Abstract
OBJECTIVE
To determine the effects of soy milk consumption compared with cow’s milk on inflammation, coagulation, and oxidative stress among patients with diabetic nephropathy.
RESEARCH DESIGN AND METHODS
This randomized, crossover clinical trial was conducted on 25 type 2 diabetic patients with nephropathy. This study had two trial phases, each for 4 weeks and one washout period for 2 weeks. Patients were randomly assigned to consume a diet containing soy milk or a diet containing cow’s milk.
RESULTS
Soy milk consumption resulted in a significant reduction in d-dimer level (percent change: −3.77 vs. 16.13%; P < 0.05). This significant effect remained even after adjusting for confounding factor (carbohydrate intake). However, soy milk consumption had no significant effects on tumor necrosis factor-α, interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and malondialdehyde levels. The result was near to significance regarding the effect of soy milk consumption on hs-CRP (percent change: −35.45 vs. 36.76%; P = 0.05). However, this effect was not significant after adjusting for the confounding variable (carbohydrate intake).
CONCLUSIONS
Soy milk consumption could decrease serum d-dimer level among type 2 diabetic patients with nephropathy. However, markers of inflammation and oxidative stress did not change following soy milk intake among these patients.
Nephropathy is the major cause of disability and death among diabetic patients (1). Inflammation and oxidative stress are relevant factors in the pathogenesis of diabetic nephropathy (DN) (2). Glomerulosclerosis, which is associated with a high amount of coagulating factors, is a major reason for nephropathy (3). A novel therapy for DN might be anti-inflammatory treatment, which could be improving renal function (4). Lifestyle modifications are first steps in controlling the complications involved in DN (5).
Recently, human studies have shown the association between dietary pattern and dietary components with inflammatory and coagulating markers (6–8). In general, healthy dietary pattern may be favorably associated with inflammatory markers (9–11). Soy protein intake potentially modulates inflammatory response among type 2 DN (12). Some data are available regarding the beneficial effects of soy products on inflammatory markers (13–18), coagulation (14), and oxidative stress indices (15).
In most previous studies, only soy ingredients were recommended for patients with DN (12). However, whole soy consumption, which has a low content of phosphorus in compare with cow’s milk, may be more effective in patients with DN (16).
Isoflavones in liquid kinds of soy foods may be absorbed faster than other soy foods (17). Furthermore, dairy consumption should be limited in DN patients for reducing the phosphorus intake (18). Thus, the low phosphorus content of soy milk compared with cow’s milk make soy milk as a better choice for patients with DN (16).
According to our knowledge, there are few studies regarding the effect of soy milk consumption on new cardiovascular risk factors among DN patients. Therefore, this study was conducted to determine the effect of soy milk consumption on inflammation, coagulation, and markers of oxidative stress among type 2 DN patients.
RESEARCH DESIGN AND METHODS
Type 2 diabetic patients with nephropathy were recruited from subjects attending to the clinic of Azzahra Hospital and the clinic of Isfahan University of Medical Sciences. The inclusion criteria were stages 1 and 2 of DN, fasting blood glucose >126 mg/dL, hypoglycemic agents, or insulin intake, proteinuria >300 mg/day, and glomerular filtration rate >90 mL/min (12,19). The diabetic nephropathy condition was confirmed by the specialist in the current study. Study procedure was explained for participants, and informed written consent was taken from all the patients. Exclusion criteria in this study were pregnancy or lactating in women, changing the dosage of medications, allergy or intolerance to soy milk or cow’s milk, and avoidance of soy milk or cow’s milk consumption. The sample size for this study was calculated based on this equation (20): n = ([Z1−α/2 + Z1−β]2 × S2)/2Δ2, where α (type 1 error) is 0.05, β (type 2 error) is 0.2, S is the variance of C-reactive protein (CRP; S = 3.3), and Δ represents differences in the means of CRP (Δ = 1.5). The rationale to use variance of CRP equal to 3.3 and differences in the means of CRP equal to 1.5 is based on the results of a previously published study (12). Thus, the power for detecting differences between the treatment conditions for various outcomes in the current study was 80%. Therefore, according to this formula, we needed 19 patients to complete the trial. We recruited 32 diabetic patients to compensate for any possible exclusion. This study was approved by the research council and ethics committee of Isfahan University of Medical Sciences (research project number 185190). In addition, this study is registered in the Iranian Registry of Clinical Trials (identification number IRCT201105282839N2) and at http://www.clinicaltrials.gov (registration number NCT01419912).
