Abstract
Background
Prostate cancer is the most common cancer among elderly men in the US, and immunotherapy has been shown to be a promising strategy to treat patients with metastatic castration-resistant prostate cancer. Efforts to identify novel prostate specific tumor antigens will facilitate the development of effective cancer vaccines against prostate cancer. Prostate-specific G-protein coupled receptor (PSGR) is a novel antigen that has been shown to be specifically over-expressed in human prostate cancer tissues. In this study, we describe the identification of PSGR-derived peptide epitopes recognized by CD8+ T cells in an HLA-A2 dependent manner.
Methodology/Principal Findings
Twenty-one PSGR-derived peptides were predicted by an immuno-informatics approach based on the HLA-A2 binding motif. These peptides were examined for their ability to induce peptide-specific T cell responses in peripheral blood mononuclear cells (PBMCs) obtained from either HLA-A2+ healthy donors or HLA-A2+ prostate cancer patients. The recognition of HLA-A2 positive and PSGR expressing LNCaP cells was also tested. Among the 21 PSGR-derived peptides, three peptides, PSGR3, PSGR4 and PSGR14 frequently induced peptide-specific T cell responses in PBMCs from both healthy donors and prostate cancer patients. Importantly, these peptide-specific T cells recognized and killed LNCaP prostate cancer cells in an HLA class I-restricted manner.
Conclusions/Significance
We have identified three novel HLA-A2-restricted PSGR-derived peptides recognized by CD8+ T cells, which, in turn, recognize HLA-A2+ and PSGR+ tumor cells. The PSGR-derived peptides identified may be used as diagnostic markers as well as immune targets for development of anticancer vaccines.
Introduction
Prostate cancer has become the most common cancer among men in the US and is the second leading cause of death from cancer in American men [1]. The standard of care for most patients with prostate cancer is surgery and/or radiation therapy. However, disease recurrence after surgery or radiation still takes place in up to 30% of patients. Although androgen-deprivation therapy is an effective treatment against recurrent disease, most of these patients eventually develop androgen-refractory prostate cancer, which is insensitive to traditional treatment. Therefore, more effective and less toxic therapies are urgently needed. Immunotherapy has been shown to be a promising approach to the treatment of prostate cancer, especially for patients with metastatic castration-resistant prostate cancer [2]–[4]. Harnessing the immune system to eradicate malignant cells is a promising approach for cancer therapy, but until recently it has been met with only sporadic clinical success [4]–[6]. Recent Food and Drug Administration (FDA) approvals of the immunotherapy-based vaccine/drug sipuleucel-T (Provenge) and ipilimumab (Yervoy) represent milestones in the field of cancer immunotherapy [7], [8]. Furthermore, a phase III clinical trial of the gp100 peptide for melanoma also produced highly encouraging clinical results [9]. However, the clinical benefits reported for these agents have fallen far short of complete responses and permanent cures. In the case of sipuleucel-T, the survival benefit for patients was only 4.1 months, without objective tumor regression or substantial changes in prostate specific antigen (PSA) levels. A recent study using animal models further reveals the importance of tumor-specific antigens in eliciting immune responses against a developing tumor [10], spurring more efforts to identify such antigens for cancer immunotherapy. Furthermore, since some major rejection antigens may be lost or altered due to T cell selection and killing [11], the best strategy is to target multiple tumor antigens that are present on individual tumors for immunotherapy.
To date, a number of prostate specific tumor antigens have been well-defined, including PSA [12], [13], prostein [14], [15], prostate stem cell antigen (PSCA) [16], prostate-specific membrane antigen (PSMA) [17]–[19], prostatic acid phosphatase (PAP) [20] and transient receptor potential p8 (trp-p8) [21]. Furthermore, HLA-class I-restricted epitopes derived from these tumor antigens have been described [22]. One drawback of single tumor antigen-based immunotherapy is that immune escape may occur. Hence, identification of additional prostate cancer-specific antigens for development of more effective and antigen-specific vaccines for patients with metastatic prostate cancer is needed. Prostate-specific G-protein coupled receptor (PSGR) is a member of the G-protein coupled odorant receptor family, and is highly expressed in prostate cancer cells compared with normal prostate cells [23]–[25], suggesting that PSGR may be targeted for the development of novel immunotherapeutic strategies against prostate cancer.
