Four years ago, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was stopped early as the result of increased mortality associated with intensive glycemic treatment of a population with type 2 diabetes (T2DM) and high cardiovascular (CV) risk (1). Further reports have appeared from ACCORD (2) and other studies of high-risk populations, notably the Action in Diabetes and Vascular Disease: Preterax and Diamacron MR Controlled Evaluation (ADVANCE) (3) and the Veterans Affairs Diabetes Trial (VADT) (4). These studies provide a body of evidence that intensive treatment of hyperglycemia in T2DM does not in all cases lead to an acceptable balance of benefits to risks. Although important questions remain, both the main findings and secondary and epidemiological analyses from these studies suggest possible changes of our therapeutic approach. This article summarizes the original findings of these trials, discusses recent reports from ACCORD, and describes the authors’ experiences in implementing the intensive regimen at a major ACCORD site. It will not cover all publications on CV risk in T2DM. Rather, we aim to present an updated view of challenges and opportunities in treating high-risk patients with T2DM, drawing mainly on lessons from ACCORD and including some testable hypotheses. The opinions expressed are our own and not meant to represent those of any professional or investigative group.
Mixed results of ACCORD, ADVANCE, and VADT
The UK Prospective Diabetes Study (UKPDS) (5) enrolled participants soon after diagnosis of T2DM and did not require them to have other CV risk factors. At the end of randomized comparison of intensive versus standard glycemic treatment, improvements of microvascular end points were found but not a reduction of CV events or mortality. However, the numbers of participants and the rates of CV events were relatively low and thus the statistical power was limited. Later studies were designed to test the effect of intensive glycemic treatment with protocols more likely to provide a definitive answer to this question. That is, much larger populations were enrolled in ADVANCE (n = 11,140) and ACCORD (n = 10,251), and both these trials and the VADT selected participants with strong CV risk profiles or prior events and thus predictably high future event rates (6–8). The mean duration of known diabetes was 8 years in the ADVANCE cohort, 10 years in ACCORD, and 11.5 years in VADT. The percentages of participants with known major CV disease were 32, 35, and 40%, respectively. The larger numbers of participants and selection of individuals with evidence of high CV risk increased the statistical power of these trials.
Aggressive glycemic control tactics were used in each trial, aiming for nearly normal glycemic control, and all three achieved very good glycemic control in these challenging populations. Intensive treatment led to A1C levels averaging 0.7% (6.5 vs. 7.3%), 1.1% (6.4 vs. 7.5%), and 1.5% (6.9 vs. 8.4%) lower than standard treatment in ADVANCE (3), ACCORD (1), and VADT (4), respectively. One or more microvascular end points were improved in all three trials. The most consistent effects were shown for renal outcomes. Variously defined renal end point events were reduced 21% (P = 0.006) in ADVANCE (3), 21% (P = 0.0005) and 32% (P = 0.0013) in ACCORD (1), and 33% (P = 0.01) in VADT (4). However, the primary CV outcomes were not improved by intensive glycemic therapy. In ACCORD, but not the other trials, a 22% increase of all-cause mortality accompanied intensive glycemic treatment (1).
Results of these trials have been analyzed together with data from the UKPDS by a collaborating group of the trial investigators (9). This careful meta-analysis found a 9% reduction of major CV events accompanying intensive glycemic treatment (hazard ratio [HR] 0.91 [95% CI 0.84–0.99]) but no significant effect on all-cause mortality (1.04 [0.90–1.20]) or CV mortality (1.10 [0.84–1.42]). The small improvement of the composite CV outcome was driven by a 15% reduction of fatal or nonfatal myocardial infarction (MI) (0.85 [0.76–0.94]). A separate meta-analysis of the same trials using different methods produced similar results, notably a 10% reduction of CV disease (relative risk 0.90 [95% CI 0.83–0.98]) but no effect on all-cause (0.97 [0.76–1.24]) or CV mortality (0.98 [0.84–1.15]) (10). Both of these meta-analyses showed an increased risk of severe hypoglycemia with intensive glycemic management (HR 2.48; relative risk 2.03). Two other meta-analyses, which added additional randomized studies to the large trials, concluded that available data show limited and inconclusive evidence of risk reduction for CV end points but confirmed a substantial increase of severe hypoglycemia (11,12).