Study procedure
A randomized, crossover clinical trial with two interventional periods and one washout period was conducted to compare the effects of soy milk and cow’s milk consumption among type 2 diabetic patients with nephropathy. Subjects were randomly assigned to two groups, and they were asked to follow a diet containing cow’s milk or soy milk for 4 weeks. After the interventional period, a washout period was conducted for 2 weeks. Then groups followed the alternate treatment for 4 weeks. All data were collected before and after each trial phase. They were requested not to change their diet and physical activity habits during the study and asked to record their physical activity and food intake for 1 day in each phase of the study. Their complications and medications were monitored carefully. During the current study, soy milk and cow’s milk were provided for all patients.
Anthropometric assessments
All patients were assessed for anthropometric measurements including height and body weight at baseline and weeks 4, 6, and 10 of the study period. Heights in standing position and without shoes and body weight while participants were minimally clothed without shoes using digital scales were measured. BMI was calculated as body weight (kg)/height2 (m).
Diet
Each subject received a usual diet for patients with nephropathy containing 0.8 g/kg protein, 2,000 mg/day sodium, and 2,000 mg/day potassium (12). Calorie requirements of each patient were calculated individually based on the equation of Institute of Medicine Food and Nutrition Board (21). Furthermore, in the soy milk and cow’s milk intervention period, one glass (240 mL) of soy milk and cow’s milk was provided for each patient. A 1-day dietary record was completed by all subjects in each phase of study to determine the adherence to the prescribed diet and soy milk or cow’s milk intake. The soy milk and cow’s milk were produced by Soya Sun Company and Mihan-Dairy Company in Iran, respectively.
Biochemical analysis
Blood samples were obtained and analyzed from fasting participants at the onset of the study and then after 4, 6, and 10 weeks. Interleukin-6 (IL-6), tumor necrosis factor (TNF-α), d-dimer, and high-sensitivity CRP (hs-CRP) were measured by using an enzyme-linked immunosorbent assay method and with commercial reagents (Boster Biological Technology for IL-6 and TNF-α, Hyphen BioMed for d-dimer, and Pars Azmoon for hs-CRP). Fibrinogen concentrations were measured by using the Clauss method with commercial reagents (Mahsa-yaran, Tehran, Iran). Malondialdehyde (MDA) was measured by using the colorimetry method. We did not use any commercial reference in the MDA method. First, a standard curve was drawn from a material named malondialdehyde bis (diethyl acetal) for its different concentrations. We separated 0.5 mL serum and centrifuged with trichloroacetic acid. We then separated 0.5 mL from this supernatant and mixed it with 0.5 mL thiobarbituric acid. We put this mixture in a 100°C temperature for 10 min. Serum MDA would react with thiobarbituric acid and provide a pink material that was written by spectrophotometer. The detection limit of methods used to determine hs-CRP was 0.124 mg/L, for IL-6, it was 0.03 pg/mL, for TNF-α, 0.13 pg/mL, for MDA, 1.56 nmol/mL, and for d-dimer, 45 ng/mL.
Soy milk and cow’s milk analysis
Ultra heat-treated soy milk was provided by Soy Max (Tehran, Iran), and ultra heat-treated low-fat milk was provided by Mihan (Tehran, Iran). Both of the products are registered with the health ministry of Iran. We used three random samples for analyzing the macronutrients. Milk fat was determined using Gerber’s method (22), whereas the method of Folch (23) was adopted for determination of soy milk fat. The micro-Kjeldal method was used to analyze the protein content (24), and carbohydrate was measured using Fehling’s solution titration method (25). Each experiment was repeated three times, and the mean of nine replicates was considered as the concentration of each tested element in soy milk or cow’s milk.
