Abstract
OBJECTIVE
Metabolic syndrome (MetS) is becoming a serious public health concern in many countries, including South Korea, which has faced remarkable changes in lifestyles and disease patterns in recent decades. We examined sex and socioeconomic status (SES) disparities in MetS and its components among South Koreans using recent, nationally representative data.
RESEARCH DESIGN AND METHODS
Data from the 2007−2008 Korea National Health and Nutrition Examination Surveys for 7,289 adults 19−65 years of age were used to examine the patterns of MetS components (defined using International Diabetes Federation criteria), and regression models were used to study the association of MetS with SES, indicated by education and family income levels.
RESULTS
MetS prevalence increased with age, from 4.6% at age 19−29 years to 25.0% at age 50−65 years. More men had MetS than women (15.8 vs. 11.6%); men had worse levels of all MetS components. In women, the low-income and low-education group was more likely to have MetS (odds ratio 2.75 [95% CI 1.75–4.31]); the high-income and high-education group was 52% less likely to have MetS (0.48 [0.25–0.89]) compared with the middle-income and middle-education group. The most common combination of MetS components was central obesity + low HDL cholesterol (HDL-C) + hypertriglyceridemia, which occurred in 15.5% of all MetS patients and in 3.4% of all South Korean adults (4.1% in men and 2.9% in women).
CONCLUSIONS
Those who were older and male as well as low-SES female had higher rates of MetS and its components in South Korea. The SES-MetS association was not found in men. Central obesity + low HDL-C + hypertriglyceridemia was the most common MetS pattern regardless of the SES.
Socioeconomic status (SES) is inversely associated with obesity (1), cardiovascular diseases (CVDs) (2) such as heart disease (3), and stroke (4) in developed countries. Higher income and education levels seem to be protective against chronic diseases as well as premature mortality (5,6). Some previous studies have examined the associations between the degenerative disease of metabolic syndrome (MetS) and SES in various populations (7–11). MetS is becoming a major public health concern worldwide as a result of the rapid global increase in obesity (12). Rapid socioeconomic transition in the past two decades in South Korea has resulted in some remarkable changes in people's lifestyles and, consequently, some changes in disease patterns (13). Several previous South Korean studies report that the MetS prevalence has increased (8,14,15).
We suspect that the patterns of the MetS component combination may vary across populations due to environmental, behavioral, and other biological factors. Understanding these varying patterns could improve our understanding of the etiology of MetS and guide related prevention and treatment efforts. To our knowledge, one gap in the literature is that no published studies have examined the patterns of the compositions of MetS components in South Koreans, although limited research has been conducted in other populations (16,17). A good understanding of the composition of various components is important because individual or particular combinations of components relate to the risk of other specific diseases and need different management and treatment.
This study assessed the association between sociodemographic-economic factors and the various components of MetS using nationally representative data collected from South Korean adults 19–65 years of age. We also studied the composition of various MetS components. The findings will help guide future interventions to reduce metabolic risks in the target population.
RESEARCH DESIGN AND METHODS
Study population and database
The data for this study were derived from the fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV, 2007−2008). The KNHANES is a series of population-based, cross-sectional surveys that selected a representative group by using a stratified, multistage sampling design according to geographic area, age, and sex. Since 2007, the KNHANES has become a year-round investigation with rolling survey sampling. Stratification was conducted based on the country’s 29 areas, including 11 metropolitan cities and provinces, the administrative unit, and the dwelling type. The survey included a health interview survey, a health examination survey, and a nutrition survey.
We used data from the health interview survey to get information regarding sociodemographic characteristics. Anthropometry, blood pressure (BP) measures, and laboratory tests of the subjects were obtained by direct health examination in a mobile examination center. Detailed descriptions of the study design and data collection have been published (18). The KNHANES IV was approved by the Korea Centers for Disease Control and Prevention Institutional Review Board.
We merged the 2007 and 2008 data and focused on adults 19−65 years of age, a sample of 8,169 (3,540 men and 4,629 women) with complete demographic data. Pregnant women (n = 74) were excluded because their metabolic risk factors were different. Only 7,486 of the 8,169 subjects had waist circumference (WC) data. Complete data on MetS components such as BP, glucose, triglyceride (TG), and HDL cholesterol (HDL-C) were available for 7,289 (3,126 men and 4,163 women) subjects, our final study sample.
