) had ABI more than 0.90. ABI >1.40 was observed in only one patient (0.4%), and the rest had ABI below 1.40. No patients had their ankle systolic blood pressure 250 mmHg or more.
Association of clinical characteristics with ABI and ankle systolic blood pressure
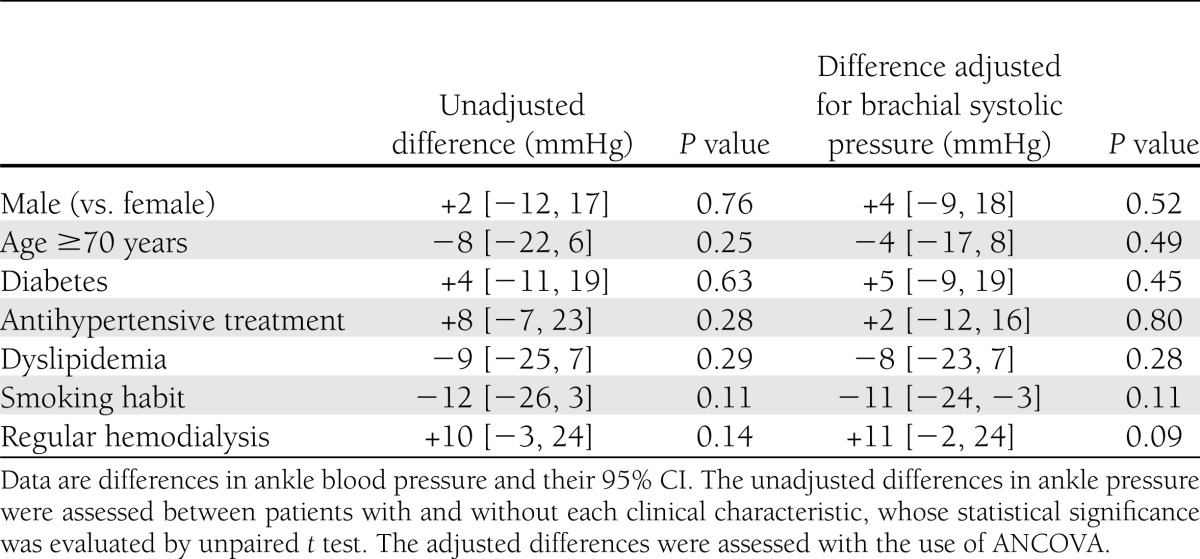
Table 1 shows the association of clinical characteristics with ABI. Patients with diabetes and regular hemodialysis had slightly, but not significantly, elevated ABI compared with those without these comorbidities; the differences and 95% CI were only 0.04 [−0.05, 0.14] and 0.06 [−0.02, 0.15], respectively. Neither did other clinical characteristics have any significant association with ABI (Table 1).
Table 1.
Association of clinical characteristics with ABI
Similar findings were observed when we investigated the association of diabetes and regular hemodialysis with ankle systolic blood pressure. As shown in Table 2, patients with diabetes and regular hemodialysis had slightly, but not significantly, higher ankle systolic blood pressure compared with those without these comorbidities; their adjusted differences and 95% CI were 5 [−9, 19] and 11 [−2, 24] mmHg, respectively. Neither did any other comorbidity provide significant difference (Table 2).
Table 2.
