Abstract
OBJECTIVE
To assess the efficacy and safety of switching from sitagliptin to liraglutide in metformin-treated adults with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In an open-label trial, participants randomized to receive either liraglutide (1.2 or 1.8 mg/day) or sitagliptin (100 mg/day), each added to metformin, continued treatment for 52 weeks. In a 26-week extension, sitagliptin-treated participants were randomly allocated to receive instead liraglutide at either 1.2 or 1.8 mg/day, while participants originally randomized to receive liraglutide continued unchanged.
RESULTS
Although 52 weeks of sitagliptin changed glycosylated hemoglobin (HbA1c) by −0.9% from baseline, additional decreases occurred after switching to liraglutide (1.2 mg/day, −0.2%, P = 0.006; 1.8 mg/day, −0.5%, P = 0.0001). Conversion to liraglutide was associated with reductions in fasting plasma glucose (FPG) (1.2 mg/day, −0.8 mmol/L, P = 0.0004; 1.8 mg/day, −1.4 mmol/L, P < 0.0001) and body weight (1.2 mg/day, −1.6 kg; 1.8 mg/day, −2.5 kg; both P < 0.0001) and with an increased proportion of patients reaching HbA1c <7% (from ∼30% to ∼50%). Overall treatment satisfaction, assessed by the Diabetes Treatment Satisfaction Questionnaire, improved after switching to liraglutide (pooled 1.2 and 1.8 mg/day, 1.3; P = 0.0189). After switching, mostly transient nausea occurred in 21% of participants, and minor hypoglycemia remained low (3–4% of participants). Continuing liraglutide treatment at 1.2 mg/day and 1.8 mg/day for 78 weeks reduced HbA1c (baseline 8.3 and 8.4%, respectively) by −0.9 and −1.3%, respectively; FPG by −1.3 and −1.7 mmol/L, respectively; and weight by −2.6 and −3.1 kg, respectively, with 9–10% of participants reporting minor hypoglycemia.
CONCLUSIONS
Glycemic control, weight, and treatment satisfaction improved after switching from sitagliptin to liraglutide, albeit with a transient increase in gastrointestinal reactions.
Although glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1RAs) and dipeptidyl peptidase-4 (DPP-4) inhibitors both have a glucose-dependent mechanism of action, distinct differences have emerged in phase 3 trials that, for the most part, have lasted as long as 6 months. In independent trials in patients with type 2 diabetes, the GLP-1RA liraglutide produced reductions in glycosylated hemoglobin (HbA1c) as great as 1.6%, in body weight of ∼3 kg, and in systolic blood pressure of 2–7 mmHg; it also improved β-cell function (1,2). Two other GLP-1RA regimens, exenatide twice daily and exenatide once weekly, decreased HbA1c by 0.8–0.9% and 1.3–1.9%, respectively, and produced weight reductions similar to liraglutide (up to 3 kg) (3–8). In contrast, smaller reductions in HbA1c (0.4–1.0%) and negligible weight changes have been reported with currently available DPP-4 inhibitors (9–17).
The greater efficacy of GLP-1RAs is probably related to the pharmacological levels of these agonists stimulating GLP-1 receptor activity (18). In contrast, DPP-4 inhibitors modestly affect the levels of endogenous GLP-1, thus producing smaller glycemic reductions and negligible weight effects (19).
Although longer-term, head-to-head trials with the two incretin classes are scarce, we recently reported the results of a 52-week, head-to-head comparison of liraglutide and sitagliptin added to metformin in participants with type 2 diabetes (20). The results showed that liraglutide (1.2 or 1.8 mg/day) produced significantly greater sustained decreases than sitagliptin 100 mg/day in HbA1c (−1.3 and −1.5%, respectively, vs. −0.9%), fasting plasma glucose (FPG) (−1.7 and −2.0 mmol/L, respectively, vs. −0.6 mmol/L), and body weight (−2.8 and −3.7 kg, respectively, vs. −1.2 kg), with a comparable rate of hypoglycemia although an initially higher frequency of nausea in the liraglutide groups (20). Furthermore, a significantly greater percentage of patients achieved the American Diabetes Association target of HbA1c <7.0% with liraglutide (1.2 and 1.8 mg) than with sitagliptin (50.3 and 63.3% for liraglutide 1.2 and 1.8 mg/day, respectively, vs. 27.1% for sitagliptin) (20). Apart from the higher incidence of gastrointestinal events with liraglutide, as expected with GLP-1RAs, the overall frequencies of adverse events (AEs), serious AEs (SAEs), and minor hypoglycemia were generally comparable between liraglutide and sitagliptin groups (20,21). Significantly greater reductions in HbA1c, FPG, and body weight relative to sitagliptin were also reported with the GLP-1RA exenatide once weekly in a 26-week, head-to-head trial (HbA1c, −0.9% vs. −1.5%; FPG, −0.9 vs. −1.8 mmol/L; body weight, −0.8 vs. −2.3 kg) (6).