The mean of the components of soy milk and cow’s milk, respectively, in this study was as follows: carbohydrate: 3.5 and 4.9 (g); fat: 1 and 1.5 (g); protein: 2.5 and 3.3 (g); sodium: 40 and 50 (mg); potassium: 110 and 156 (mg); phosphorus: 53 and 120 (mg); and calcium: 40 and 100 (mg).
Statistical analysis
At first, normal distribution of all variables was checked with the Kolmogorov-Smirnov test. The distribution of hs-CRP, TNF-α, and d-dimer was not normal. Thus, we reported geometric means regarding these variables. End point and baseline of treatment values were used to calculate the percent change of each variable. The paired t test was used for comparing percentage change of variables, end values, and baseline values. We used ANCOVA in the adjusted models, which were adjusted for sex and carbohydrate intake. The P values for the possible interaction of time × sex × variables were also reported. Statistical significance was defined as P < 0.05. Statistical analysis was performed by using SPSS for Windows version 18.0 (SPSS, Chicago, IL). We used the Nutritionist IV program to estimate dietary intake of patients.
RESULTS
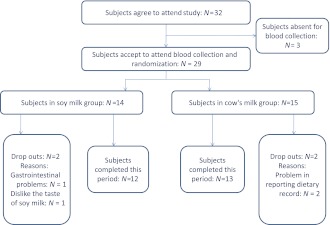
From 32 patients that enrolled into this study, 29 patients attended randomization and blood collection. Therefore, 14 and 15 subjects were randomly assigned to the soy milk and cow’s milk groups, respectively. Two patients withdrew during the soy milk period due to the taste of soy milk and gastrointestinal problems following soy milk consumption. Problems in reporting dietary record caused the exclusion of two patients during the cow’s milk period. Finally, 25 participants completed this study (Fig. 1). The mean ± SD age and BMI of patients were 51 ± 10 years and 28 ± 4 kg/m2, respectively. A total of 60% of the subjects were females. Only 24% of patients had an academic degree, 40% of the study population was in menopause, and 32% of participants used insulin. Dietary intake of subjects in different phases of trial was assessed. No significant difference was shown between the two periods of intervention regarding the amount of nutrient intakes except for fiber and carbohydrate.
Figure 1.
Patient flow diagram.
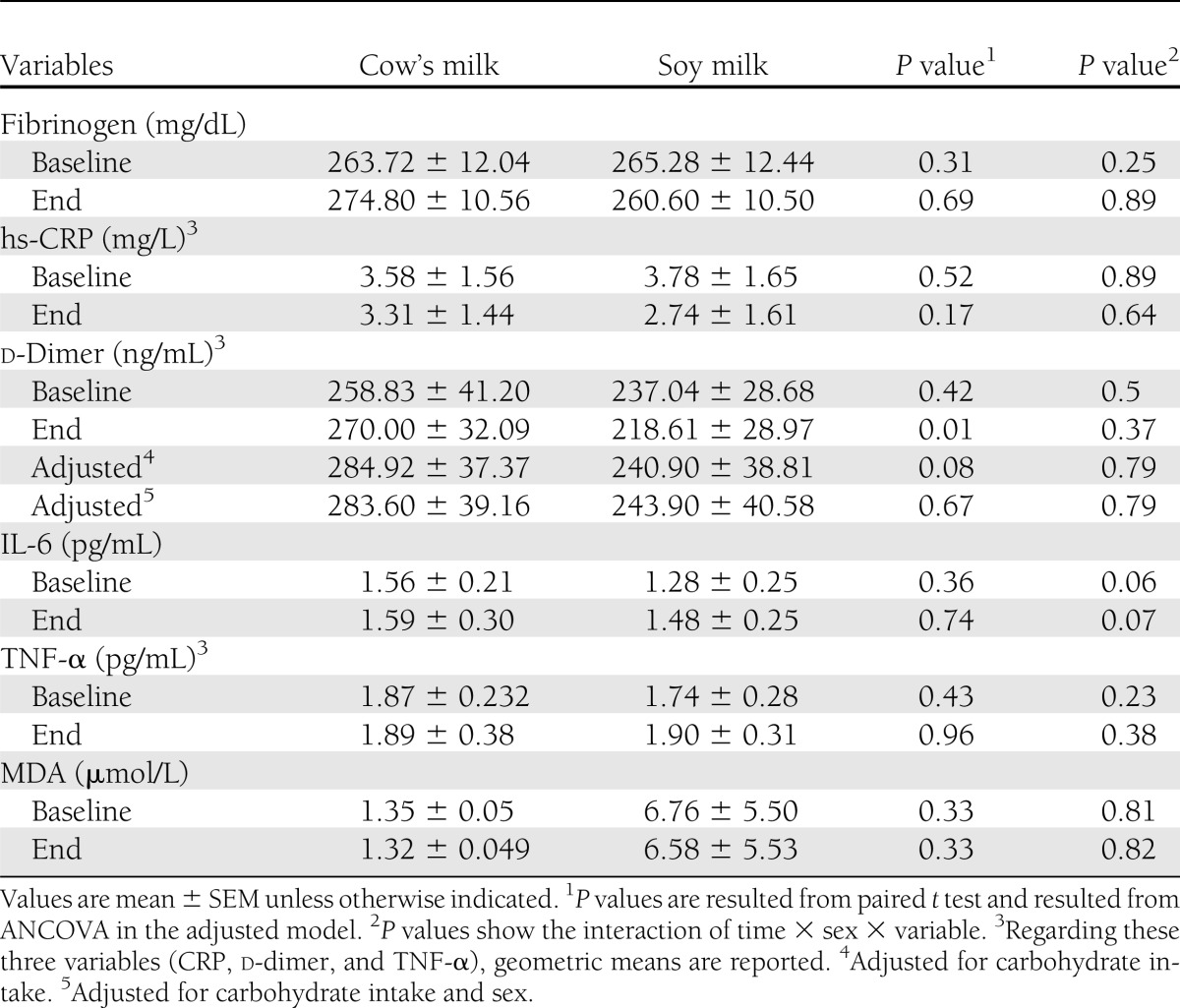
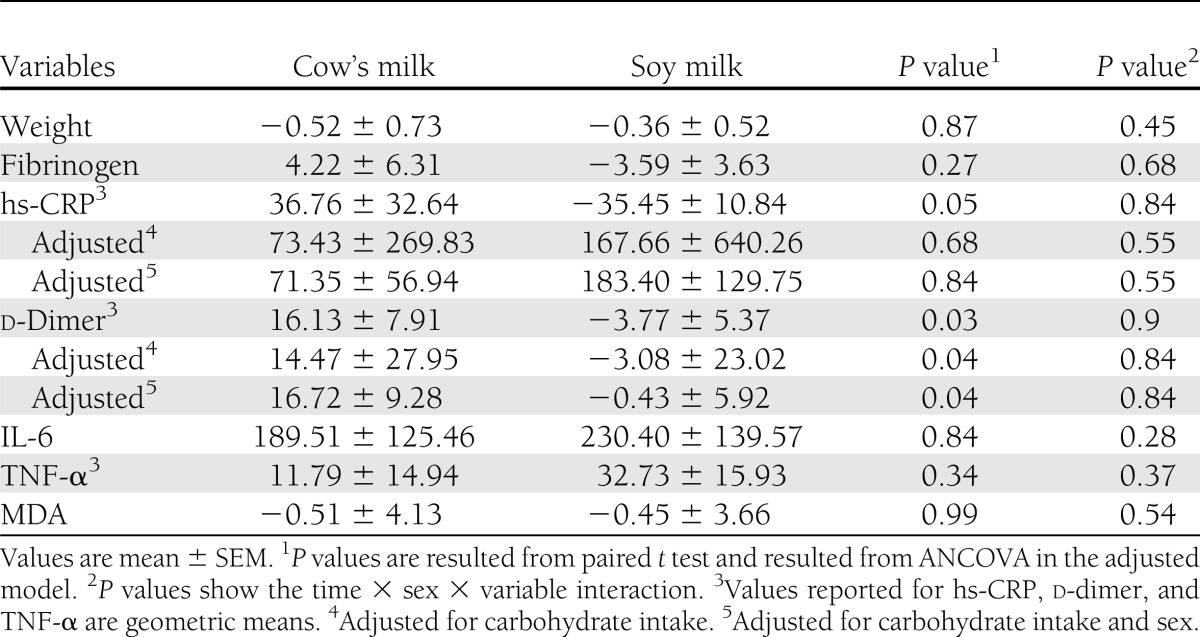