Association of clinical characteristics with ankle systolic blood pressure
Association of clinical characteristics with arterial calcification
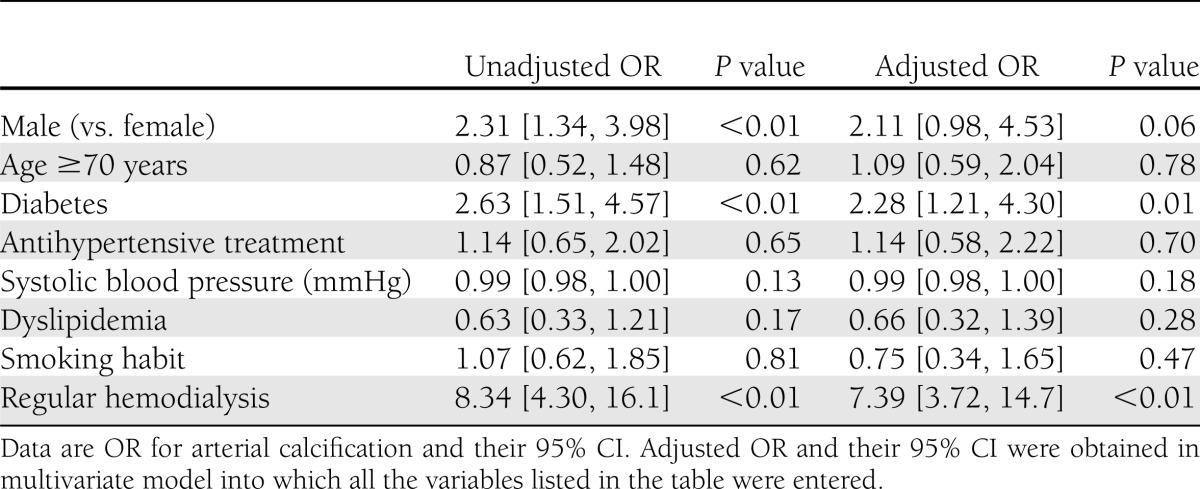
Calcification of tibial arteries was found in 186 patients (69%), and we assessed the association of clinical characteristics with the arterial calcification. As shown in Table 3, diabetes and regular hemodialysis, as well as male sex, had a significant association with arterial calcification in the univariate model. These two variables were also significantly associated with arterial calcification in a multivariate model, in which adjusted ORs were 2.33 [1.24, 4.36] (P = 0.01) and 7.40 [3.72, 14.7] (P < 0.01), respectively. Other variables had no significant association with the arterial calcification.
Table 3.
Association of clinical characteristics with arterial calcification
We subsequently performed category analysis to assess the effect of diabetic duration and dialysis vintage on the outcome. In brief, diabetic patients were categorized into two groups with the use of the median values of diabetic duration (20 years) and were compared with nondiabetic population in the multivariate model. Similarly, patients receiving regular hemodialysis were classified with the median dialysis vintage (5 years) and were compared with nondialysis receivers. As a result, longstanding diabetes and hemodialysis were both independently associated with an increased risk of arterial calcification (P < 0.01). The adjusted OR [95% CI] for diabetes with duration ≥20 years was 4.65 [2.03, 10.6], whereas that for diabetic duration <20 years was 1.57 [0.78, 3.15]. On the other hand, the adjusted OR for regular hemodialysis with vintage <5 years and ≥5 years was 4.45 [1.90, 10.4] and 10.1 [3.61, 28.3], respectively.
Association of angiographic findings with ABI and ankle systolic blood pressure
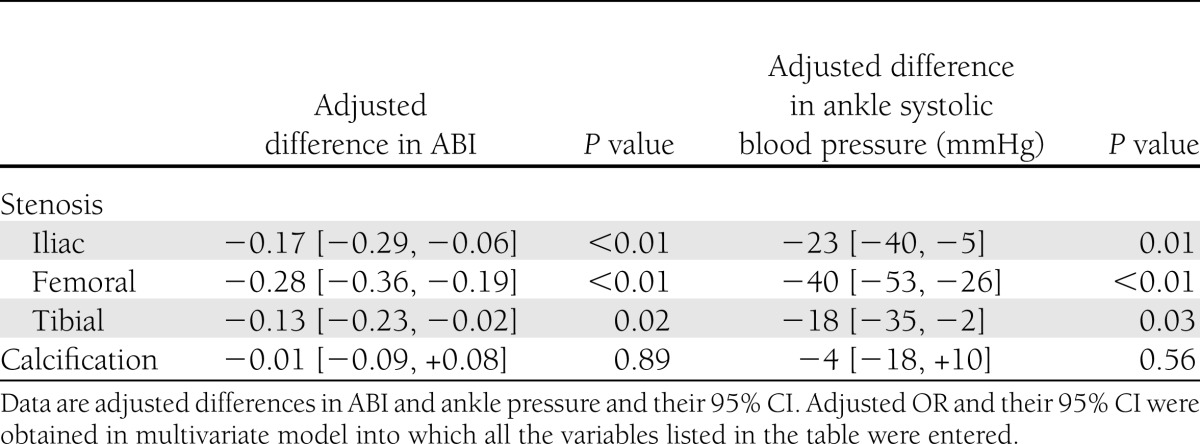
We finally investigated the association of angiographic characteristics with ABI and ankle systolic blood pressure. The stenosis of iliac, femoral, and tibial arteries was found in 45, 171, and 222 patients (17, 64, and 83%, respectively), and patients with arterial stenosis in each segment had lower levels of ABI and ankle systolic blood pressure than those without the stenosis. As shown in Table 4, multivariate analysis revealed that all of these arterial stenoses were independently associated with reduced ABI and ankle systolic blood pressure. The adjusted reduction by each segmental stenosis ranged from −0.13 to −0.28 in ABI and from −18 mmHg to −40 mmHg in ankle systolic blood pressure (Table 4).