Why intensive glucose lowering late in the course of T2DM had only a weakly protective effect on CV outcomes in these high-risk populations may partly be explained by long-term follow-up of the UKPDS population (13). Twenty years after randomization and 10 years after the end of randomized treatment, the frequency of MI was 15% lower and all-cause mortality 13% lower in the prior intensive treatment group. Similar results were reported from long-term follow-up of participants with type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) trial (14). No significant effect on CV outcomes was apparent at the end of 6.5 years of intensive versus standard glycemic treatment in the DCCT, but 10 years later the composite of CV death, nonfatal MI, or nonfatal stroke was reduced 57% in the prior intensive treatment group. Apparently, the CV effects of a few years of intensive glycemic treatment early in diabetes may be small or unapparent, but the resulting tissue changes can persist and medical benefits may become evident more than 10 years later. The persistent effect of early treatment also suggests that prior poor metabolic control may result in anatomic changes that are not easily reversed. Thus, late-stage, high-risk patients of the kind selected for ADVANCE, ACCORD, and VADT may be less likely to benefit from glycemic control after years of hyperglycemia. Earlier treatment may be more effective even though its effects are delayed.
This realization has been sobering, but the unexpected occurrence of higher mortality accompanying the intensive treatment strategy in ACCORD has led to even greater concern and much commentary. Earlier diagnosis and better early treatment to forestall both microvascular and CV complications deserve consideration, but this is beyond the scope of this review. However, an equally pressing question is how best to treat people with long-term T2DM and established CV risk factors. The main proposals from various commentaries (12–22) include the following: 1) individualize therapeutic targets and tactics for different groups of patients with type 2 diabetes; 2) reduce the intensity of treatment for high-risk people by aiming to keep A1C between 7 and 8%—the target range for the standard treatment group in ACCORD; and 3) consider older age, longer duration of diabetes, presence of known CV disease or other major illness, and prior severe hypoglycemic events as important predictors of risk. A statement of the American Diabetes Association (ADA) regarding therapeutic targets reads as follows (23): “Less stringent A1C goals (such as <8%) may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, and extensive comorbid conditions, and those with longstanding diabetes in whom the general goal is difficult to attain despite DSME [diabetes self-management education], appropriate glucose monitoring, and effective doses of multiple glucose-lowering agents including insulin.” A more recent statement of the ADA together with the European Association for the Study of Diabetes (EASD) extends this statement by emphasizing the need to adapt therapeutic tactics for a given patient (24): “In a shared decision-making approach, clinician and patient act as partners, mutually exchanging information and deliberating on options, in order to reach a consensus on the therapeutic course of action.” Another recent description of individualizing therapeutic tactics for specific patients also deserves repeating here (25): “Strategies to minimize the risk of hypoglycemia while improving glycemic control include addressing the problem of hypoglycemia at each patient contact, applying the principles of aggressive therapy through education of patients, encouraging frequent self-monitoring of blood glucose, setting up flexible regimens of treatment with insulin or other drugs and providing individualized glycemic goals and ongoing professional support, and considering both the conventional risk factors for hypoglycemia and the risk factors for hypoglycemia-associated autonomic failure.”
How to individualize treatment? Looking for clues from ACCORD
This guidance is appropriate but lacks specificity. To be more exact in identifying populations and individuals suited to one or another glycemic target, or one or another therapeutic method, more information about both benefits and risks is needed. Analyses from ADVANCE and VADT are helpful, but the extensive database from ACCORD may be the most important single resource (19) because this was the one trial showing increased mortality. With the assumption that increased mortality associated with intensive treatment in ACCORD was not a statistical anomaly as has been suggested (26) but reflected a true difference in risk, examination of data from ACCORD continues. Several publications already available offer helpful insights.
Intensive treatment led to benefits as well as risks
Increased mortality during intensive treatment in ACCORD has largely obscured the favorable effects reported from secondary end points and substudies.
Nonfatal MI.
The primary outcome showed no significant between-treatment difference at the end of randomized treatment (1) or after 1.5 additional years of follow-up during which participants previously using the intensive strategy changed to the standard strategy (2). This composite comprised CV death, nonfatal MI, and nonfatal stroke. Nonfatal stroke was least frequent and showed no trend toward between-treatment differences. Nonfatal MI was most frequent, and risk of its occurrence in the intensive therapy group was 21% lower at the transition (HR 0.79 [95% CI 0.81–1.03], P = 0.01) and 18% lower at the end of the study (0.82 [0.70–0.96], P = 0.01). In contrast, the risk of CV death was 27% (nonsignificantly) higher (1.27 [ 0.99–1.63], P = 0.07) at the transition and 29% higher (1.29 [1.04–1.60], P = 0.02) at the end. Whether these reciprocal changes in nonfatal MI and CV death, evident both after 3.5 and 5 years of follow-up, were related is unknown. Possibly, some MIs that might have been nonfatal during standard treatment became fatal because of intensive therapy. Alternatively, sudden death not related to MI (e.g., caused by arrhythmia) may have been increased by intensive treatment whereas, as a separate occurrence, nonfatal MI was decreased by intensive therapy.