Although metformin is the established first-line therapy in type 2 diabetes, there is no consensus regarding optimal second-line therapy or an effective alternative agent when the first second-line therapy used fails to provide adequate glycemic control. Although head-to-head studies have reported that treatment with GLP-1RAs produce greater glycemic and weight benefits compared with DPP-4 inhibitors (6,20,21), there are very limited data available exploring the effects of switching from a DPP-4 inhibitor to a GLP-1RA (22). To address this important clinical question, we conducted an exploratory investigation during which we assessed the efficacy and safety of switching participants from sitagliptin to liraglutide after 52 weeks in a 26-week study extension. The efficacy and safety of continuing liraglutide therapy for 78 weeks were also examined.
RESEARCH DESIGN AND METHODS
Study design and outcome measures
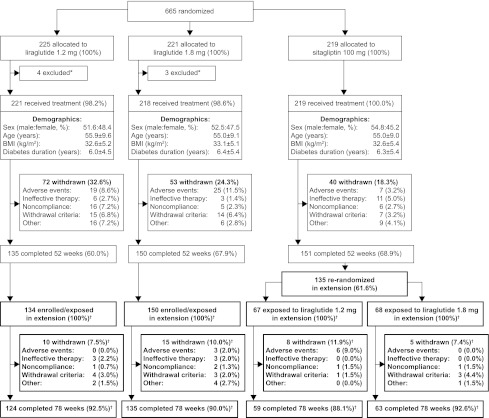
The 52-week study design and patient inclusion and exclusion criteria were reported previously (21). Briefly, individuals with type 2 diabetes, inadequately controlled with metformin ≥1,500 mg/day after ≥3 months (HbA1c, 7.5–10%), were randomly allocated to receive either injectable liraglutide 1.2 or 1.8 mg/day or oral sitagliptin 100 mg/day, each added to metformin. After 52 weeks, sitagliptin-treated participants were again randomly allocated (1:1) to receive either liraglutide 1.2 or 1.8 mg/day, through a weekly dose escalation of 0.6 mg/day, and were treated for another 26 weeks. The participants who were randomly assigned to liraglutide at baseline continued the same treatment for the entire 78 weeks (Fig. 1).
Figure 1.
Trial flow diagram. Data are n (%) participants unless otherwise noted. *Participants were withdrawn if they fulfilled withdrawal criteria, decided against participation, or did not attend any postrandomization visits. Participant disposition during the study extension is shown in bold. †Numbers and percentages of participants are from the extension analysis set.
Withdrawal criteria were almost identical to those at 52 weeks (20), with one additional withdrawal criterion (FPG >10.0 mmol/L, with no treatable intercurrent cause) applicable during the extension. Efficacy and safety end points were identical to those evaluated at 52 weeks and were assessed as reported previously (20). The protocol, including the extension, was approved by the appropriate institutional review board. The study followed Good Clinical Practice guidelines and conformed to the Declaration of Helsinki, with participants providing written, informed consent. The 78-week trial started on 6 June 2008 and ended on 3 June 2010.
Statistical methods
For participants receiving liraglutide from baseline to week 78, efficacy was analyzed with the full analysis set, all randomly allocated participants who were exposed to at least one dose of the trial drug, which was identical to the safety analysis set in this trial. For participants switching from sitagliptin to liraglutide (weeks 52–78), efficacy and safety were analyzed with the extension analysis set, all full analysis set 52-week completers exposed to the trial products during the extension.
As described previously (23), the validated Diabetes Treatment Satisfaction Questionnaire (DTSQ), status version, was used to assess participant satisfaction with treatment (24,25). As in the main trial period, participants from Slovakia, Serbia, and Slovenia were also excluded from the DTSQ analyses during the extension (82 of 419 [19.6%]) because of the lack of linguistically validated questionnaires. The two liraglutide switch groups (those switching from sitagliptin to either 1.2 or 1.8 mg/day liraglutide) were pooled into one group during a post hoc analysis of DTSQ scores. This was done to increase the power of the analysis and to allow a clearer interpretation of score changes between weeks 52 and 78 as differences between oral and injectable treatments. Missing postbaseline data were imputed by means of the last observation carried forward method.