In an attempt to determine whether PSGR can be recognized by T cells, we describe the identification of PSGR-derived T cell epitopes for T cell recognition by an immuno-bioinformatics approach. Twenty-one peptides that were predicted to bind to the HLA-A2 molecule were selected and synthesized. All these peptides were evaluated in vitro for their ability to stimulate T cells in PBMCs from both healthy subjects and prostate patients based on interferon-γ (IFN-γ) release measured by ELISA or ELISPOT assays. Three peptides were found to induce IFN-γ release in peripheral T cells from both healthy subjects and prostate cancer patients. Importantly, these peptide-specific T cells could recognize HLA-A2+, PSGR-expressing LNCaP cells in an HLA-class I-dependent manner.
Materials and Methods
Healthy Donors and Prostate Cancer Patients
Ten HLA-A2+ prostate cancer patients and ten HLA-A2+ healthy subjects were enrolled in this study after written informed consent was obtained. All protocols were approved by the Institutional Review Board (IRB) of Baylor College of Medicine before commencing studies. 20 mL of peripheral blood was obtained from each person, and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Lymphoprep (Nycomed Pharma AS; Oslo, Norway). The freshly isolated PBMCs were cryopreserved for later use in 1 mL freezing medium containing 90% FCS and 10% dimethyl sulfoxide (DMSO) at −140°C. The expression of HLA-A2 molecules on PBMCs obtained from cancer patients and healthy subjects was verified by flow cytometry with FITC-labeled HLA-A2 mAb BB7.2 (BD Pharmingen, San Diego, CA, USA).
Cell Lines
T2 cells (an HLA-A2+ TAP-deficient cell line), PC3 cells (an HLA-A2-negative prostate cancer cell line), and LNCaP cells (an HLA-A2 positive prostate carcinoma cell line) were all purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). All cell lines were maintained in RPMI-1640 medium (Mediatech; Manassas, VA, USA), supplemented with 10% FBS, 1% L-glutamine, and 1% penicillin and streptomycin.
Peptides
Twenty-one PSGR-derived peptides (Table 1) were predicted using BIMAS (http://www-bimas.cit.nih.gov/molbio/hla_bind/), SYFPEITHI (http://www.syfpeithi.de/), and Rankpep (http://bio.dfci.harvard.edu/Tools/rankpep.html) based on the HLA-A2 binding motif. Only epitopes that were predicted by at least two of these algorithms were selected for further testing. The peptides were synthesized by a solid-phase method using a peptide synthesizer (AApptec, Inc.; Louisville, KY, USA), purified by reverse-phase high-performance liquid chromatography and validated by mass spectrometry. The synthesized peptides were dissolved in DMSO at a concentration of 10 mg/mL and stored at −80°C until further use.
Table 1. The predicted HLA-A2 binding peptides derived from the prostate-specific G-protein coupled receptor (PSGR).
| Peptide # | HLA restriction | Position | Sequence |
| PSGR1 | HLA-A2 | 287–295 | VLNPIVYGV |
| PSGR2 | HLA-A2 | 188–196 | KLACDDIRV |
| PSGR3 | HLA-A2 | 276–284 | ILANIYLLV |
| PSGR4 | HLA-A2 | 28–36 | WLAFPLCSL |
| PSGR5 | HLA-A2 | 220–228 | YLLILKTVL |
| PSGR7 | HLA-A2 | 181–189 | CLHQDVMKL |
| PSGR10 | HLA-A2 | 213–222 | SLLISFSYLL |
| PSGR11 | HLA-A2 | 245–254 | HVCAVFIFYV |
| PSGR12 | HLA-A2 | 21–30 | GLEEAQFWLA |
| PSGR13 | HLA-A2 | 156–165 | ALMAPLPVFI |
| PSGR14 | HLA-A2 | 275–284 | VILANIYLLV |
| PSGR15 | HLA-A2 | 221–230 | LLILKTVLGL |
| PSGR16 | HLA-A2 | 37–46 | YLIAVLGNLT |
| PSGR17 | HLA-A2 | 66–75 | CMLSGIDILI |
| PSGR18 | HLA-A2 | 100–109 | LLQMFAIHSL |
| PSGR19 | HLA-A2 | 56–65 | SLHEPMYIFL |
| PSGR20 | HLA-A2 | 117–126 | LLAMAFDRYV |
| PSGR21 | HLA-A2 | 41–50 | VLGNLTIIYI |
| PSGR22 | HLA-A2 | 250–259 | FIFYVPFIGL |
| PSGR23 | HLA-A2 | 139–148 | TLPRVTKIGV |
| PSGR24 | HLA-A2 | 253–262 | YVPFIGLSMV |
In vitro Stimulation of Peptide-specific T cells in PBMCs
PBMCs (1×105 cells/well) from either healthy subjects or prostate cancer patients were incubated with standard peptide concentrations of 20 µg/mL per peptide [26]–[28] in 96-well U-bottom microplates (BD, Franklin Lakes, NJ, USA) in 200 µL of T-cell medium (TCM), consisting of RPMI 1640 (Mediatech, Manassas, VA, USA), 10% human AB serum (Valley Biomedical, Winchester, USA), 50 µM of 2-mercaptoethanol, 100 U/mL of interleukin-2 (IL-2), and 0.1 mM MEM nonessential amino acid solution (Invitrogen, Grand Island, NY, USA). Half of the TCM was removed and replaced with fresh TCM containing peptides (20 µg/mL) every 5 days. After 14 days of culture, the cells were harvested and tested for their ability to produce IFN-γ in response to T2 cells (1×104 cells/well), which were pre-loaded with either PSGR peptide (5 µg/mL) or a control peptide (an irrelevant HLA-A2 binding peptide: NLLTHVESL ) as a negative control. After 18 hours of incubation, supernatants were collected, and IFN-γ release was determined by ELISA assay.