Albuminuria.
The prespecified composite microvascular end points showed no clear benefit of intensive glycemic therapy in the general population of ACCORD (27). Components of these composites were assessed as secondary end points. Some, notably those related to clinically important loss of function (significant loss of vision or marked reduction of creatinine clearance), showed no treatment-related differences. However, new appearance of albuminuria and progression of existing albuminuria were both significantly reduced with intensive treatment. Incident microalbuminuria was 21% reduced (HR 0.79 [95% CI 0.69–0.90], P = 0.0005) at the end of randomized treatment and 15% (0.85 [0.77–0.94], P = 0.0012) at the end of the study. Progression to macroalbuminuria was 32% lower (0.68 [0.54–0.86], P = 0.0013) at the transition and 29% lower (0.71 [0.59–0.86], P = 0.0003) at the end.
Retinopathy.
A subgroup of 2,856 participants in ACCORD was evaluated by fundus photography for progression of diabetic retinopathy at randomization and after 4 years of treatment (28). About half had visible retinopathy at randomization. Worsening of vision was not reduced with intensive therapy, although a nonsignificant desirable trend occurred (HR 0.88 [95% CI 0.77–10.1], P = 0.06). Progression of retinopathy was reduced 33% by intensive therapy (0.67 [0.51–0.87], P = 0.003).
Brain volume.
Cognitive function and brain volume assessed by magnetic resonance imaging were assessed in 2,977 participants at baseline and 20 and 40 months after starting randomized treatment. At baseline, previous reports of an association between worse cognitive performance and higher A1C were confirmed in this population (29). After 40 months of randomized treatment, no between-group difference in worsening of cognitive test scores over time was apparent, but an expected decline of brain volume was significantly attenuated (P = 0.0007) in the intensive treatment group (30).
To summarize, significant reductions of nonfatal MI, new or worsening albuminuria and retinopathy, and brain shrinkage resulted from the intensive treatment strategy in ACCORD. Corresponding protection of CV function, renal function, vision, and cognition was not shown during the 3- to 5-year period of follow-up. Whether such medical benefits would appear later, as suggested by the changes of physiological/anatomical markers, is as yet unknown.
Baseline characteristics associated with increased mortality with intensive treatment
An exploratory subgroup analysis sought baseline factors independently associated with increased mortality during subsequent intensive treatment in ACCORD (31). Three possibly predictive characteristics emerged: A1C levels higher than 8.5%, self-reported history of neuropathy, and self-reported history of aspirin use. An elevated A1C reflecting poor glycemic control on entry to the trial seems a logical predictor because previous difficulty in managing diabetes might reflect severe physiological abnormalities, other medical conditions, or behavioral and environmental barriers to safe application of an intensive strategy. The significance of historical reports of neuropathy or aspirin use is less clear. Perhaps they indicate significant burdens of microvascular or CV disease, respectively. The possibility that a history of neuropathy predicted risk from intensive treatment because it was associated with autonomic neuropathy was not supported by a subsequent analysis (32). Although cardiac autonomic neuropathy was associated with higher risk of death, the increase did not differ between treatment groups.
On-treatment factors associated with increased mortality
Proposed mechanisms include drug effects, weight gain, A1C reduction, and hypoglycemia. No clear evidence points to specific drugs, drug combinations, or weight gain as mediators of mortality during intensive therapy to date, but analyses continue. On the other hand, new information is available for two other candidates: rapid and sustained reduction of A1C and severe hypoglycemia.
Reduction of A1C.