Changes from week 52 to 78 within groups were analyzed by paired t test. Logistic regression was used to analyze the participant proportions achieving HbA1c targets and composite end point (HbA1c <7.0%, with no weight gain and no confirmed major or minor hypoglycemia), with treatment and country as fixed effects and baseline HbA1c and body weight (for composite) as covariates. The proportions of participants achieving glycemic and composite targets at weeks 52 and 78 were compared with the McNemar test for matched pairs.
Changes from baseline to week 78 were analyzed with ANCOVA, with treatment and country as fixed effects and baseline value as a covariate. Hypoglycemia and serum calcitonin were analyzed as reported previously (21). Mean ± SD values are presented for baseline and week 52 data to demonstrate parameter variability within the population, whereas changes during weeks 52–78 and 0–78 are reported as mean ± SE to illustrate the accuracy of the estimates. P < 0.05 was considered significant.
RESULTS
The groups were well matched for demographic and other characteristics at baseline (21). Of 436 total participants completing 52 weeks of treatment, 419 (96.1%) entered the extension, with 381 of the 419 participants (90.9%) completing 78 weeks in total (Fig. 1). Of the 284 combined liraglutide continuers (1.2 and 1.8 mg/day) entering the extension, 259 (91%) finished 78 weeks of treatment. The most common withdrawal reasons during the extension from the liraglutide continuing groups (1.2 and 1.8 mg/day) were meeting the withdrawal criteria (1.8 and 1.4%, respectively) and ineffective therapy (1.3 and 1.4%, respectively). Of the 135 participants who were switched to liraglutide after week 52, 122 (90%) completed the extension. The most common withdrawal reasons from the switch groups (sitagliptin to liraglutide 1.2 mg and sitagliptin to liraglutide 1.8 mg/day) were AEs (9 and 0%, respectively) and meeting the withdrawal criteria (1.5 and 4.4%, respectively). No participants withdrew from either switch group because of ineffective therapy during the extension.
Participants switching from sitagliptin to liraglutide
Efficacy.
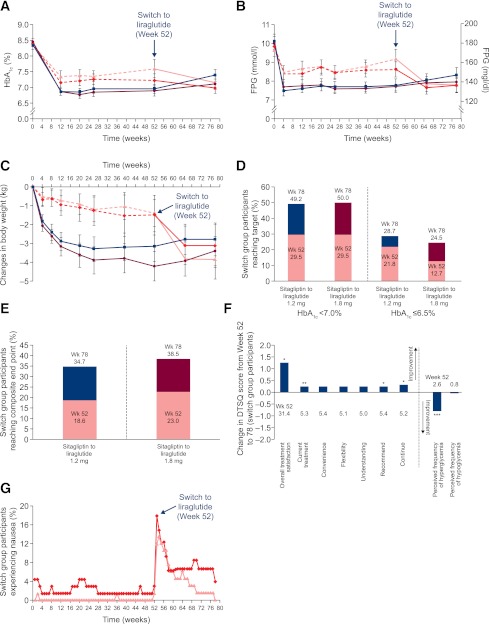
Although 52 weeks of treatment with sitagliptin changed baseline HbA1c by −0.9%, switching to liraglutide (1.2 or 1.8 mg/day) for the next 26 weeks was associated with additional changes from the 52-week HbA1c values (Fig. 2A): −0.2% ± 0.1% from 7.23% ± 0.9% (P = 0.006) for liraglutide 1.2 mg/day and −0.5% ± 0.1% from 7.6% ± 1.2% for liraglutide 1.8 mg/day (P = 0.0001). FPG also decreased in both switch groups during weeks 52–78 (Fig. 2B): from 8.6 ± 1.7 mmol/L (154.8 ± 30.6 mg/dL) by −0.8 ± 0.2 mmol/L (−14.4 ± 3.6 mg/dL) for liraglutide 1.2 mg (P = 0.0004) and from 9.2 ± 2.1 mmol/L (165.6 ± 37.8 mg/dL) by −1.4 ± 0.3 mmol/L (−25.2 ± 5.4 mg/dL) for liraglutide 1.8 mg/day (P < 0.0001).
Figure 2.