Effects of the interventions on inflammation, oxidative stress, and coagulating markers are shown in Table 1. Statistical analyses showed that soy milk compared with cow’s milk consumption resulted in a significant reduction in end values of d-dimer among DN patients. This significant result did not remain after adjustment for carbohydrate intake (adjusted end values of d-dimer in soy milk and cow’s milk: 218.61 ± 138.95 vs. 284.92 ± 183.75 ng/mL; P = 0.08, respectively). Percent changes of markers are shown in Table 2. Analysis showed significant difference in percent changes regarding d-dimer level in the two groups. The significant effect of soy milk consumption on d-dimer remained even after adjustment for carbohydrate intake (adjusted percent change of d-dimer in soy milk and cow’s milk: −3.08 ± 23.02 vs. 14.47 ± 27.59%; P = 0.04, respectively). The effects of soy milk consumption on hs-CRP level was near significant (P = 0.051). This result was not significant after adjustment for carbohydrate intake. There were no significant differences in percent change in FBS in the two interventional periods (percent change in FBS: 10.84 ± 10.47 in cow’s milk vs. −0.5 ± 4.95 in soy milk; P = 0.26). HbA1c was not also significantly different between these two interventional periods (percent change in HbA1c: −8.15 ± 3.60% vs. −7.00 ± 2.10%; P = 0.14).
Table 1.
Baseline and end values of new cardiovascular risk factors among type 2 diabetic patients following consumption of the soy milk and cow’s milk
Table 2.
Differences in percent changes of new cardiovascular risk factors among type 2 diabetic patients following consumption of the soy milk and cow’s milk
CONCLUSIONS
The results of this study revealed that soy milk versus cow’s milk consumption for 4 weeks reduced serum d-dimer level. Although most of the previous studies have shown the beneficial effects of soy products consumption on inflammation (26), there are few studies regarding the effects of soy milk on inflammation, oxidative stress, and coagulation.