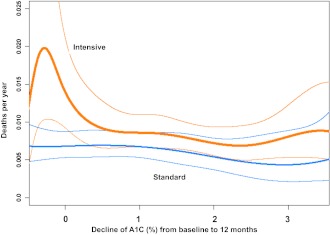
Epidemiological analysis of relationships between aggressive reduction of A1C and mortality produced surprising findings (33). Examining all participants in ACCORD together confirmed an association of higher on-treatment A1C with greater risk of death as in other populations. However, when the intensive- and standard-treatment groups were studied separately, the patterns were quite different (interaction P = 0.0007) (Fig. 1). With the intensive strategy, a log-linear relationship was apparent. Risk was lowest when updated average on-treatment A1C was near 6% and increased steadily at higher levels of A1C. With the standard strategy, the relationship was not clearly linear but closer to a shallow U-shaped curve with lowest risk between 7 and 8% A1C. The relationships between these curves suggest that the excess risk with intensive compared with standard treatment occurred among participants whose average A1C values were above 7%. When mortality rates were plotted against the change of A1C after beginning randomized treatment, clear differences between treatment groups were again present (Fig. 2). In the standard group, individuals with greater than 2% reduction of A1C from baseline after 4–12 months of standard treatment had the same risk of death later in the trial as those whose A1C did not change from baseline. In contrast, in the intensive treatment group increased risk was evident only for participants whose A1C declined little or not at all after entering the trial. Thus, the participants at highest risk were those who attempted intensive therapy but failed to improve glycemic control and continued to have A1C greater than 7%.
Figure 1.
The risk of all-cause mortality during randomized treatment in ACCORD is shown for each treatment group by spline curves over a range of average A1C from 6 to 9%. Values are adjusted for covariates by a proportional hazards model. Bold colored lines represent each treatment group and finer colored lines the 95% CI. The figure is adapted with permission from Riddle et al. (33).
Figure 2.
All-cause yearly mortality rates during randomized treatment in ACCORD are shown for each treatment group over a range of decreases of A1C during the first year of treatment. Covariate-adjusted values are calculated by a Poisson regression model. Bold colored lines represent each treatment group and finer colored lines the 95% CI. The figure is adapted with permission from Riddle et al. (33).
Hypoglycemia.
Hypoglycemia can have serious consequences, including seizures, motor vehicle accidents and other injuries, and cardiac arrhythmias. Epidemiological studies also show hypoglycemia is associated with increased risk of death, but whether as a cause or mainly by association with other factors is unknown (34–36). Thus it is natural to suspect hypoglycemia as a culprit during intensive treatment in ACCORD (37). The annual incidence of events requiring medical assistance was higher with intensive than with standard treatment, 3.14 vs. 1.03% (38). However, severe hypoglycemia was only rarely linked with deaths for which the cause could be assessed. Hypoglycemia was judged as definitely contributing to death for 1 participant in the intensive group, probably contributing to death in 1 in the intensive and 2 in the standard group, and possibly connected with death in 25 in the intensive and 13 in the standard group (39). Individuals using the intensive strategy who were known to have prior severe hypoglycemia were less likely to die later than those using the standard strategy with a prior severe event (39). Among participants who had prior severe hypoglycemia, the annual mortality rate was 2.8% in the intensive treatment group and 4.9% in the standard treatment group (HR 0.55 [95% CI 0.31–0.99]). This paradox—that intensive treatment resulted in more severe events but these could not be convincingly linked to the increase risk of death—remains unexplained.
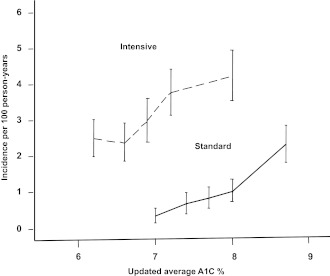
Two additional analyses point toward a novel hypothesis. One concerns the relation of severe hypoglycemia to the updated average A1C in each treatment group. Whereas severe hypoglycemia was more common at low levels of A1C in type 1 diabetes in the DCCT, higher average A1C levels were associated with more hypoglycemia in each treatment group in ACCORD (38) (Fig. 3). The intensive treatment group had a higher incidence of severe events over most of the A1C range compared with the standard group, but the slopes of the curves were similar. The excess of events with the intensive treatment strategy was most evident in the range of average A1C between 7 and 8%, again drawing attention to participants who were unable to reduce A1C below 7%. This subgroup had the highest incidence of severe hypoglycemia and also the highest risk of death. Nevertheless, the question remains: Why were individuals who previously had severe hypoglycemia at greater risk of later death if they were in the standard group than if they were attempting the intensive strategy?
Figure 3.
The incidence of hypoglycemia requiring medical assistance during randomized treatment in ACCORD is shown for each treatment group by quintiles of updated average A1C. Vertical bars show 95% CI. The figure is adapted with permission from Bonds et al. (39).