Selected efficacy and safety parameters. Mean HbA1c (A), FPG (B), and change in body weight (C) during 78 weeks for participants originally randomly allocated to receive liraglutide and participants switched to liraglutide after 52 weeks (Wk). D: Proportions of switch group participants (%) reaching target HbA1c <7.0% or ≤6.5% at weeks 52 and 78. E: Proportions of switch group participants reaching composite end point of HbA1c <7.0%, with no weight gain and no confirmed major or minor hypoglycemia, at weeks 52 and 78. F: Changes in the DTSQ scores from week 52 to week 78 (pooled liraglutide switch groups). *P < 0.05, **P < 0.01, ***P < 0.0001. G: Nausea incidence during weeks 0–78. For participants switched to liraglutide at week 52 in panels A, B, and C, the dashed lines represent the main trial period, whereas solid lines represent the extension (weeks 53–78). For D and E, estimates are from a logistic regression model, with treatment and country as fixed effects and baseline HbA1c and body weight (for composite) as covariates. Error bars are 1.96 × SE, corresponding to the 95% CI. Filled blue squares indicate liraglutide 1.2 mg/day, filled maroon circles indicate liraglutide 1.8 mg/day, filled red diamonds indicate sitagliptin to liraglutide 1.2 mg/day, and filled pink triangles indicate sitagliptin to liraglutide 1.8 mg/day.
Significant weight reductions occurred after switching to liraglutide for 26 weeks (Fig. 2C): from 92.8 ± 20.6 kg by −1.6 ± 0.4 kg for liraglutide 1.2 mg/day and from 91.6 ± 18.7 kg by −2.5 ± 0.4 kg for liraglutide 1.8 mg/day (both P < 0.0001). Consistent with this observation, significant decreases in waist circumference also occurred after 26 weeks of switching to either dose of liraglutide (Supplementary Table 1).
The proportion of participants achieving HbA1c <7.0% (American Diabetes Association target) increased significantly after switching to liraglutide for both groups (P = 0.0005 for 1.2 mg/day; P = 0.0026 for 1.8 mg/day) (Fig. 2D). A greater percentage of participants reached the American Association of Clinical Endocrinologists target of HbA1c ≤6.5% after switching to liraglutide, with a significant increase observed only for the liraglutide 1.8 mg/day group (P = 0.0117) (Fig. 2D). Furthermore, changing therapy to liraglutide (both groups) was associated with a significant increase in the percentage of participants reaching the composite end point of HbA1c <7.0%, with no weight gain and no confirmed major or minor hypoglycemia (P = 0.0018 for liraglutide 1.2 mg/day; P = 0.0192 for liraglutide 1.8 mg/day) (Fig. 2E).
Treatment satisfaction was assessed as reported previously in the pooled participant population switched to liraglutide (Fig. 2F) (23). From weeks 52 to 78, overall treatment satisfaction improved significantly for patients switching to liraglutide (+1.3, P = 0.0189), driven mainly by improvements in the categories “current treatment” (+0.3, P = 0.0066), “recommend” (+0.3, P = 0.0176), and “continue” (+0.3, P = 0.0292). The “perceived frequency of hyperglycemia” also improved significantly after switching to liraglutide (−0.8, P < 0.0001), while treatment “convenience,” “flexibility,” “understanding of diabetes,” and “perceived frequency of hypoglycemia” remained relatively unchanged.
Switching to liraglutide significantly increased β-cell function, as determined by homeostasis model assessment of β-cell function (both groups), and significantly reduced the homeostasis model assessment of insulin resistance (sitagliptin to liraglutide 1.8 mg/day) (Supplementary Table 1). In addition, transition to liraglutide was associated with significant reductions in fasting triglycerides and total cholesterol (sitagliptin to liraglutide 1.8 mg/day) and in LDL cholesterol (both groups) (Supplementary Table 1). Mean heart rate increased slightly in both switch groups during weeks 52–78, but the elevation was statistically significant only for the sitagliptin to liraglutide 1.8 mg/day group (Supplementary Table 1). There were no significant changes in blood pressure in either switch group from week 52 to week 78 (Supplementary Table 1).
Safety.
During weeks 52–78, most treatment-emergent AEs (TEAEs) in participants switched to liraglutide 1.2 or 1.8 mg/day were mild or moderate (≥91%). The proportion of patients reporting SAEs in both switch groups was low and ranged between 2.9 and 6.0%, with no consistent pattern with respect to system organ class (Supplementary Table 2). One death (acute renal failure) occurred in a 56-year-old woman who had previously received sitagliptin 100 mg/day for 385 days and was switched to liraglutide 1.2 mg/day and treated for 31 days. The event was classified by the investigator as unlikely to be related to the trial drug.
Gastrointestinal TEAEs were reported most frequently in both switch groups (32.8 and 36.8% of participants for sitagliptin to liraglutide 1.2 mg/day and sitagliptin to liraglutide 1.8 mg/day, respectively). Nausea was the most common gastrointestinal AE, occurring in about 21% of participants in each switch group. Nausea was generally transient after switching to liraglutide and declined a few weeks thereafter in both switch groups (Fig. 2G). No major hypoglycemic episodes (defined as participant unable to self-treat) occurred after changing treatment to liraglutide. The rates of minor hypoglycemia (defined as confirmed plasma glucose <3.1 mmol/L [56 mg/dL]) remained low after switching therapy: 0.031 and 0.060 episodes/participant/year for sitagliptin to liraglutide 1.2 mg/day and sitagliptin to liraglutide 1.8 mg/day, respectively.