Table 4.
Association of angiographic characteristics with ABI and ankle systolic blood pressure
On the other hand, arterial calcification had no significant association with either ABI (P = 0.89) or ankle systolic blood pressure (P = 0.56) (Table 4). Neither was its association with ankle systolic blood pressure statistically significant after additional adjustment for brachial systolic blood pressure (P = 0.96).
CONCLUSIONS
Ankle systolic blood pressure and ABI can noninvasively assess hemodynamics in the lower limb and are now widely used in clinical practice to screen and determine PAD (1). These measurements are also used in the detection of CLI; when patients have unhealing foot ulceration or rest pain, one can suspect it of resulting from lower-limb ischemia if these measurements are reduced. The current study demonstrated that arterial stenosis in each segment of the lower body was independently associated with a reduced ankle systolic blood pressure and ABI level in CLI patients. These findings suggest that accumulating arterial stenoses of the leg would additively reduce these measurements and that smaller values of the measurements would clinically reflect more widely distributed arterial stenoses.
On the other hand, it has been pointed out that these measurements can be falsely elevated in patients with diabetes and end-stage renal disease because of their noncompressible vessels caused by vascular calcification (2,8). However, it still remains unrevealed whether these false elevations could be similarly observed in CLI patients. Furthermore, although it is often mentioned that ankle systolic blood pressure in CLI patients is typically lower than 70 mmHg, its true distribution in CLI patients has been seldom reviewed and so far is not fully understood. According to a very few previous clinical trials, which disclosed its distribution in their patient characteristics, elevated ankle systolic blood pressure might be more common than expected (13). To clarify these issues, we limited the study population to CLI patients and investigated the distribution of these measurements and the association of clinical characteristics with these measurements. We hypothesized that diabetes and regular hemodialysis would increase the risk of arterial calcification and would consequently be associated with a false elevation of ankle systolic blood pressure and ABI in CLI patients.
The current study found that mean measurements of ankle systolic blood pressure and ABI were as high as 85 mmHg and 0.59 and that a substantial part of the patients had ankle systolic blood pressure ≥70 mmHg and/or ABI >0.90. However, in contrast with our initial hypothesis, neither diabetes nor regular hemodialysis had any significant association with these elevated measurements, although these two comorbidities, especially with a long duration and vintage, significantly increased the risk of arterial calcification. The lack of significant association was also observed between arterial calcification and elevated ankle systolic blood pressure and ABI levels in the population. These findings suggest that both diabetes and regular hemodialysis are associated with arterial calcification as reported previously (18,19), but that the presence of arterial calcification is not associated with elevated ankle systolic blood pressure or ABI in CLI patients.
Our current findings were in contrast with those in some previous literature (20), which demonstrated that diabetes was a dominant risk factor for elevated ABI levels. There would be two possible explanations of this discrepancy between our findings and theirs. One possible explanation would be the way of ABI measurement. In that previous study, they measured ABI by detecting the signals with photoplethysmography at the third finger and great toe, whereas in the current study we measured ABI with the use of the automated oscillometric device. There are three common methods of assessing ABI: oscillometric, Doppler, and photoplethysmographic techniques. Although these measurements provide similar results in some articles (15,21,22), it is possible that these three methods provide different results in calcified arteries. The oscillometric technique might be less subject to arterial calcification, whereas use of different methods might provide higher pressure measurements in calcified arteries.