The other analysis bears on this point. The association between mortality risk and self-reported nonsevere hypoglycemia was examined in a subgroup of participants on either regimen who had previously had a severe event (39). Not surprisingly, the incidence of documented nonsevere events was greater in the intensive treatment group. However, participants with more nonsevere events with glucose confirmed <3.9 mmol/L (70 mg/dL) had lower risk of later death, independent of which treatment regimen was used. The hypothesis that prior nonsevere hypoglycemia can be protective was explored in a more comprehensive subsequent report that included data from more than 10,000 participants in ACCORD (40). After adjustment for covariates, the frequency of nonsevere events was again inversely associated with mortality. That is, individuals with more mild to moderate hypoglycemia had lower risk of death. This association was especially strong in the intensively treated group. Together, these findings pose the possibility that “hypoglycemic preconditioning” modified the risk of death associated with severe hypoglycemia in ACCORD. Hypoglycemia-associated autonomic failure resulting in reduced secretion of catecholamines and fewer symptoms during subsequent hypoglycemic events (25,41) can result in accidents and injuries. But how is it related to the risk of fatal arrhythmias? Perhaps participants who did not reduce A1C below 7% were more vulnerable to arrhythmias or other negative consequences of an isolated severe event because they usually had high glucose levels and less often experienced mild hypoglycemia.
Evolution of intensive therapy during ACCORD
Patterns in the whole ACCORD population
The ACCORD participants’ mean age was 62 years and median A1C 8.1% (mean 8.3%) at enrollment (1). About 60% were already taking metformin, 50% a sulfonylurea, 20% a thiazolidinedione, and 35% insulin. In the first year, participants assigned to intensive treatment were prescribed combinations of oral agents to improve glycemic control according to general guidance in the ACCORD protocol (42). Insulin was added when needed, usually first as basal insulin but with prandial injections later in many cases. By the end of treatment, 95% of intensively treated participants had been prescribed metformin, 87% a secretagogue, 92% a thiazolidinedione, 23% an α-glucosidase inhibitor, 77% some form of insulin therapy, and 55% a regimen including prandial insulin (1). The median A1C of the intensive group decreased to 6.7% at 4 months and by 1 year after randomization stabilized at a plateau in the 6.4–6.5% range. In contrast, the standard group attained median A1C 7.5% at 4 months and maintained this level to the end of treatment.
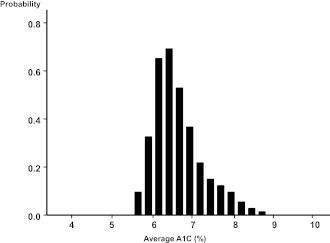
At the end of 1 year, the upper interquartile boundary for A1C in the intensive treatment group in ACCORD was about 7%, indicating that ∼25% of participants had values above 7% (1). A similar proportion of “high-outliers” persisted throughout 3.5 years of intensive treatment, represented by a tail of the distribution curve of A1C levels attained (33) (Fig. 4). After cessation of the intensive treatment strategy, levels of A1C for participants previously assigned to intensive treatment increased gradually over the last 1.5 years of observation. Even so, at the end of the trial, the former intensive group had median A1C 7.2%—still lower than 7.6% for the standard group (2).
Figure 4.
The frequency of occurrence of average updated A1C values over the observed range at the time of all visits during randomized treatment in ACCORD is shown for participants in the intensive treatment group. The figure is adapted with permission from Riddle et al. (33).
Because ACCORD compared two treatment strategies rather than specific drug regimens, investigators were “given flexibility to individualize interventions” to achieve treatment goals for each participant (42). For the intensive treatment group, decisions on addition or discontinuation of drugs or changes of dosage were to be made at each visit. In practice, this required addition of all classes of drugs that seemed safe and appropriate for most intensive group participants in the first 1–2 years. Thereafter, the emphasis was on individualized adjustments of dosage and instruction of participants on the medication and lifestyle regimen assigned, especially in the case of insulin therapy.
Individualization of treatment at one ACCORD site: observations and tactics.
Our ACCORD site at Oregon Health & Science University (OHSU) enrolled more than 240 participants, half to intensive treatment. Four endocrinologists and an adult nurse practitioner with experience in diabetes were actively involved in glycemic management. Because the costs of treatment and access to the investigators were not barriers, this setting tested the efficacy of current treatment methods under better-than-usual conditions. At the outset we at OHSU, like others in ACCORD, expected that most methods widely used for intensive insulin treatment would be applicable in ACCORD. Over time, because of the small margin for error when approaching A1C 6% and also the high-risk characteristics of this population, we modified some tactics.