Mean serum calcitonin levels remained below the upper limit of normal for both sexes, and no cases of malignant thyroid neoplasm were reported. No cases of pancreatitis were reported after switching to liraglutide.
Participants continuing liraglutide treatment
Efficacy.
For patients randomly allocated to receive liraglutide at baseline and continuing the same treatment for 78 weeks, liraglutide (1.2 and 1.8 mg/day) reduced HbA1c (baseline, 8.4% ± 0.8% and 8.4% ± 0.7%, respectively) by −0.9% ± 0.1% and −1.3% ± 0.1%, respectively; FPG (baseline, 10.1 ± 2.4 mmol/L [181.8 ± 43.2 mg/dL] and 9.9 ± 2.4 mmol/L [178.2 ± 43.2 mg/dL], respectively) by −1.3 ± 0.2 mmol/L [−23.4 ± 3.2 mg/dL] and −1.7 ± 0.2 mmol/L [−29.7 ± 3.2 mg/dL], respectively; and body weight (baseline, 93.7 ± 18.4 and 94.6 ± 18.1 kg, respectively) by −2.6 kg and −3.1 kg, respectively (Fig. 2 and Supplementary Table 3). Target HbA1c values of <7.0% and ≤6.5% were achieved by 34.7 and 12.0%, respectively, of participants continuing on liraglutide at 1.2 mg/day and by 51.2 and 26.6%, respectively, of participants continuing on liraglutide at 1.8 mg/day. Consistent with the observed weight decrease, a reduction in waist circumference also occurred in both groups continuing liraglutide therapy (Supplementary Table 3).
After 78 weeks, 27.7 and 43.7% of participants treated with liraglutide 1.2 and 1.8 mg/day, respectively, reached the composite end point of HbA1c <7.0%, with no weight gain and no hypoglycemia. Overall, both doses of liraglutide did not produce any notable changes in blood pressure and lipid parameters after 78 weeks (Supplementary Table 3). Similar to the 52-week results, however, a slight elevation in heart rate was observed at week 78, that was most pronounced with liraglutide 1.8 mg/day (Supplementary Table 3). In addition, 78 weeks of continuous liraglutide treatment produced improvements in the homeostasis model assessment of β-cell function (both groups) and fasting insulin (1.8 mg/day group) parameters (Supplementary Table 3). For DTSQ items, improvements from baseline were generally sustained at week 78 (Supplementary Table 3).
Safety.
During weeks 0–78, the vast majority (≥97%) of TEAEs in the liraglutide 1.2 and 1.8 mg/day continuing groups were mild or moderate, and small percentages of participants reported SAEs (5.4 and 8.7%, respectively; Supplementary Table 4). Gastrointestinal disorders were the most common TEAEs in both liraglutide groups (mostly transient nausea was reported by 20.9 and 20.6% of participants, respectively). Two deaths occurred in participants originally randomly allocated to receive liraglutide during weeks 0–78, both in the 1.8 mg/day group. One previously reported death from pancreatic carcinoma (diagnosed after 8 days of liraglutide treatment) occurred during the first 52 weeks and was classified by the investigator as unlikely to be related to the trial drug. The second death (bile duct cancer) occurred during the extension in a participant treated for 401 days (diagnosed after 316 days) and with a known choledocal stenosis in the medical history and cholecystectomy with stent implant since 2006. The event was classified by the investigator as unlikely to be related to the trial drug.
During the 78 weeks, two events of major hypoglycemia occurred in a 39-year-old female participant originally randomly allocated to receive liraglutide 1.2 mg/day. The first episode occurred during the main trial period and was described previously (21). The second episode occurred during the extension, after 486 treatment days. Finger-stick glucose concentration was too low to register. The participant was hospitalized overnight for glucose stabilization; no seizures or loss of consciousness occurred. The participant recovered after 1 day, and the event was classified by the investigator as serious and possibly related to the trial drug. The rates of minor hypoglycemia, after exclusion of an extreme outlier in the liraglutide 1.8 mg/day group with 23 minor episodes, were 0.156 and 0.130 episodes/participant/year for liraglutide 1.2 and 1.8 mg/day, respectively.
