Abstract
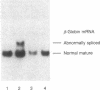
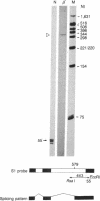
beta-Thalassemia genes, although often mild in their effects, are common among American Blacks. We have begun a systematic molecular analysis of beta-thalassemia mutations in this group. DNA polymorphisms in the beta-globin gene cluster were examined among 22 beta-thalassemia chromosomes. Six different haplotypes were observed. beta-globin genes of two of these were cloned, and their phenotypes were examined both in heterologous cells upon transient expression and in vivo. The gene found in the most common haplotype (9 of 22 chromosomes) contained a single base substitution (A----G) at position -29 within the highly conserved proximal promoter element (the "TATA" box). This mutant gene directed beta-globin RNA at 25% of normal levels both in heterologous cells and in vivo. It was associated with a mild beta +-thalassemia phenotype. A different gene, isolated from an apparently rare haplotype (1 of 22 chromosomes), had a single base substitution (A----G) within the acceptor splice site of the second intervening sequence. This mutation abolished normal RNA splicing so that the only RNA made from the gene in vitro was an alternatively spliced RNA, which could not encode beta-globin. The mild deficit in beta-globin production attributable to the -29 A----G mutant allele most likely accounts for the frequently mild nature of beta-thalassemia among American Blacks.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Serjeant G. R., Theisen C. E., Dover G. J., Kazazian H. H., Jr Origin of the beta S-globin gene in blacks: the contribution of recurrent mutation or gene conversion or both. Proc Natl Acad Sci U S A. 1984 Feb;81(3):853–856. doi: 10.1073/pnas.81.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Orkin S. H., Kazazian H. H., Jr, Goff S. C., Boehm C. D., Waber P. G., Sexton J. P., Ostrer H., Fairbanks V. F., Chakravarti A. Evidence for multiple origins of the beta E-globin gene in Southeast Asia. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6608–6611. doi: 10.1073/pnas.79.21.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman A. S., McCurdy P. R., Manos O., Sherman A. Homozygous beta thalassemia in American Blacks: the problem of mild thalassemia. J Lab Clin Med. 1973 Jun;81(6):857–866. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Cheng T. C., Beamer W. G., Phillips J. A., 3rd, Bartke A., Mallonee R. L., Dowling C. Etiology of growth hormone deficiency in little, Ames, and Snell dwarf mice. Endocrinology. 1983 Nov;113(5):1669–1678. doi: 10.1210/endo-113-5-1669. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dobkin C., Pergolizzi R. G., Bahre P., Bank A. Abnormal splice in a mutant human beta-globin gene not at the site of a mutation. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1184–1188. doi: 10.1073/pnas.80.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- George D. L., Phillips J. A., 3rd, Francke U., Seeburg P. H. The genes for growth hormone and chorionic somatomammotropin are on the long arm of human chromosome 17 in region q21 to qter. Hum Genet. 1981;57(2):138–141. doi: 10.1007/BF00282009. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. E., Humphries R. K., Ley T., Cline A., Kantor J. A., Nienhuis A. W. "Silent" nucleotide substitution in a beta+-thalassemia globin gene activates splice site in coding sequence RNA. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2318–2322. doi: 10.1073/pnas.80.8.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Lee K. Y., Furbetta M., Angius A., Cao A. Polymorphism of DNA sequence in the beta-globin gene region. Application to prenatal diagnosis of beta 0 thalassemia in Sardinia. N Engl J Med. 1980 Jan 24;302(4):185–188. doi: 10.1056/NEJM198001243020401. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Kazazian H. H., Jr Polymorphism and molecular pathology of the human beta-globin gene. Prog Hematol. 1983;13:49–73. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Ostrer H., Goff S. C., Sexton J. P. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature. 1982 Dec 23;300(5894):768–769. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Little P. F., Kazazian H. H., Jr, Boehm C. D. Improved detection of the sickle mutation by DNA analysis: application to prenatal diagnosis. N Engl J Med. 1982 Jul 1;307(1):32–36. doi: 10.1056/NEJM198207013070106. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Sexton J. P., Cheng T. C., Goff S. C., Giardina P. J., Lee J. I., Kazazian H. H., Jr ATA box transcription mutation in beta-thalassemia. Nucleic Acids Res. 1983 Jul 25;11(14):4727–4734. doi: 10.1093/nar/11.14.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Sexton J. P., Goff S. C., Kazazian H. H., Jr Inactivation of an acceptor RNA splice site by a short deletion in beta-thalassemia. J Biol Chem. 1983 Jun 25;258(12):7249–7251. [PubMed] [Google Scholar]
- Pardoll D. M., Rodeheffer R. J., Smith R. R., Charache S. Aplastic crisis due to extensive bone marrow necrosis in sickle cell disease. Arch Intern Med. 1982 Nov;142(12):2223–2225. [PubMed] [Google Scholar]
- Pierce H. I., Kurachi S., Sofroniadou K., Stamatoyannopoulos G. Frequencies of thalassemia in American blacks. Blood. 1977 Jun;49(6):981–986. [PubMed] [Google Scholar]
- Poncz M., Ballantine M., Solowiejczyk D., Barak I., Schwartz E., Surrey S. beta-Thalassemia in a Kurdish Jew. Single base changes in the T-A-T-A box. J Biol Chem. 1982 Jun 10;257(11):5994–5996. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Tuan D., Biro P. A., deRiel J. K., Lazarus H., Forget B. G. Restriction endonuclease mapping of the human gamma globin gene loci. Nucleic Acids Res. 1979 Jun 11;6(7):2519–2544. doi: 10.1093/nar/6.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]