1) Stepwise initiation of prandial insulin.
To avoid overwhelming participants who were already taking a long list of drugs, mealtime insulin was usually started with a single injection before the dominant meal, rather than with all meals at once. The aim was to simplify adherence and decision making by the participant. This approach has subsequently been documented in clinical studies (43).
2) Limited reliance on postprandial glucose testing and carbohydrate counting.
Early guidance in ACCORD favored glucose testing both before and after meals, together with frequent use of carbohydrate counting, to guide insulin dosing. Because this seemed inconsistently effective, our group at OHSU eventually stressed testing before meals and at bedtime and based adjustments of mealtime insulin on these values and the anticipated size of each meal for most intensive participants. We also did not emphasize correction doses for preprandial glucose elevations. A similar approach based on preprandial testing and semiquantitative adjustment of prandial dosing without carbohydrate-counting has recently been reported (44).
3) Accurate information.
Over time a particularly strong emphasis was placed on determining the actual medication-taking and eating practices of each participant, rather than relying upon information reported from the study records. This proved remarkably challenging and required both skill and persistence (45). The dosage and timing of insulin recommended at prior visits was often not being used, and perhaps had never been attempted. Some participants never varied doses from day to day; others changed them routinely. Unusual patterns of meals, with variations in number, timing, and composition from day to day, were common. When glycemic patterns were other than expected, obtaining accurate information on medication use became the highest priority. This seemed necessary both to improve glycemic control and to reduce risks, especially hypoglycemia resulting from inconsistent use of insulin.
4) Modeling decision making for participants.
After the first year or two, intensive management relied more on appropriate adjustments of dosage than on adding new medications. Accordingly the investigators concluded that safely maintaining control of A1C increasingly depended on daily decision making by the participants, rather than prescriptive instructions (46,47). Continuity of the relationship between participants and investigators appeared to facilitate such individualized daily therapy.
Interpretation and conclusions
Both the published reports and our own observations from ACCORD have influenced our view of managing high-risk patients with T2DM. Although the very ambitious 6% A1C target in ACCORD was not reached by most participants seeking it, the success of half of them in maintaining A1C at 6.4% or less for up to 6 years was a considerable accomplishment. It showed that even with treatment methods that are already obsolescent, near-normal glycemic control was often possible when necessary resources were provided. However, this achievement often depended on systematic individualization of tactics.
The 1.1% reduction of A1C beyond the 7.5% median level maintained by the standard strategy led to improvements in retinopathy and albuminuria, nonfatal MI, and age-related loss of brain volume. These desirable changes support the hypothesis from epidemiological data that reducing A1C can improve some medical outcomes even in this high-risk population. The meta-analyses including data from other trials reinforce this conclusion. At the same time, evidence for an excess of all-cause and CV mortality in ACCORD cannot be dismissed. Isolated severe hypoglycemia at present appears the leading candidate for a mediating cause of the excess mortality, but other potential factors have not been excluded and current evidence is indirect and inconclusive.
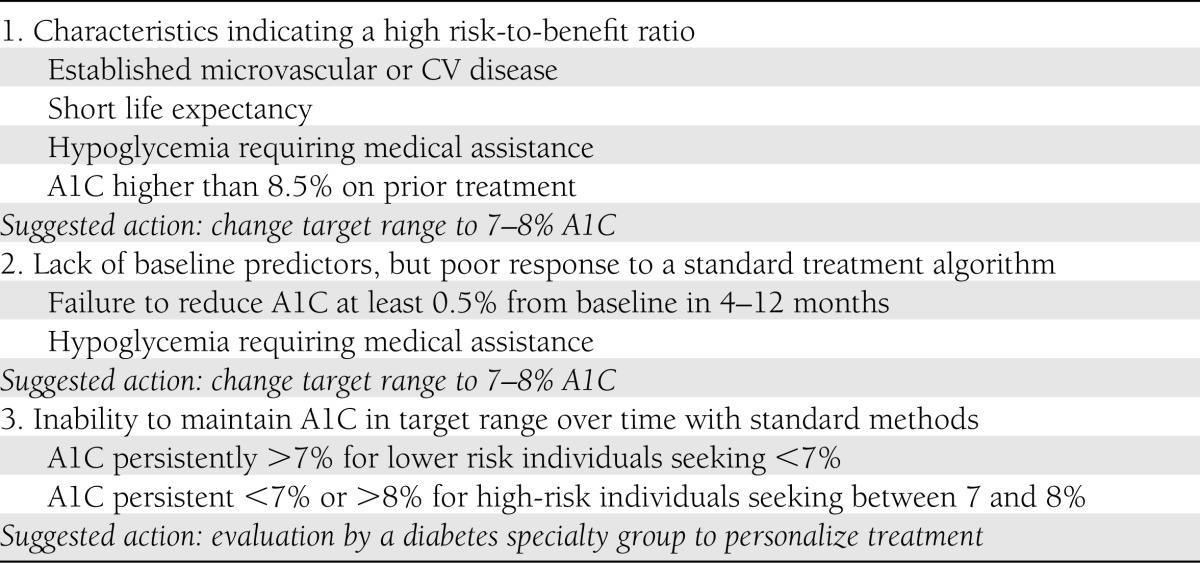
The problem, then, is weighing benefit versus risk. We do not believe current evidence justifies abandoning the general goal of attaining A1C <7%. Rather, individualization of both targets and tactics is needed to minimize risks. Three separate strategies emerge from experience in ACCORD (Table 1).