Rapid Expansion Protocol (REP) for PSGR Peptide-specific T-cells
PSGR peptide specific T cells were expanded by a rapid expansion protocol (REP) as previously described [29] with a slight modification. Briefly, on day 0, 0.1−0.5×106 PSGR peptide specific T cells were cultured in a T25 flask with 20 mL RPMI-1640 supplemented with 10% human AB serum, 50 µM of 2-mercaptoethanol, and 30 ng/mL OKT3 antibody (Ortho Biotech, Bridgewater, NJ), together with 20×106 irradiated allogeneic PBMCs and 5×106 irradiated Epstein Barr Virus (EBV) transformed B-cells as feeder cells. Flasks were incubated upright at 37°C in 5% CO2. IL-2 (300 IU/mL) was added on day 1, and on day 5, half of the cell culture supernatant was removed and replenished with fresh medium containing 300 IU/mL IL-2. 14 days after initiation of the REP, cells were harvested and cryopreserved for future experiments.
ELISA Assay
Cytokine release was measured by coating 96-well ELISA plates (Thermo Fisher Scientific; Rochester, NY, USA) with 1 µg/mL anti-human IFN-γ (Pierce Biotechnology; Rockford, IL, USA) overnight at 4°C. The plate was washed six times with PBS containing 0.05% Tween-20 (wash solution) to remove unbound coating antibody, and blocked with 1% BSA/PBS at room temperature for 2 hrs. Afterwards, 50 µL supernatant was added to each well and incubated at room temperature for 1 hr, then 50 µL of 0.5 µg/mL biotinylated anti-human IFN-γ (Pierce Biotechnology; Rockford, IL, USA) was added and plates were incubated for an additional 1 hr at room temperature. After incubation, plates were washed and incubated for 30 min with Poly-HRP-Streptavidin (Thermo Fisher Scientific; Rochester, NY, USA) diluted 1∶5000 in PBS/1% BSA. Plates were washed and 100 µL of TMB substrate solution (Sigma-Aldrich Co.; St. Louis, MO, USA) was added per well. The colorimetric reaction was stopped using 2N H2SO4 and plates were read at 450 nm using an ELISA plate reader.
IFN-γ ELISPOT Assay
The IFN-γ ELISPOT assay was performed as previously described [27] to quantify peptide-specific cytotoxic T-lymphocytes (CTLs) after in vitro expansion. Briefly, 96-well ELISPOT plates (Millipore; Bedford, MA, USA) were coated overnight at 4°C with 7.5 µg/mL anti-human IFN-γ (Pierce Biotechnology; Rockford, IL, USA). Plates were washed six times with sterile PBS to remove unbound coating antibody. T cells were seeded at 1×105 cells per well and incubated with T2 cells alone, T2 cells pulsed with a PSGR peptide (5 µg/mL) or an irrelevant peptide as a negative control. Cells stimulated with 5 µg/mL OKT3 antibody (Ortho Biotech; Bridgewater, NJ, USA) were used as positive control. After incubating samples for 18 - 20 hrs at 37°C and 5% CO2, plates were washed with wash solution. 0.75 µg/mL Biotinylated anti-human IFN-γ (Pierce Biotechnology; Rockford, IL, USA) was added, and plates were incubated for 2 hrs at room temperature. After incubation, plates were washed with wash solution and incubated further with Poly-HRP-Streptavidin (Thermo Fisher Scientific; Rochester, NY, USA) diluted 1∶1000 in PBS/1% BSA for 1 hr. Plates were washed and 200 µL of 4-chloro-1-naphthol substrate (Sigma-Aldrich Co.; St. Louis, MO, USA) was added to each well. Finally, plates were washed under running tap water and dried at room temperature. IFN-γ spot-forming cells (SFC) were enumerated using an ELISPOT reader (C.T.L. Technologies, Minneapolis, MN, USA).