During the 78 weeks, mean serum calcitonin levels remained below the upper limit of normal for both sexes in the two treatment groups, and no cases of malignant thyroid neoplasm were reported. One case of pancreatitis (nonacute) occurred during the first 52 weeks and was described previously; no additional cases occurred after week 52 (20).
CONCLUSIONS
Switching from sitagliptin after 52 weeks to liraglutide for the next 26 weeks, both in combination with metformin, was associated with significant improvements in glycemic control, body weight, β-cell function, and overall treatment satisfaction. Apart from transient increases in gastrointestinal reactions, as is common when initiating GLP-1RA therapy, no additional or unexpected safety or tolerability concerns were raised by switching to either dose of liraglutide. Participants treated with liraglutide for 78 weeks had clinically significant reductions in HbA1c (0.9–1.3%) and weight (2.5–3.1 kg), with safety and tolerability profiles consistent with previous reports and no new safety concerns (1).
These results are consistent with other long-term studies of liraglutide. In one trial, 2 years of treatment with liraglutide 1.2 or 1.8 mg/day changed HbA1c by −0.6 and −0.9%, respectively, and body weight by −2.3 and −2.8 kg, respectively (26). In another, 26-week improvements in HbA1c were sustained for 2 years of treatment with liraglutide, and weight reductions were also maintained after 2.5 years (−3.0 kg with 1.2 mg/day and −2.9 kg with 1.8 mg/day) (27).
The greater efficacy of liraglutide than sitagliptin observed in the first 52 weeks of this study is consistent with other head-to-head trials comparing a GLP-1RA with sitagliptin (the DURATION-2 [Duration therapy Utilization: Researching changes in A1c, weight, and other factors Through Intervention with exenatide ONce weekly] trial of exenatide once weekly vs. sitagliptin and the T-emerge-4 trial of taspoglutide vs. sitagliptin) (6,28). The DURATION-2 trial also included a switch phase after 26 weeks. The 26-week reductions in HbA1c (−0.3%), FPG (−0.7 mmol/L), and body weight (−1.1 kg) after switching from sitagliptin to exenatide once weekly in the DURATION-2 trial extension were similar to the reductions observed in participants switched to liraglutide 1.2 mg/day in our study; however, greater mean decreases in these glycemic control indicators and weight occurred in patients switched to liraglutide 1.8 mg/day (Fig. 2A–C) (22).
These glycemic and nonglycemic improvements after switching therapy are likely due to the different modes of action exhibited by DPP-4 inhibitor sitagliptin and the GLP-1RA liraglutide. Direct stimulation of GLP-1 receptors by GLP-1RAs dosed to pharmacological levels results in glucose-dependent insulin secretion, inhibition of glucagon release, increased satiety, and reduced food intake, thus translating to effective glycemic control and weight loss observed in multiple studies (18,19). In contrast, DPP-4 inhibitors act indirectly by preventing enzymatic degradation of the incretin hormones, GLP-1 and glucose-dependent insulinotropic polypeptide (18,19). Although DPP-4 inhibitors also stimulate glucose-dependent insulin secretion and inhibit glucagon release, lower levels of endogenous GLP-1 achievable with these agents translate into reduced glycemic efficacy and negligible weight effects relative to GLP-1RAs.
The assessment of treatment satisfaction can provide valuable information on patient adherence and thus may help predict long-term treatment outcomes (29). Our results show that the switch from an oral therapy to an injectable was associated with an increase in overall treatment satisfaction, while patients’ perceptions of treatment convenience and flexibility remained essentially unchanged. The improved treatment satisfaction may result from weight loss or actual or perceived improvements in treatment efficacy. These results complement the greater treatment satisfaction with liraglutide compared with sitagliptin reported in the first 52 weeks of this trial (23), and similar results have been reported in another study comparing sitagliptin with an injectable GLP-1RA (30).
The 78-week safety profile of liraglutide was generally consistent with that observed during the development program, with gastrointestinal disorders being the most prevalent AEs (1). The liraglutide continuing groups had more gastrointestinal events than the sitagliptin group during the initial 4–8 weeks of the 78 treatment weeks. Similarly, gastrointestinal events were the most frequently reported events after switching to liraglutide, as expected with GLP-1RAs (1,2). In accordance with previous reports, nausea incidence peaked transiently after switching and declined after several weeks (1,21).
Postmarketing reports of pancreatitis with exenatide, and later sitagliptin, resulted in the addition of pancreatitis as a withdrawal criterion in this trial as well, and a single case of chronic pancreatitis was reported during the first year (20). In addition, during the trial, calcitonin levels remained below the upper normal limits for both sexes with both liraglutide and sitagliptin, consistent with findings from all phase 3 trials with liraglutide (31).