Table 1.
Proposed indicators of need to individualize therapeutic targets or tactics for high-risk type 2 diabetes
Individualizing the A1C target before treatment
As generally agreed, patients with the prominent risk factors emphasized in the ADA and ADA/EASD statements (notably, long-duration diabetes, severe vascular complications, short life expectancy, prior severe hypoglycemia) should not seek A1C <7%, but rather the range from 7 to 8%. These characteristics should be more specifically defined, but the principle is clear. Individuals without these warning signs might still begin efforts to reach A1C <7%.
Individualizing the A1C target by response to treatment
Observing the early response to therapy is also desirable. Most people starting with elevated A1C obtain substantial improvement by using standard, evidence-based therapies under conditions of usual clinical practice. However, some will not respond as expected for physiological or behavioral reasons not identified in advance. The mortality risk associated with the intensive treatment in ACCORD was concentrated in the subgroup of participants who did not reduce A1C more than 0.5% after 4–12 months of treatment. This lack of success—no greater than 0.5% reduction from a baseline above 7.5%—appears to indicate that continuing to seek A1C <7% may be hazardous and the 7–8% target range more appropriate for that person.
Personalizing therapy for individuals unsuccessful with algorithmic methods.
People without evident risk-factors who substantially reduce A1C by algorithm-driven treatment but do not maintain levels in the chosen target range of A1C may benefit from individualization of tactics independent of the target (24,48,49). “Personalized” treatment aims to adapt tactics to each person’s characteristics, both biological and behavioral, to optimize the benefits and limit risks of therapy. Although not entirely defined as yet, this would include assessing the person’s physiological responses, barriers to success, and preferences; proposing a personalized pharmacological and behavioral regimen; and (especially for the use of insulin) modeling the process of making good decisions in daily self-care. It may be assumed that, unlike type 1 diabetes, T2DM rarely requires such personalized attention. Experience in recent trials argues otherwise. This approach requires long-term follow-up by an experienced specialty team as an adjunct to primary care. Whether such resources can be provided for high-risk individuals may depend on the long-term medical outcomes of ACCORD and other large trials.
Testable hypotheses
Like those of other commentators, our views derive from secondary or exploratory analyses and thus are based on hypotheses. Here we highlight three hypotheses that deserve further investigation.
A physiological hypothesis
Hypoglycemic preconditioning underlies the ACCORD paradox. Severe hypoglycemia is most dangerous when it is an isolated event. Repeated mild hypoglycemia reduces the risk of arrhythmias and other serious consequences of subsequent severe hypoglycemia.
A behavioral hypothesis
Failure to respond quickly to usual treatment predicts increased risk. If 4–12 months of evidence-based treatment does not reduce A1C at least 0.5%, reassessing targets and tactics will reduce risk.
A health system hypothesis
Failure to maintain A1C goals within a selected target range by algorithm-based tactics increases risks and calls for personalized treatment by specialized personnel. Integrating an algorithm-driven primary care system with a diabetes specialty group by automatic triggers for personalized attention will improve medical outcomes and reduce costs.
In summary, A1C <7% remains a target for some people with long-duration diabetes, but those at highest risk should be identified and assigned different goals, usually a target range between 7 and 8%. High risk status can be identified by baseline characteristics or by failure to rapidly improve control with standard therapies. Individuals not maintaining appropriate A1C goals with algorithmic treatment may benefit from a personalized regimen with structured follow-up. These tentative conclusions are based on testable hypotheses.
