Abstract
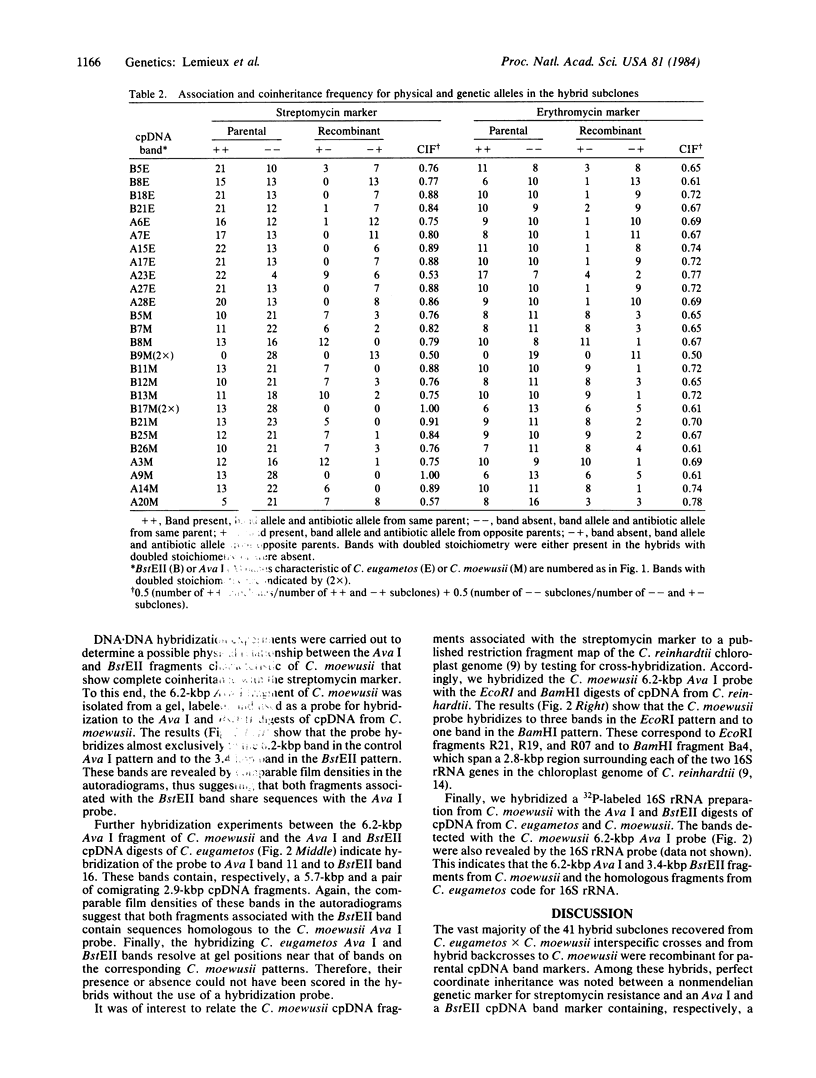
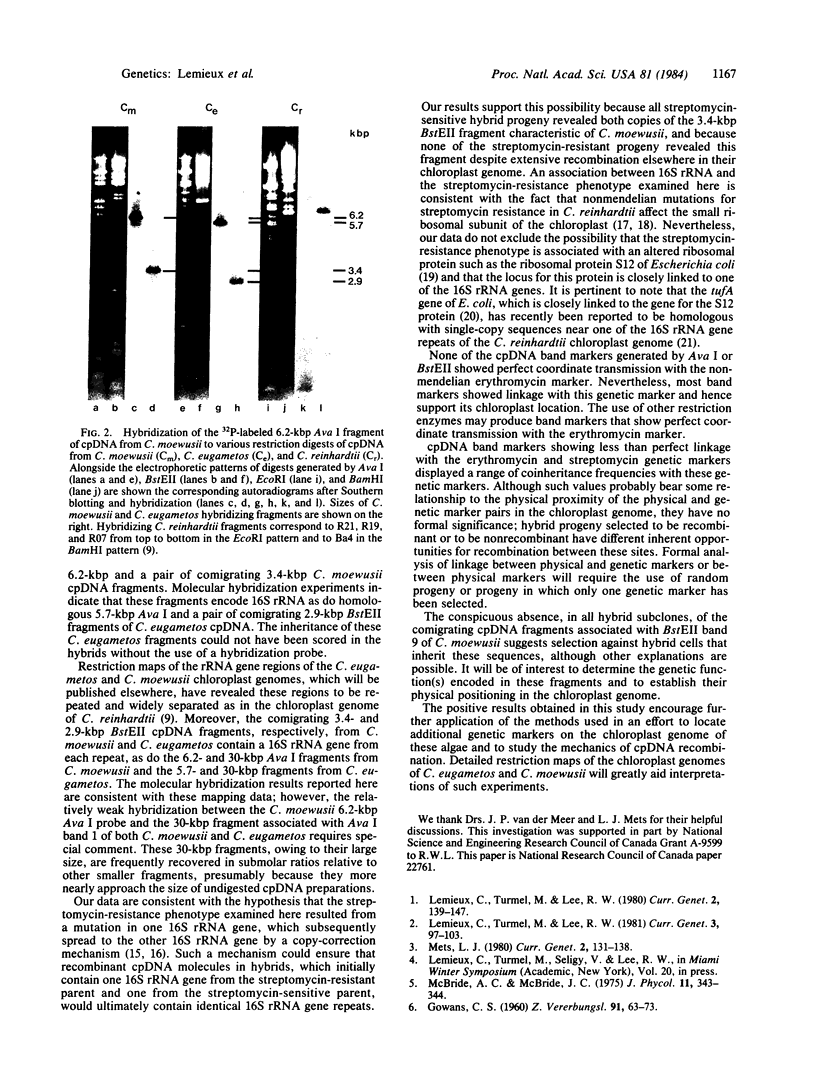
Differences in the distribution of Ava I and BstEII restriction sites in the chloroplast DNA (cpDNA) of Chlamydomonas eugametos and C. moewusii have been used to detect extensive cpDNA recombination in the hybrid progeny of these interfertile algae. In the present study, the inheritance of these restriction-site differences was tested for recombination with nonmendelian genetic markers for resistance to streptomycin and erythromycin in interspecific crosses and in hybrid backcrosses to C. moewusii. Most of the restriction-pattern markers appear linked to the antibiotic-resistance markers, thus supporting the chloroplast localization of the resistance markers. The streptomycin marker, in particular, shows perfect coordinate inheritance with an Ava I band containing one cpDNA fragment and a BstEII band containing two comigrating cpDNA fragments. Molecular hybridization experiments using DNA from the Ava I band as a probe show sequence homology between this DNA, the two comigrating BstEII fragments, and cpDNA fragments from C. reinhardtii containing the genes for 16S rRNA. The results show the feasibility of using C. eugametos-C. moewusii hybrids to identify cpDNA sequences that either contain or are closely linked to nonmendelian genetic markers.