Measures of MetS components
Anthropometric measures.
Standing height (Seca 225; SECA, Hamburg, Germany) and weight (GL-6000–20; CASKOREA Co., Ltd., Seoul, Korea) were obtained using standardized techniques and equipment. WC was measured at the midpoint between the subcostal bottom and the top of the iliac crest using a fiberglass tape (Seca 200). Measurements were recorded to the nearest 0.1 cm or 0.1 kg.
BP.
Three BP determinations were obtained by standard methods with the subject in a sitting position using a mercury sphygmomanometer. After rest, while seated, BP was measured three times at 30-s intervals. The average of the second and third measurements was the variable for the analysis.
Laboratory tests.
All blood specimen collection and processing instructions are described in the KNHANES Laboratory/Medical Technologists Procedures Manual (13). Blood samples were collected in the morning after fasting for at least 8 h. Fasting blood glucose, TG, and HDL-C levels were measured using an automated hematology analyzer (ADVIA 1650; Siemens, Tarrytown, NY) in a central, certified laboratory.
Classification of MetS
The 2006 International Diabetes Federation (IDF) definition for MetS was used. For a person to be defined as having the MetS, they must have central obesity based on WC with cut-points specific to South Koreans (WC ≥90 cm in men and ≥85 cm in women) (19) plus any two of the following four factors: 1) elevated TG, ≥150 mg/dL (1.7 mmol/L), or specific treatment for this lipid abnormality; 2) reduced HDL-C, <40 mg/dL (1.03 mmol/L) in men and <50 mg/dL (1.29 mmol/L) in women, or specific treatment for this lipid abnormality; 3) elevated BP, systolic BP ≥130 or diastolic BP ≥85 mmHg, or treatment of previously diagnosed hypertension; and 4) elevated fasting plasma glucose, ≥100 mg/dL (5.6 mmol/L), or previously diagnosed type 2 diabetes. The total number of these metabolic disturbances was calculated for each subject.
Socioeconomic characteristics
SES was defined based on education, household per capita income, and a combination of income and education. The quartiles of average household monthly income were defined by KNHANES (18). We defined three incomes groups, low (Q1 + Q2), middle (Q3), and high (Q4), and three education groups, <high school (HS) education (12 years), HS, and >HS education.
Next, we defined nine income-education groups, but we only present the results for three combinations to indicate the differences of extreme SES, namely, low income and low education (worse SES), middle income and middle education (middle SES) and high income and high education (best SES). In addition, urban-rural residence was classified based on the South Korean administrative units primarily according to population size (urban ≥50,000) (20). Rural areas (and associated populations) are those dominated by primary or secondary industries such as agriculture and manufacturing. Tertiary, service-related industries dominate the urban areas.
Statistical analysis
The key outcome variables of this study were MetS and combinations of various MetS components. The key exposure variables were sex and SES. All analyses were two tailed, and a P value <0.05 was considered statistically significant. The primary sampling units, strata, and sampling weights were taken into account using STATA release 11.0 survey-related commands. This gave nationally representative estimates and a correct estimate of the related variances.
First, we described the sex and SES differences in metabolic outcomes. Between-group differences in means were tested using Student t tests, and those in categorical variables (e.g., MetS and the various MetS component combinations) were analyzed using χ2 tests. We also tested trends across household income and education categories.
Next, we fit logistic regression models to examine the association between sex and SES and metabolic outcomes (outcome variables). All models included age, sex, education, income, and residence; however, we did not control for lifestyle variables such as smoking, eating, or alcohol drinking because there are studies suggesting that they are in the causal pathways between SES and MetS (21,22). Further, we tested the association between MetS and combinations of SES (different levels of education plus income, see above). The middle-income and middle-education group was treated as the reference.
RESULTS
Prevalence of MetS and its components by age and sex
The subjects’ mean age was 40.1 years. Most participants (83.4%) lived in urban areas. Men had higher incomes and education than women (P < 0.05). The prevalence of MetS increased with age regardless of sex, from 4.6% at age 19−29 years to 25.0% at age 50−65 years (trend test, P < 0.05). More men than women had MetS (15.8 vs. 11.6%; P < 0.05), but after age 50, more women than men had MetS. Especially, postmenopausal women were more likely than premenopausal women to have MetS (odds ratio [OR] 2.02 [95% CI 1.48–2.76]) and its components as well.