Another possible explanation of the different findings in our current study from those in previous reports would be systemic distribution of noncompressible vessels in CLI patients. Previous studies often recruited PAD patients, including those with only intermittent claudication and with no symptoms, or patients suspected of PAD (20), whereas we limited our study population to CLI patients. It is well known that patients with CLI suffer more progressed atherosclerosis and more severe arterial calcification compared with patients with other PAD (23). In the current study population, the prevalence of tibial calcification had a considerable overlap (∼90%) with that of the arterial calcification elsewhere in the lower body (data not shown). Although we did not have any data about arterial calcification of the upper limb in the recruited patients, it would be of no surprise if patients with calcified tibial arteries were very likely to have other arteries, including those in the upper limb, similarly calcified (24–26). It might be that CLI patients whose ankle systolic blood pressure was falsely evaluated because of calcified tibial arteries were more likely to have falsely elevated brachial systolic blood pressure as a result of calcified brachial arteries (27–31). The coexisting false elevation of brachial systolic blood pressure would cancel the false increase in ABI caused by tibial calcification, and this is one possible explanation of no significant association between tibial calcification and increased ABI level.
Some may argue that these hypotheses do not explain the absence of a significant association between tibial calcification and ankle systolic blood pressure measurements. However, in clinical practice, brachial blood pressure is used as a substitute for systemic blood pressure; patients’ systemic blood pressure is generally evaluated and controlled on the basis of the measurements of brachial blood pressure (32–34). No one ever knows in clinical practice whether their elevated brachial pressure reflects the presence of true hypertension or is caused by calcification of brachial arteries (27–31). Patients with truly elevated brachial pressure would be more likely to have their ankle pressure elevated by their hypertension too. On the other hand, as discussed above, patients with falsely elevated brachial pressure by arterial calcification would be more likely to suffer widely distributed calcification in the systemic artery (27) and therefore to have their ankle pressure falsely elevated. As a result, it would be clinically impossible to distinguish the false elevation of ankle pressure induced by arterial calcification from the true elevation as a result of hypertension. This would possibly be why we failed to observe significant difference in ankle systolic blood pressure between those with and without calcified tibial arteries.
In conclusion, both diabetes and regular hemodialysis were significantly associated with arterial calcification, but not with elevated measurements of ankle systolic blood pressure or ABI, in CLI patients. Furthermore, these noninvasive measurements were not affected by the presence of calcification in tibial arteries in these patients, whereas accumulating arterial stenoses in the lower body had additive effects on reduced levels of these measurements.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
M.T. and H.K. researched data and wrote the manuscript. O.I., N.K., T.M., and M.I. contributed to the discussion. I.S. reviewed and edited the manuscript. H.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Shinsuke Nanto (Department of Advanced Cardiovascular Theraputics, Osaka University Graduate School of Medicine) and Masaaki Uematsu (Cardiovascular Division, Kansai Rosai Hospital) for help with preparing the manuscript.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007;33(Suppl. 1):S1–S75 [DOI] [PubMed] [Google Scholar]
- 2.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes—a review. Diabet Med 2010;27:4–14 [DOI] [PubMed] [Google Scholar]
- 3.Heald CL, Fowkes FG, Murray GD, Price JF, Ankle Brachial Index Collaboration Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006;189:61–69 [DOI] [PubMed] [Google Scholar]
- 4.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ 1996;313:1440–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowkes FG, Murray GD, Butcher I, et al. Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 2003;107:753–756 [DOI] [PubMed] [Google Scholar]