Overall study design limitations included the absence of a placebo group and the lack of double blinding. Limitations that applied specifically to the switch period were the small number of patients per switch group and the lack of a control group (e.g., a sitagliptin continuing group) to serve as a reference and comparison for the observed efficacy changes in the groups switched to liraglutide. Because the number of patients was small, it was decided not to keep a sitagliptin continuing group and instead switch all sitagliptin recipients to liraglutide; however, another 2-year study with sitagliptin added to metformin has shown that efficacy tends to decline after the first year (32), and thus no further improvement in sitagliptin-treated patients would have been expected had they not switched to liraglutide.
In conclusion, switching treatment from sitagliptin to liraglutide was associated with improvements in glycemic control, body weight, and treatment satisfaction, while the rates of hypoglycemia remained low. Overall, the switch was well tolerated, although it was accompanied by a transient rise in gastrointestinal reactions (mainly nausea), as expected with GLP-1 receptor agonists. The additional glycemic and weight reductions observed after switching second-line treatment to liraglutide show that this switch may be especially beneficial for those patients not achieving glycemic goals with sitagliptin, without the need for a third-line antihyperglycemic agent. It is reasonable to hypothesize that similar findings may be observed when comparing other long-acting GLP-1RAs and DPP-4 inhibitors. This exploratory study sets the stage for larger, more robust studies to examine the effects of switching from one second-line type 2 diabetes therapy to another.
Supplementary Material
Acknowledgments
The study was funded by Novo Nordisk A/S, Bagsvaerd, Denmark.
R.E.P. has attended advisory panels or acted as a consultant for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, Takeda, sanofi-aventis, MannKind, Roche, and Zealand and has received research grants or participated in clinical trials for Eli Lilly & Co., GlaxoSmithKline, MannKind, Merck & Co., Novartis Pharmaceuticals Corporation, Novo Nordisk Inc, Pfizer Inc., sanofi-aventis, Takeda, and Roche. M.A.N. has received research grants from Bayer Vital Pharma, Eli Lilly & Co., Menarini/Berlin-Chemie, Merck Sharp & Dohme, Novartis Pharma, and Novo Nordisk A/S and has accepted honoraria for membership on advisory boards and for consulting and has received honoraria for speaking on incretin-based antidiabetes medications from Amylin Pharmaceuticals, AstraZeneca, Bayer Vital Pharma, Menarini/Berlin-Chemie, Biovitrum, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Hoffmann-La Roche, Novartis Pharma, Novo Nordisk A/S, sanofi-aventis, and Takeda. T.B. has attended advisory panels for Amylin Pharmaceuticals; has received research support from Animas Corporation, Becton Dickinson, CPEX Pharmaceuticals, Dexcom, Eli Lilly & Co., GlaxoSmithKline, Medtronic MiniMed, Merck, Novo Nordisk Inc., Resmed, and sanofi-aventis; and has attended speakers’ bureaus for Amylin Pharmaceuticals Inc., Dexcom, Eli Lilly & Co., Medtronic MiniMed, Novo Nordisk Inc., Roche Diagnostics, and sanofi-aventis. E.M. has attended advisory panels for Merck Sharp & Dohme, Novartis, Novo Nordisk, and sanofi-aventis. S.Fi. has acted as consultant and has attended speakers’ bureaus for Novo Nordisk A/S. A.J.G. is an advisor and speaker for GlaxoSmithKline, Merck & Co., Novo Nordisk, and Sankyo. A.B.T. is an employee of and shareholder in Novo Nordisk and was directly involved in study conduct. S.Fu. is an employee of and shareholder in Novo Nordisk. M.D. has acted as consultant, advisory board member, and speaker for Eli Lilly & Co., Merck Sharp & Dohme, Novartis, Novo Nordisk A/S, Roche, and sanofi-aventis; has acted as a speaker for Servier; and has received grants in support of investigator and investigator-initiated trials from Eli Lilly & Co., GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Novo Nordisk A/S, Pfizer, sanofi-aventis, and Servier. No other potential conflicts of interest relevant to this article were reported.
R.E.P. participated in study design, enrolled patients, reviewed and interpreted the clinical trial report and data, and reviewed and edited the manuscript. M.A.N., T.B., E.M., S.Fi., A.J.G., and M.D. were investigators, and they participated in the interpretation of data, as well as in review of the manuscript. A.B.T. was International Medical Director for the trial, and she participated in trial conduct, planning of analyses, data interpretation, and drafting and revision of the manuscript. S.Fu. participated in data interpretation and review of the manuscript. R.E.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
These data have previously appeared in abstract form in Pratley R, Nauck M, Bailey T, et al.; 1860-LIRA-DPP-4 Study Group. Switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide further improves glycemic control and weight loss in patients with type 2 diabetes (Abstract). Diabetes 2011;60(Suppl. 1):A307.