Acknowledgments
Preparation of the manuscript was supported in part by the Rose Hastings and Russell Standley Memorial Trusts.
M.C.R. has received research grant support from Amylin, Eli Lilly, and sanofi-aventis and honoraria for consulting from Amylin, Eli Lilly, sanofi-aventis, Hoffmann-La Roche, and Valeritas. D.M.K. has received honoraria for speaking from Amylin. No other potential conflicts of interest relevant to this article were reported.
The authors thank Andrew Ahmann, MD, Kathryn Hanavan, RN, MSN, ANP, and Diana Negreanu, MD, all employees of Oregon Health & Science University, for participating in discussions on this topic during the ACCORD trial.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerstein HC, Miller ME, Genuth S, et al. ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 6.ADVANCE Management Committee Study rationale and design of ADVANCE: action in diabetes and vascular disease—preterax and diamicron MR controlled evaluation. Diabetologia 2001;44:1118–1120 [DOI] [PubMed] [Google Scholar]
- 7.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99(Suppl.):21i–33i [DOI] [PubMed] [Google Scholar]
- 8.Abraira C, Duckworth W, McCarren M, et al. VA Cooperative Study of Glycemic Control and Complications in Diabetes Mellitus Type 2 Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications 2003;17:314–322 [DOI] [PubMed] [Google Scholar]
- 9.Turnbull FM, Abraira C, Anderson RJ, et al. Control Group Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 10.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med 2009;151:394–403 [DOI] [PubMed] [Google Scholar]
- 11.Hemmingsen B, Lund SS, Gluud C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 2011;343:d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 2011;343:d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 14.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch IB. Piecing the puzzle together: ACCORDing to whom? J Clin Endocrinol Metab 2008;93:1161–1163 [DOI] [PubMed] [Google Scholar]
- 16.Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med 2008;358:2630–2633 [DOI] [PubMed] [Google Scholar]
- 17.Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association. American College of Cardiology Foundation. American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montori VM, Fernández-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med 2009;150:803–808 [DOI] [PubMed] [Google Scholar]
- 19.Riddle MC. Counterpoint: Intensive glucose control and mortality in ACCORD—still looking for clues. Diabetes Care 2010;33:2722–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 21.Genuth S, Ismail-Beigi F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab 2012;97:41–48 [DOI] [PubMed] [Google Scholar]
- 22.Colagiuri S. Optimal management of type 2 diabetes: the evidence. Diabetes Obes Metab 2012;14(Suppl. 1):3–8 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2004;350:2272–2279 [DOI] [PubMed] [Google Scholar]
- 26.Lachin JM. Point: Intensive glycemic control and mortality in ACCORD—a chance finding? Diabetes Care 2010;33:2719–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ismail-Beigi F, Craven T, Banerji MA, et al. ACCORD trial group Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew EY, Ambrosius WT, Davis MD, et al. ACCORD Study Group. ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al. Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 2009;32:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND investigators Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calles-Escandón J, Lovato LC, Simons-Morton DG, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pop-Busui R, Evans GW, Gerstein HC, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riddle MC, Ambrosius WT, Brillon DJ, et al. Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoungas S, Patel A, Chalmers J, et al. ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 35.Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011;124:1028–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301:1556–1564 [DOI] [PubMed] [Google Scholar]
- 37.Cryer PE. Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med 2011;124:993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller ME, Bonds DE, Gerstein HC, et al. ACCORD Investigators The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaquist ER, Miller ME, Bonds DE, et al. ACCORD Investigators The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care 2012;35:409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Riddle MC, Kendall DM, et al. ACCORD Study Group Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(Suppl.):34i–43i [DOI] [PubMed] [Google Scholar]
- 43.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011;13:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008;31:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 2004;27:1218–1224 [DOI] [PubMed] [Google Scholar]
- 46.Riddle MC. A strategy for chronic disease. Lancet 1980;2:734–736 [DOI] [PubMed] [Google Scholar]
- 47.Montori VM, Gafni A, Charles C. A shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect 2006;9:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klonoff DC. Personalized medicine for diabetes. J Diabetes Sci Tech 2008;2:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pozzilli P, Leslie RD, Chan J, et al. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician’s personalized approach. Diabetes Metab Res Rev 2010;26:239–244 [DOI] [PubMed] [Google Scholar]