- 7.Kitahara T, Ono K, Tsuchida A, et al. Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am J Kidney Dis 2005;46:688–696 [DOI] [PubMed] [Google Scholar]
- 8.Leskinen Y, Salenius JP, Lehtimäki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis 2002;40:472–479 [DOI] [PubMed] [Google Scholar]
- 9.Suominen V, Rantanen T, Venermo M, Saarinen J, Salenius J. Prevalence and risk factors of PAD among patients with elevated ABI. Eur J Vasc Endovasc Surg 2008;35:709–714 [DOI] [PubMed] [Google Scholar]
- 10.Ix JH, Katz R, Peralta CA, et al. A high ankle brachial index is associated with greater left ventricular mass MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;55:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suominen V, Uurto I, Saarinen J, Venermo M, Salenius J. PAD as a risk factor for mortality among patients with elevated ABI—a clinical study. Eur J Vasc Endovasc Surg 2010;39:316–322 [DOI] [PubMed] [Google Scholar]
- 12.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000;31:S1–S296 [PubMed] [Google Scholar]
- 13.Nehler MR, Brass EP, Anthony R, et al.; Circulase investigators. Adjunctive parenteral therapy with lipo-ecraprost, a prostaglandin E1 analog, in patients with critical limb ischemia undergoing distal revascularization does not improve 6-month outcomes. J Vasc Surg 2007;45:953–960 [DOI] [PubMed]
- 14.Koji Y, Tomiyama H, Ichihashi H, et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of the presence of coronary artery disease in subjects with a high risk of atherosclerotic cardiovascular disease. Am J Cardiol 2004;94:868–872 [DOI] [PubMed] [Google Scholar]
- 15.Richart T, Kuznetsova T, Wizner B, Struijker-Boudier HA, Staessen JA. Validation of automated oscillometric versus manual measurement of the ankle-brachial index. Hypertens Res 2009;32:884–888 [DOI] [PubMed] [Google Scholar]
- 16.Soga Y, Iida O, Hirano K, Suzuki K, Yokoi H, Nobuyoshi M. Restenosis after stent implantation for superficial femoral artery disease in patients treated with cilostazol. Catheter Cardiovasc Interv 2012;79:541–548 [DOI] [PubMed] [Google Scholar]
- 17.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus: The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Diabetol Int 2010;1:2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg 2011;41:110–116 [DOI] [PubMed] [Google Scholar]
- 19.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002;39:695–701 [DOI] [PubMed] [Google Scholar]
- 20.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg 2008;48:1197–1203 [DOI] [PubMed] [Google Scholar]
- 21.Beckman JA, Higgins CO, Gerhard-Herman M. Automated oscillometric determination of the ankle-brachial index provides accuracy necessary for office practice. Hypertension 2006;47:35–38 [DOI] [PubMed] [Google Scholar]
- 22.Harrison ML, Lin HF, Blakely DW, Tanaka H. Preliminary assessment of an automatic screening device for peripheral arterial disease using ankle-brachial and toe-brachial indices. Blood Press Monit 2011;16:138–141 [DOI] [PubMed] [Google Scholar]
- 23.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol 2008;51:1967–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol 2004;24:331–336 [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the Healthy Women Study. Arterioscler Thromb Vasc Biol 1999;19:2189–2198 [DOI] [PubMed] [Google Scholar]
- 26.Odink AE, van der Lugt A, Hofman A, et al. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam study. Atherosclerosis 2007;193:408–413 [DOI] [PubMed] [Google Scholar]
- 27.Sheckman P, Klassen G. Pseudohypertension secondary to a noncompressible brachial artery. Can Med Assoc J 1974;111:1227–1228 [PMC free article] [PubMed] [Google Scholar]
- 28.Foran TG, Sheahan NF, Cunningham C, Feely J. Pseudo-hypertension and arterial stiffness: a review. Physiol Meas 2004;25:R21–R33 [DOI] [PubMed] [Google Scholar]
- 29.Littenberg B, Wolfberg C. Pseudohypertension masquerading as malignant hypertension. Case report and review of the literature. Am J Med 1988;84:539–542 [DOI] [PubMed] [Google Scholar]
- 30.Taguchi JT, Suwangool P. “Pipe-stem” brachial arteries. A cause of pseudohypertension. JAMA 1974;228:733. [PubMed] [Google Scholar]
- 31.Kuriyama S, Tomonari H, Utsunomiya Y, Kinoshita N, Matsumoto H. Pseudohypertension in hemodialyzed arteriosclerotic patients. Nephron 1994;66:479–480 [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancia G, Laurent S, Agabiti-Rosei E, et al. European Society of Hypertension Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009;27:2121–2158 [DOI] [PubMed] [Google Scholar]
- 34.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]