The authors thank the 1860-LIRA-DPP-4 Study Group, their staff, clinical trial personnel, and study participants. The authors thank Irina Nayvelt, PhD, and John Smith, PhD, from Novo Nordisk, for medical writing and editorial assistance. The authors also thank Helle Hartvig from Novo Nordisk for analyzing the data and reviewing the manuscript.
Footnotes
Clinical trial reg. no. NCT00700817, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2113/-/DC1.
*A complete list of investigators for the 1860-LIRA-DPP-4 Study Group can be found in the Supplementary Data online.
References
- 1.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab 2009;11(Suppl. 3):26–34 [DOI] [PubMed] [Google Scholar]
- 2.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 3.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 5.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM, Wysham C, Macconell L, et al. DURATION-2 Study Group Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Nauck M, Forst T, et al. Efficacy and safety of exenatide once weekly versus liraglutide in subjects with type 2 diabetes (DURATION-6): a randomised, open-label study (Abstract). Diabetologia 2011;54(Suppl. 1):S38 [Abstract 75] [DOI] [PubMed] [Google Scholar]
- 9.Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008;24:537–550 [DOI] [PubMed] [Google Scholar]
- 10.Scott R, Loeys T, Davies MJ, Engel SS, Sitagliptin Study 801 Group Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008;10:959–969 [DOI] [PubMed] [Google Scholar]
- 11.Arechavaleta R, Seck T, Chen Y, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2011;13:160–168 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Hissa MN, Garber AJ, et al. Saxagliptin 014 Study Group The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009;32:1649–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollander P, Li J, Allen E, Chen R, CV181-013 Investigators Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 2009;94:4810–4819 [DOI] [PubMed] [Google Scholar]
- 14.Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 2011;13:258–267 [DOI] [PubMed] [Google Scholar]
- 15.Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011;13:65–74 [DOI] [PubMed] [Google Scholar]
- 16.Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res 2007;39:218–223 [DOI] [PubMed] [Google Scholar]
- 17.Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res 2009;41:368–373 [DOI] [PubMed] [Google Scholar]
- 18.Nauck MA. Unraveling the science of incretin biology. Am J Med 2009;122(Suppl.):S3–S10 [DOI] [PubMed] [Google Scholar]
- 19.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 20.Pratley R, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 2011;65:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 [DOI] [PubMed] [Google Scholar]
- 22.Wysham C, Bergenstal R, Malloy J, et al. DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med 2011;28:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies M, Pratley R, Hammer M, Thomsen AB, Cuddihy R. Liraglutide improves treatment satisfaction in people with Type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet Med 2011;28:333–337 [DOI] [PubMed] [Google Scholar]
- 24.Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med 1990;7:445–451 [DOI] [PubMed] [Google Scholar]
- 25.Bradley C. The DTSQ; revised March 2011. Available from http://www.healthpsychologyresearch.com/Admin/uploaded/Summary/dtsq%20summary%20rev_24.3.11.pdf Accessed December 19, 2011
- 26.Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B, LEAD-3 (Mono) Study Group Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab 2011;13:348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauck M, Hermansen K, Frid A, et al. Sustained glycaemic control with 2 years liraglutide and glimepiride treatment (both combined with metformin), achieved with weight loss and less hypoglycaemia with liraglutide; data from the LEAD-2 trial (Abstract). In IDF 2009 20th World Congress Abstract Book Montreal: IDF, 2009; p. 473 (Poster P-1402) [Google Scholar]
- 28.Bergenstal RM, Forti A, Chiasson J, et al. Once weekly taspoglutide, a human GLP-1 analog, is superior to sitagliptin in improving glycaemic control and weight loss in patients with type 2 diabetes (T2D): results from the T-emerge 4 trial (Abstract). Diabetes 2010;59(Suppl. 1):A16 (Abstract 58-OR)
- 29.Peyrot M, Rubin RR. How does treatment satisfaction work? Modeling determinants of treatment satisfaction and preference. Diabetes Care 2009;32:1411–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best JH, Rubin RR, Peyrot M, et al. Weight-related quality of life, health utility, psychological well-being, and satisfaction with exenatide once weekly compared with sitagliptin or pioglitazone after 26 weeks of treatment. Diabetes Care 2011;34:314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab 2011;96:853–860 [DOI] [PubMed] [Google Scholar]
- 32.Seck T, Nauck M, Sheng D, et al. Sitagliptin Study 024 Group Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract 2010;64:562–576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.