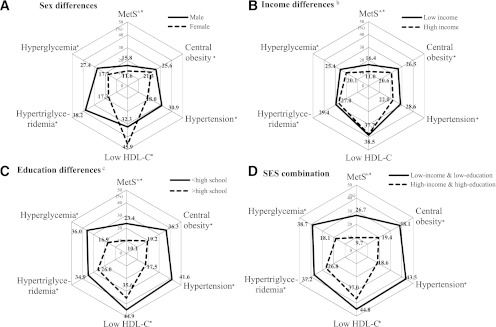
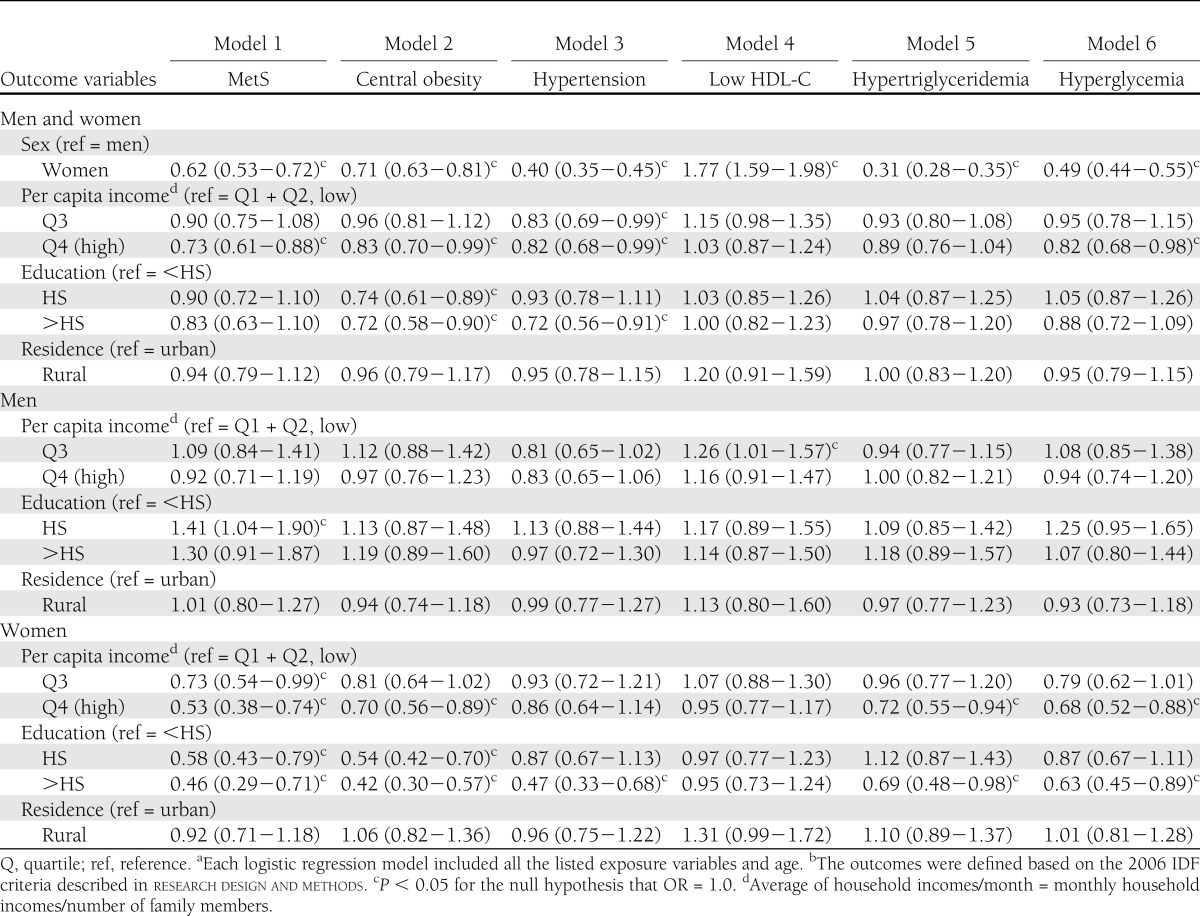
Men suffered worse metabolic outcomes than women, with the exception of low HDL-C prevalence (all P < 0.05) (data not shown). Figure 1A shows the sex-specific prevalence of MetS and its components (P < 0.05). Logistic regression models showed that women had lower rates of MetS (38%), central obesity (29%), hypertension (60%), hypertriglyceridemia (69%), and hyperglycemia (51%) than men (P < 0.05), whereas they had a higher low HDL-C rate (77%; P < 0.05) than men (Table 1).