RNA Extraction and RT-PCR
RNA extraction and RT-PCR was carried out as reported previously [30]. In brief, total RNA was extracted from prostate cancer cells with 1 mL Trizol reagent (Invitrogen; Carlsbad, CA, USA). Three micrograms of RNA was reverse-transcribed to cDNA in 30 µl volume and 1 µl of each cDNA was used in subsequent PCR reaction with a pair of PSGR specific primers: Primer 1: 5′-GAAGATCTATGAGTTCCTGCAACTTC-3′, primer 2: 5′-CCCAAGCTT TCACTTGCCTCCCACAG-3′. β-actin was used as loading control: primer 1: 5′-CATGATGGAGTTGAAGGTAGTTTCG-3′; Primer 2: 5′-CAGACTATGCTGTCCCTGTACGC-3′. The PCR reaction was carried out under the following conditions: 94°C for 2 min, 94°C for 30 s, 56°C for 30 s, 72°C for 1 min 20 s, total 35 cycles, 72°C for 10 min, and β-actin was run for 25 cycles. Equal amounts of PCR products were then loaded and detected by gel electrophoresis.
Cytotoxicity Assay
PSGR derived peptide-specific T cells were tested for cytotoxicity against both PC3 and LNCaP by a lactate dehydrogenase (LDH) assay (Promega; Madison, WI, USA). The assay was performed in accordance with the manufacturer’s instructions. LDH release was calculated based on the following formula:
Cytotoxicity (%) = (Experimental – Effector Spontaneous – Target Spontaneous LDH release)/(Target Maximum – Target Spontaneous LDH release) × 100.
Spontaneous release was determined by using the supernatant of the target cells alone or effector cells alone, and the maximum release was determined by using the supernatant of target cells incubated with a lysis solution included in LDH kit. To determine if T cell recognition is HLA-I restricted, anti-HLA-I, anti-HLA-II, or anti-CD19 mAb (all from ATCC, Manassas, VA, USA) were added into wells at the initiation of the culture.
Intracellular IFN-γ Cytokine Staining
PSGR-derived peptide specific T cells (0.5−1×106 ) were cultured with 0.5×106 T2 cells pulsed with or without peptide (5 µg/mL) in the presence of GolgiStop (BD Pharmingen, San Diego, CA, USA) in a 48-well plate for 4 hrs at 37°C. Cells were stained with anti-CD8 and anti-IFN-γ and analyzed using a FACScalibur machine.
Statistics
Student’s t-test was used to analyze quantitative differences between the experimental wells and controls in ELISA and ELISPOT assays. P<0.05 was considered significant.
Results
Induction of PSGR Derived Peptide-specific CTLs in Healthy Donors
To determine whether PSGR-reactive T cell precursors are present in healthy subjects, we obtained PBMCs from 10 HLA-A2+ healthy donors and stimulated them in vitro with each of the 21 PSGR-derived peptides containing HLA-A2-binding motif (Table 1). After 2 weeks of peptide stimulation, supernatants from peptide-stimulated T cells were analyzed by ELISA assay to detect IFN-γ release in response to T2 cells pulsed with or without corresponding peptides. As shown in Table 2, 13 PSGR-derived peptides were capable of inducing peptide-specific T-cell responses in at least one of 10 healthy subjects. Importantly, PSGR3, PSGR4 and PSGR14 could induce T cell responses in 7 out of 10 healthy subjects, indicating that these 3 peptides are immunogenic and potentially capable of expanding antigen-specific T cells in healthy subjects.
Table 2. Induction of peptide-specific T cells from the PBMCs of ten HLA-A2+ healthy subjects.