Recently, findings suggested that imbalance between the coagulation pathways in DN might damage vascular endothelial cells (27). Also, d-dimer is correlated with renal dysfunction in type 2 diabetic patients (28). In this study, we evaluated serum fibrinogen and d-dimer levels in DN patients, and a significant reduction was observed in the percent change of d-dimer levels in the soy milk group. The change of fibrinogen was not significantly different between the two groups. Previous studies showed conflicting results about the effect of soy interventions on serum fibrinogen (29). One study has demonstrated that genistein consumption could decrease fibrinogen concentrations among postmenopausal women (30). In another study, soy flour consumption did not affect fibrinogen level (31). Although participants were different in the mentioned studies, different types of products, sample size, and study durations might be responsible for these controversial results.
However, in the current study, no distinct difference on inflammatory factors was observed between the two groups. Contrary to our findings, one study reported the beneficial effect of soy containing isoflavones on IL-6 and TNF-α levels among postmenopausal women (30). The findings from a longitudinal study that was conducted in type 2 DN for 4 years have shown that soy protein intake significantly reduced CRP level (12). Results of present study showed near-significant reduction in serum CRP level following soy milk consumption; however, it might be significant if the duration of our intervention was >4 weeks.
Oxidative stress has an important role in pathology of some diseases (31). One study revealed that soy product consumption for 8 weeks could reduce MDA levels in patients with the metabolic disorder (13). Our findings did not show a significant reduction on oxidative stress indices following soy milk consumption. This might be due to the types of soy products and study duration compared with others. The role of soy foods on health is controversial. Gut microflora, ethnic origin, and dietary context may affect absorption, metabolism, and bioavailability of isoflavones (32). Also, bioavailability of isoflavones is associated with gut transit time, fecal digestion rates, lactase, some dietary factors, and storage conditions of soy milk (33). Lactase may be needed in hydrolyzation of isoflavone glucosides in the small intestine (34,35). Therefore, these factors might have a role in not seeing any major changes in the current study.