Figure 1.
Comparison of prevalence (%) of metabolic outcomes by sex and SES among 19- to 65-year-old South Koreans: KNHANES 2007−2008. A: Male, solid line; female, dotted line. B: Low income, solid line; high income, dotted line. C: <HS, solid line; >HS, dotted line. D: Low income and low education, solid line; high income and high education, dotted line. aOutcomes were defined based on the 2006 IDF definition. bIncome was the quartiles of average household monthly income: low (Q1 + Q2), middle (Q3), and high (Q4). cEducation levels: <HS, HS, and >HS education. *P < 0.05, between-group difference was significant.
Table 1.
Associations (OR and 95% CI) between sociodemographic characteristics and MetS and its components among 19- to 65-year-old South Korean adults: KNHANES 2007−2008a,b
Association between SES and metabolic outcomes
Our results show strong associations between SES and metabolic outcomes, particularly for women. Higher SES groups seemed to have lower risks. The mean BMI, WC, BP, TG, and fasting blood glucose and the prevalence of MetS and its components were highest among the low-income and the <HS groups (P < 0.05). However, HDL-C level was highest among the high-income and HS education groups. Low HDL-C rate was the highest among the middle-income and <HS education groups. The mean metabolic disturbances were highest among low-income and <HS education groups (trend test, P < 0.05) (data not shown). Figure 1C and D shows the education and SES differences in the prevalence of MetS and its components (P < 0.05); no differences in low HDL-C and hypertriglyceridemia prevalence were observed among the income groups (P > 0.05) (Fig. 1B). The low-income and <HS education groups had the highest mean number of metabolic disturbances. Our models included age, sex, education, income, and residence and showed that 1) high-income participants had lower rates of MetS (27%), central obesity (17%), hypertension (18%), and hyperglycemia (18%) than low-income participants (P < 0.05); and 2) high-education participants had a lower central obesity rate (28%; P < 0.05) than low-education participants. However, when using sex-stratified analysis, there was no clear association between MetS components and income or education in men, whereas most components, except in low HDL-C, had an inverse relation to income or education in women (P < 0.05) (Table 1).
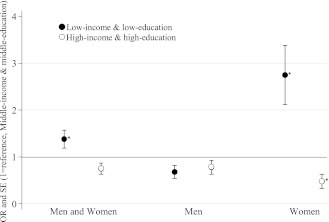
We also assessed the associations between the joint effect of income and education with MetS (Fig. 2). After controlling for age, sex, and urban-rural residence, the low-income and low-education group had a higher MetS rate (OR 1.38 [95% CI, 1.05–1.81]) than the middle-income and middle-education group (reference group), whereas the high-income and high-education group did not differ from the reference group (0.75 [0.55–1.04]). However, the associations were significant only in women regardless of their menopausal status. Compared with the reference group, low-income and low-education women were approximately three times more like to have MetS (2.75 [1.75–4.31]), whereas the high-income and high-education women's risk was lower by 52% (0.48 [0.25–0.89]).
Figure 2.
ORs (±SE) for MetS by education and family income among 19- to 65-year-old South Koreans: KNHANES 2007−2008. Low income and low education, ●; high income and high education, ○. Logistic regression model included age, sex, and urban-rural residence. Middle income and middle education was the reference group. Income was the quartiles of average household monthly income: low (Q1 + Q2), middle (Q3), and high (Q4). Education levels: <HS, HS, and >HS education. *P < 0.05 for the null hypothesis that OR = 1.0.