Keywords: interspecific crosses, organelle genetics, physical mapping, heterologous hybridization probing
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- GOWANS C. S. Some genetic investigations on Chlamydomonas eugametos. Z Vererbungsl. 1960;91:63–73. doi: 10.1007/BF00889999. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Grant D. M., Rabert D. K., Harris E. H., Boynton J. E., Gillham N. W. Mutants of Chlamydomonas reinhardtii with physical alterations in their chloroplast DNA. Plasmid. 1982 Mar;7(2):133–151. doi: 10.1016/0147-619x(82)90073-7. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J. Transformation of the green alga Chlamydomonas reinhardii with yeast DNA. Nature. 1982 Mar 4;296(5852):70–72. doi: 10.1038/296070a0. [DOI] [PubMed] [Google Scholar]
- Schlanger G., Sager R. Localization of five antibiotic resistances at the subunit level in chloroplast ribosomes of Chlamydomonas. Proc Natl Acad Sci U S A. 1974 May;71(5):1715–1719. doi: 10.1073/pnas.71.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Watson J. C., Surzycki S. J. Extensive sequence homology in the DNA coding for elongation factor Tu from Escherichia coli and the Chlamydomonas reinhardtii chloroplast. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2264–2267. doi: 10.1073/pnas.79.7.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]