We attempted to follow patients in each phase and used a dietary record to assess the food intake. However, dietary assessment might have some biases. We could not measure serum or urine isoflavones levels, which could be a limitation of this study. It is suggested that the relation between serum or urine isoflavones levels and inflammatory markers or coagulating factors will be assessed in future studies. We only measured MDA for evaluating the oxidative stress. This seems weak to present some conclusions. MDA is an end product of lipid peroxidation; however, the use of this assay to assess oxidative stress status is problematic because MDA is not a specific product of lipid peroxidation. Furthermore, because of financial limitations, we could not measure F2 isoprostane, protein carbonyls, total antioxidant capacity, or plasma antioxidant enzyme activities.
Strengths of the current study are the crossover design, high percentage of patients who completed this study, and detailed data collection through face-to-face meetings. Moreover, soy milk and cow’s milk were provided for all patients.
As a summary, our findings showed that soy milk consumption for 4 weeks could decrease serum d-dimer level and had no significant effects on the inflammation and oxidative stress in patients with DN.
ACKNOWLEDGMENTS
This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
No potential conflicts of interest relevant to this article were reported.
M.S.M. and L.A. analyzed and interpreted data. A.E., M.M.N., M.M., and L.A. conceptualized and designed the study and drafted the manuscript. L.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01419912, clinicaltrials.gov.
References
- 1.Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev 2010;3:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis 2008;2:72–79 [PubMed] [Google Scholar]
- 3.Madan R, Gupt B, Saluja S, Kansra UC, Tripathi BK, Guliani BP. Coagulation profile in diabetes and its association with diabetic microvascular complications. J Assoc Physicians India 2010;58:481–484 [PubMed] [Google Scholar]
- 4.Kang YS, Lee MH, Song HK, et al. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int 2010;78:883–894 [DOI] [PubMed] [Google Scholar]
- 5.Alwakeel JS, Isnani AC, Alsuwaida A, et al. Factors affecting the progression of diabetic nephropathy and its complications: a single-center experience in Saudi Arabia. Ann Saudi Med 2011;31:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr 2009;139:335–339 [DOI] [PubMed] [Google Scholar]
- 7.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care 2007;30:967–973 [DOI] [PubMed] [Google Scholar]
- 9.Galland L. Diet and inflammation. Nutr Clin Pract 2010;25:634–640 [DOI] [PubMed] [Google Scholar]
- 10.Pot GK, Geelen A, Majsak-Newman G, et al. Increased consumption of fatty and lean fish reduces serum C-reactive protein concentrations but not inflammation markers in feces and in colonic biopsies. J Nutr 2010;140:371–376 [DOI] [PubMed] [Google Scholar]
- 11.Bhupathiraju SN, Tucker KL. Greater variety in fruit and vegetable intake is associated with lower inflammation in Puerto Rican adults. Am J Clin Nutr 2011;93:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care 2008;31:648–654 [DOI] [PubMed] [Google Scholar]
- 13.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Dietary soya intake alters plasma antioxidant status and lipid peroxidation in postmenopausal women with the metabolic syndrome. Br J Nutr 2007;98:807–813 [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto S, Fujii T, Morimiya T, Johdo O, Nakamura T. The fibrinolytic activity of a novel protease derived from a tempeh producing fungus, Fusarium sp. BLB. Biosci Biotechnol Biochem 2007;71:2184–2189 [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Liu Y, Chang H, et al. Effects of genistein on oxidative injury in endothelial cells. J Nutr Sci Vitaminol (Tokyo) 2008;54:402–408 [DOI] [PubMed] [Google Scholar]