The prevalence of various MetS compositions by sex and SES
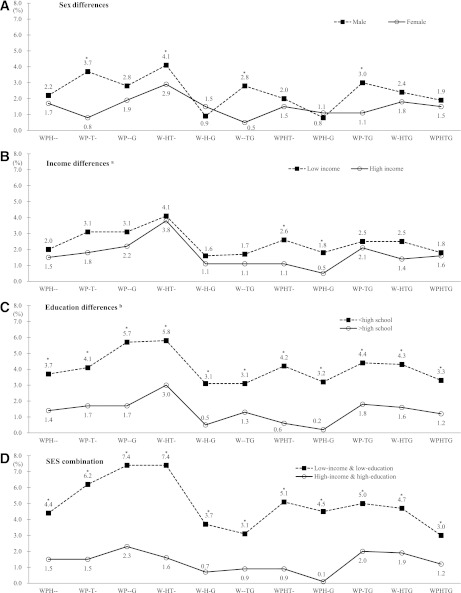
We detected some sex and SES differences in MetS compositions. The four combinations of central obesity + hypertension + hypertriglyceridemia, central obesity + low HDL-C + hypertriglyceridemia, central obesity + hypertriglyceridemia + hyperglycemia, and central obesity + hypertension + hypertriglyceridemia + hyperglycemia were significantly different between sexes (Fig. 3). However, in both men and women, the most frequently reported pattern was a combination of central obesity, low HDL-C, and hypertriglyceridemia (4.2% in men and 2.9% in women). The combination was also the highest prevalence in both low- (4.1%) and high- (3.8%) income groups, as well as in <HS (5.8%) and >HS (3.0%) education groups. In low-income and low-education participants, central obesity + low HDL-C + hypertriglyceridemia and central obesity + hypertension + hyperglycemia (7.4% in each case) were the most common, followed by central obesity + hypertension + hypertriglyceridemia (6.2%) and central obesity + hypertension + low HDL-C + hypertriglyceridemia (5.1%); whereas the high-income and high-education group had an overall lower prevalence of MetS combinations. The SES differences were statistically significant (all P < 0.05).
Figure 3.
Comparison of prevalence (%) of different combinations of MetS components by sex and SES among 19- to 65-year-old South Koreans: KNHANES 2007−2008. A: Male, ■; female, ○. B: Low income, ■; high income, ○. C: <HS, ■; >HS, ○. D: Low income and low education, ■; high income and high education, ○. G, blood glucose (hyperglycemia); H, HDL-C (low HDL-C); P, BP (hypertension); T, TG (hypertriglyceridemia); W, WC (central obesity). WPHTG, WC, BP, HDL-C, TG, and blood glucose. For example, WPHTG means that all MetS components (WC, BP, HDL-C, TG, and blood glucose) were included. W-HTG means that four groups (WC, HDL-C, TG, and blood glucose) were included and one MetS component (BP) was not included. aIncome was the quartiles of average household monthly income: low (Q1 + Q2), middle (Q3), and high (Q4). bEducation levels: <HS, HS, and >HS education. *P < 0.05, between-group difference was significant.
CONCLUSIONS
The current study examined the associations between SES and MetS and its components based on the most recent, nationally representative data from South Korea. The prevalence of MetS increased with age. MetS prevalence and the patterns of its components differed by sex and SES. More men had MetS and had worse metabolic outcomes. Groups with lower SES had worse MetS components, especially women. Better-educated and higher-income women were less likely to have those components. Finally, the most common MetS combination was central obesity + low HDL-C + hypertriglyceridemia.
Based on IDF criteria, 15.8% of men and 11.6% of women had MetS, although only 4.0% of the population had a BMI ≥30 kg/m2. After controlling for covariates, women were 38% less likely to have MetS. This sex difference was consistent with previous findings in South Korea (15) and other populations, such as those in the U.S. (23), Taiwan (24), Japan (25), and Greece (26). Some previous studies have reported that the prevalence of MetS increased dramatically in women >50 years of age due to their postmenopausal status and sudden increase of abdominal obesity (27,28). In the current study, postmenopausal women had a higher rate of MetS (OR 2.02 [95% CI 1.48–2.76]) than premenopausal women.
Low SES was associated with higher prevalence of MetS in South Korean women regardless of their menopausal status, but not in men. Similar to our findings, a previous cross-sectional study among Portuguese adults found that lower SES, defined by education and occupation, was associated with MetS, but only in women (7). In addition, other South Korean and U.S. studies have found similar results (8,9). A French study suggested an inverse association between income and MetS in women (10). In South Korea, women with higher SES tend to pursue healthier lifestyles, including eating a healthier diet and engaging in regular physical activity (8). In contrast, higher SES South Korean men have more work-related stress and sedentary lifestyles and more frequently eat out for business, resulting in increased consumption of alcohol and high-fat/calorie foods. Future longitudinal studies are needed to confirm the causality between low SES and MetS. Although several biological disparities, such as sex and menopausal status, exist, we have consistently demonstrated that SES has a significant association with MetS. We confirmed the sex- and age-related differences, which help guide MetS prevention and management efforts in the target population.
Several other studies reported an inverse association between SES and metabolic outcomes (7,9,10,29). In contrast, in Tunisian (11) and Jamaican populations (30), higher SES groups had higher MetS rates. This discrepancy could be explained by at least two factors. First, it could be attributable to variations in study samples, outcome assessment, and data analysis methods. Second, it may be due to the developmental levels of these countries. We expected that the high-SES groups in low-income countries might have higher energy intakes and less labor-demanding work and lifestyles and thus are more likely to develop obesity and MetS than low-SES groups.
In addition, education seems to have a greater impact on MetS prevalence than family income in our population. Other previous studies found similar results (10,31). Although education and income are correlated, in general, Koreans with high education might be more aware of MetS and related health risks and thus might maintain healthier lifestyles. Therefore, more effort should be made to target the low-education groups in South Korea for MetS prevention.
Another main finding of our study was in regard to the combination patterns of various MetS components. To our knowledge, only two previous studies have assessed the combination of MetS components (16,17). However, these previous studies did not consider the SES effects on the composition of different MetS components. In addition, one study (16) investigated only three combinations, and the other (17) studied only middle-aged and older adults.
In our study, the most common MetS component combination was central obesity + low HDL-C + hypertriglyceridemia; 15.5% of all MetS patients and 3.4% of all South Korean adults had the condition. Men had a higher proportion of the combination than women (4.1% in men vs. 2.9% in women); however, among only those study participants who had MetS components, 20.4% of men and 25.7% of women had the combination. This may be due to low prevalence of low HDL-C in South Korean men by high alcohol consumption as compared with women. Intriguingly, we found significant differences in MetS compositions between the <HS and >HS education groups, as well as between the SES groups; however, the order of high prevalence is similar. The central obesity + low HDL-C + hypertriglyceridemia combination was the most common, followed by central obesity + hypertension + hyperglycemia and central obesity + hypertension + hypertriglyceridemia + hyperglycemia. A good understanding of the composition of MetS components will help guide related prevention and management efforts, including treatment.
South Koreans had elevated BP and lower HDL-C compared with other populations. For example, compared with a U.S. study (32), our subjects had a higher diastolic BP and lower HDL-C level. South Koreans have a higher diastolic BP than other Asia-Pacific populations, especially among men (33). Mean diastolic BP was 78.3 mmHg in our study, and 75.7 mmHg in Australians and 75.6 mmHg in Japanese (33). A recent Korean study investigating the impact of the individual components of MetS on CVD mortality suggests that high BP is the key component for predicting CVD mortality (34). In addition, HDL-C in South Koreans is lower than that of other populations such as Australians, Japanese, and Samoans in men and women (33). The results of most previous studies are consistent regarding HDL-C (35,36). HDL-C levels can be influenced by health-related factors such as physical activity, alcohol consumption, smoking, and diet, but the genetic factor is a key determinant of HDL-C levels in South Korea (35). Whether the international cutoff point of low HDL-C is appropriate for South Koreans needs more research.
The current study has a number of strengths. First, it was based on a recent, nationally representative sample in South Korea. In addition, our analysis took into account the complex sampling design effect to provide representative estimates. Second, most previous studies have only studied MetS, but we investigated the combination of various MetS components. However, this is a cross-sectional study, which precludes inferences as to causation.