- 16.Mateos-Aparicio I, Redondo Cuenca A, Villanueva-Suárez MJ, Zapata-Revilla MA. Soybean, a promising health source. Nutr Hosp 2008;23:305–312 [PubMed] [Google Scholar]
- 17.Franke AA, Ashburn LA, Kakazu K, Suzuki S, Wilkens LR, Halm BM. Apparent bioavailability of isoflavones after intake of liquid and solid soya foods. Br J Nutr 2009;102:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nutritional care in renal disease. In Applications in Medical Nutrition Therapy. 2nd edZeman FJ, Ney DM, Eds. New Jersey, Prentice Hall, 1996, p. 276 [Google Scholar]
- 19.Meyers BD, Bennett PH. Clinical evolution of renal disease in insulin dependent and noninsulin dependent diabetes mellitus. In Principles and Practice of Nephrology. 1st ed Jacobson HR, Striker, GE, Klahr, S, Eds. Philadelphia, BC Decker, 1991, p. 464 [Google Scholar]
- 20.Fleiss JL. The Design and Analysis of Clinical Experiments. London, John Wiley and Sons, 1986, p. 263–271 [Google Scholar]
- 21.Institute of Medicine Food and Nutrition Board: Dietary Reference Intake for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC, National Academies Press, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kleyn DH, Lynch JM, Barbano DM, Bloom MJ, Mitchell MW. Determination of fat in raw and processed milks by the Gerber method: collaborative study. J AOAC Int 2001;84:1499–1508 [PubMed] [Google Scholar]
- 23.Faerk J, Skafte L, Petersen S, Peitersen B, Michaelsen KF. Macronutrients in milk from mothers delivering preterm. Adv Exp Med Biol 2001;501:409–413 [DOI] [PubMed] [Google Scholar]
- 24.Nozawa S, Hakoda A, Sakaida K, Suzuki T, Yasui A. Method performance study of the determination of total nitrogen in soy sauce by the Kjeldahl method. Anal Sci 2005;21:1129–1132 [DOI] [PubMed] [Google Scholar]
- 25.Díaz-Castelazo C, Rico-Gray V, Ortega F, Angeles G. Morphological and secretory characterization of extra floral nectaries in plants of coastal Veracruz, Mexico. Ann Bot 2005;96:1175–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong JY, Wang P, He K, Qin LQ. Effect of soy isoflavones on circulating C-reactive protein in postmenopausal women: meta-analysis of randomized controlled trials. Menopause 2011;18:1256–1262 [DOI] [PubMed] [Google Scholar]
- 27.Nasca MM, Zhou JR, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol 2008;102:84–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakabayashi I, Masuda H. Association of D-dimer with microalbuminuria in patients with type 2 diabetes mellitus. J Thromb Thrombolysis 2009;27:29–35 [DOI] [PubMed] [Google Scholar]
- 29.Imani H, Tabibi H, Atabak S, Rahmani L, Ahmadinejad M, Hedayati M. Effects of soy consumption on oxidative stress, blood homocysteine, coagulation factors, and phosphorus in peritoneal dialysis patients. J Ren Nutr 2009;19:389–395 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Cao S, Nagamani M, Anderson KE, Grady JJ, Lu LJ. Decreased circulating levels of tumor necrosis factor-alpha in postmenopausal women during consumption of soy-containing isoflavones. J Clin Endocrinol Metab 2005;90:3956–3962 [DOI] [PubMed] [Google Scholar]
- 31.Rashidi A, Nakhjavani M, Esteghamati A, et al. Association between oxidant/antioxidant markers and proteinuria in type 2 diabetes: results in 142 patients. J Nephrol 2009;22:733–738 [PubMed] [Google Scholar]
- 32.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr 2006;136:2291–2296 [DOI] [PubMed] [Google Scholar]
- 33.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer 2007;57:1–10 [DOI] [PubMed] [Google Scholar]
- 34.Rau De Almeida Callou K, Sadigov S, Lajolo FM, Genovese MI. Isoflavones and antioxidant capacity of commercial soy-based beverages: effect of storage. J Agric Food Chem 2010;58:4284–4291 [DOI] [PubMed] [Google Scholar]
- 35.Tamura A, Shiomi T, Hachiya S, Shigematsu N, Hara H. Low activities of intestinal lactase suppress the early phase absorption of soy isoflavones in Japanese adults. Clin Nutr 2008;27:248–253 [DOI] [PubMed] [Google Scholar]