In summary, we found that MetS is becoming a common chronic disease among South Korean adults, as 15.8% of men and 11.6% of women in the study had MetS, although the rate is still much lower than that of some other countries, including China (24.8%) (37) and the U.S. (39.1%) (38). Higher rates of MetS and its components were found in South Korean older adults, men, and low-SES women, demonstrating the groups that need to be a focus of attention. Among low-SES women, nearly one-third (30.6%) had MetS. Central obesity + low HDL-C + hypertriglyceridemia was the most common pattern of MetS. Multidimensional efforts are needed to deal with the growth of chronic diseases in South Korea; MetS can serve as a good indicator of the health burden the population is facing. Measures such as educational programs, screening, and prevention need to be taken, tailoring them to different SES groups by sex, as the association between SES and MetS varied by sex.
Acknowledgments
This study was supported in part by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-81335-01A1).
No potential conflicts of interest relevant to this article were reported.
H.L. designed the study, acquired data, analyzed and interpreted the data, and drafted the manuscript. T.N. assisted in the analysis and interpretation of the results and provided critical intellectual feedback to help revise the manuscript. R.C. provided critical intellectual feedback in findings interpretation and helped to revise the manuscript. Y.W. designed the study, guided the data analysis and interpretation of the results, and revised the manuscript. All authors read and approved the final version of the manuscript. Y.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 29th Annual Scientific Meeting of the Obesity Society, Orlando, Florida, 1–5 October 2011.
The authors would like to thank the staff at the Korea Centers for Disease Control and Prevention for their assistance and the anonymous reviewers for their comments and suggestions to improve the study.
References
- 1.Mackenbach JP, Stirbu I, Roskam AJ, et al. European Union Working Group on Socioeconomic Inequalities in Health Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008;358:2468–2481 [DOI] [PubMed] [Google Scholar]
- 2.Mackenbach JP, Bos V, Andersen O, et al. Widening socioeconomic inequalities in mortality in six Western European countries. Int J Epidemiol 2003;32:830–837 [DOI] [PubMed] [Google Scholar]
- 3.Petrelli A, Gnavi R, Marinacci C, Costa G. Socioeconomic inequalities in coronary heart disease in Italy: a multilevel population-based study. Soc Sci Med 2006;63:446–456 [DOI] [PubMed] [Google Scholar]
- 4.Gillum RF, Mussolino ME. Education, poverty, and stroke incidence in whites and blacks: the NHANES I Epidemiologic Follow-up Study. J Clin Epidemiol 2003;56:188–195 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88:1973–1998 [DOI] [PubMed] [Google Scholar]
- 6.Saydah S, Lochner K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep 2010;125:377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos AC, Ebrahim S, Barros H. Gender, socio-economic status and metabolic syndrome in middle-aged and old adults. BMC Public Health 2008;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park MJ, Yun KE, Lee GE, Cho HJ, Park HS. A cross-sectional study of socioeconomic status and the metabolic syndrome in Korean adults. Ann Epidemiol 2007;17:320–326 [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Räikkönen K, Gallo L, Kuller LH. Association between socioeconomic status and metabolic syndrome in women: testing the reserve capacity model. Health Psychol 2008;27:576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallongeville J, Cottel D, Ferrières J, et al. Household income is associated with the risk of metabolic syndrome in a sex-specific manner. Diabetes Care 2005;28:409–415 [DOI] [PubMed] [Google Scholar]
- 11.Allal-Elasmi M, Haj Taieb S, Hsairi M, et al. The metabolic syndrome: prevalence, main characteristics and association with socio-economic status in adults living in Great Tunis. Diabetes Metab 2010;36:204–208 [DOI] [PubMed] [Google Scholar]
- 12.James PT, Rigby N, Leach R, International Obesity Task Force The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil 2004;11:3–8 [DOI] [PubMed] [Google Scholar]
- 13.Korean Centers for Disease Control and Prevention. The Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2007-2009. Seoul, Republic of Korea, Korean Centers for Disease Control and Prevention, 2010 [Google Scholar]
- 14.Yoon YS, Lee ES, Park C, Lee S, Oh SW. The new definition of metabolic syndrome by the International Diabetes Federation is less likely to identify metabolically abnormal but non-obese individuals than the definition by the revised National Cholesterol Education Program: the Korea NHANES study. Int J Obes (Lond) 2007;31:528–534 [DOI] [PubMed] [Google Scholar]
- 15.Park HS, Oh SW, Cho SI, Choi WH, Kim YS. The metabolic syndrome and associated lifestyle factors among South Korean adults. Int J Epidemiol 2004;33:328–336 [DOI] [PubMed] [Google Scholar]
- 16.Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino RB., Sr Trajectories of entering the metabolic syndrome: the Framingham Heart Study. Circulation 2009;120:1943–1950 [DOI] [PubMed] [Google Scholar]
- 17.Lee CM, Huxley RR, Woodward M, et al. DETECT-2 Collaboration The metabolic syndrome identifies a heterogeneous group of metabolic component combinations in the Asia-Pacific region. Diabetes Res Clin Pract 2008;81:377–380 [DOI] [PubMed] [Google Scholar]
- 18.The Ministry of Health, Welfare, and Family Affairs, Centers for Disease Control and Prevention. The Guideline for the Usage of KNHANES Raw Data. Seoul, Republic of Korea, Korean Centers for Disease Control and Prevention, 2007
- 19.Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75:72–80 [DOI] [PubMed] [Google Scholar]
- 20.The National Assembly of the Republic of Korea. The Local Government Act [Internet], 2010. Available from http://likms.assembly.go.kr/ Accessed 30 May 2011
- 21.Heo M, Kim RS, Wylie-Rosett J, Allison DB, Heymsfield SB, Faith MS. Inverse association between fruit and vegetable intake and BMI even after controlling for demographic, socioeconomic and lifestyle factors. Obes Facts 2011;4:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson W, Kyvik KO, Mortensen EL, Skytthe A, Batty GD, Deary IJ. Does education confer a culture of healthy behavior? Smoking and drinking patterns in Danish twins. Am J Epidemiol 2011;173:55–63 [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Tao Q, Sun F, Zhan S. The impact of socioeconomic status on the incidence of metabolic syndrome in a Taiwanese health screening population. Int J Public Health 2012;57:551–559 [DOI] [PubMed] [Google Scholar]
- 25.Arai H, Yamamoto A, Matsuzawa Y, et al. Prevalence of metabolic syndrome in the general Japanese population in 2000. J Atheroscler Thromb 2006;13:202–208 [DOI] [PubMed] [Google Scholar]
- 26.Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J 2004;147:106–112 [DOI] [PubMed] [Google Scholar]
- 27.Kim HM, Park J, Ryu SY, Kim J. The effect of menopause on the metabolic syndrome among Korean women: the Korean National Health and Nutrition Examination Survey, 2001. Diabetes Care 2007;30:701–706 [DOI] [PubMed] [Google Scholar]
- 28.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care 2005;28:385–390 [DOI] [PubMed] [Google Scholar]
- 29.Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001-2006. Ann Epidemiol 2010;20:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson TS, Younger N, Tulloch-Reid MK, et al. Prevalence of the metabolic syndrome in Jamaican adults and its relationship to income and education levels. West Indian Med J 2010;59:265–273 [PubMed] [Google Scholar]
- 31.Yang X, Tao Q, Sun F, Zhan S. The impact of socioeconomic status on the incidence of metabolic syndrome in a Taiwanese health screening population [article online], 2012. Available from http://www.springerlink.com/content/0168389g477736l5/ Accessed 10 March 2012 [DOI] [PubMed]
- 32.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr 2008;87:1914–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CM, Huxley RR, Woodward M, et al. Detect-2 Collaboration Comparisons of metabolic syndrome definitions in four populations of the Asia-Pacific region. Metab Syndr Relat Disord 2008;6:37–46 [DOI] [PubMed] [Google Scholar]
- 34.Shin CY, Yun KE, Park HS. Blood pressure has a greater impact on cardiovascular mortality than other components of metabolic syndrome in Koreans. Atherosclerosis 2009;205:614–619 [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Han JH, Park HS. Prevalence of low HDL-cholesterol levels and associated factors among Koreans. Circ J 2006;70:820–826 [DOI] [PubMed] [Google Scholar]
- 36.Rouvre M, Vol S, Gusto G, et al. Low high density lipoprotein cholesterol: prevalence and associated risk-factors in a large French population. Ann Epidemiol 2011;21:118–127 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188 [DOI] [PubMed] [Google Scholar]
- 38.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 2005;28:2745–2749 [DOI] [PubMed]