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | |
| PSGR1 | 195 | 0 | 0 | 0 | 0 | 281 | – | – | – | – |
| PSGR2 | 0 | 0 | 455 | 0 | 0 | 0 | – | – | – | – |
| PSGR3 | 127 | 398 | 393 | 768 | 227 | 183 | 645 | 0 | 394 | 0 |
| PSGR4 | 302 | 152 | 457 | 0 | 407 | 226 | 847 | 0 | 0 | 459 |
| PSGR5 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR7 | 0 | 0 | 0 | 0 | 168 | 0 | – | – | – | – |
| PSGR10 | 917 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR11 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR12 | 0 | 139 | 0 | 246 | 0 | 432 | – | – | – | – |
| PSGR13 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR14 | 471 | 0 | 115 | 0 | 124 | 296 | 843 | 0 | 346 | 854 |
| PSGR15 | 0 | 0 | 456 | 0 | 0 | 0 | – | – | – | – |
| PSGR16 | 602 | 0 | 0 | 0 | 163 | 0 | – | – | – | – |
| PSGR17 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR18 | 176 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR19 | 0 | 0 | 0 | 0 | 195 | 0 | – | – | – | – |
| PSGR20 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR21 | 0 | 291 | 0 | 0 | 300 | 377 | – | – | – | – |
| PSGR22 | 289 | 0 | 0 | 0 | 0 | 0 | – | – | – | - |
| PSGR23 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
| PSGR24 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
Note: Values denote concentrations of IFN-γ (pg/ml) in the supernatants; –, not done.
Presence of PSGR-derived Peptide Specific CTLs in Prostate Cancer Patients
Since peptide-specific T cells against PSGR3, PSGR4 and PSGR14 were found in more than 70% of healthy subjects, we reasoned that CTL precursors that recognize these 3 peptides may also be high in PBMCs of prostate cancer patients. To test our hypothesis, we examined whether these three peptide candidates can induce peptide-specific CTLs from the PBMCs of HLA-A2+ prostate cancer patients. PBMCs from prostate cancer patients were collected and stimulated in vitro with PSGR3, PSGR4 or PSGR14 peptides. As shown in Table 3, PSGR3, PSGR4 and PSGR14 indeed induced peptide-specific CTLs from PBMCs of prostate cancer patients.
Table 3. Induction of peptide-specific T cells from the PBMCs of HLA-A2+ prostate cancer patients.
| #2 | #3 | #12 | #13 | #14 | #15 | #16 | #17 | #22 | #25 | |
| PSGR3 | 525 | 503 | 326 | 217 | 0 | 282 | 35 | 156 | 103 | 22 |
| PSGR4 | 451 | 351 | 0 | 301 | 309 | 70 | 6.7 | 102 | 56 | 27 |
| PSGR14 | 344 | 116 | 272 | 23 | 0 | 24 | 0 | 100 | 8 | 11 |
Note: Values denote concentrations of IFN-γ (pg/ml) in the supernatants.
Recognition of Prostate Cancer Cell Lines by the PSGR Derived Peptide-specific T cells
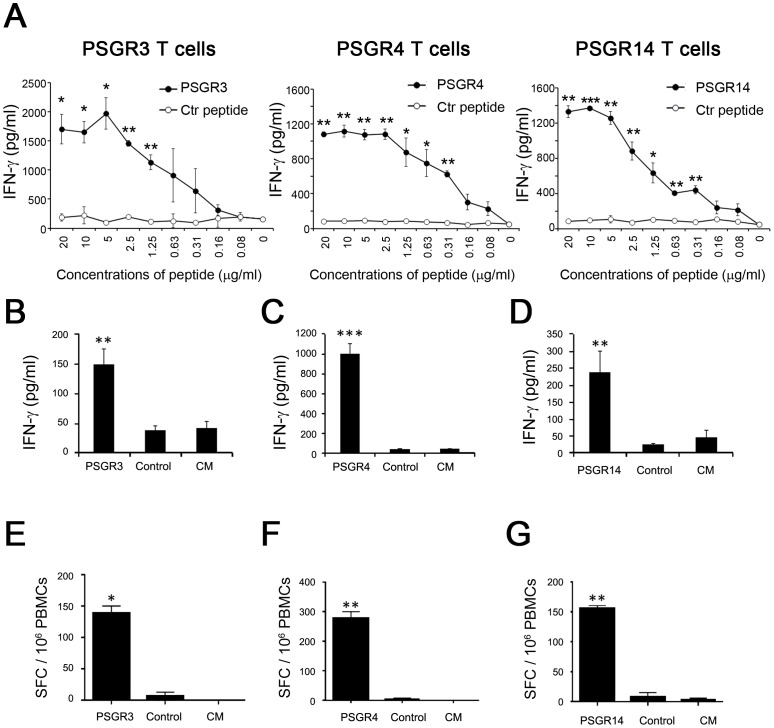
To obtain a large number of PSGR peptide-specific T cells for further analysis, we expanded PSGR peptide-specific T cells identified in Tables 2 and 3. To determine an effective concentration of peptide for loading T2 cells for T cell recognition, we performed peptide titration experiments. As shown in Figure 1A, for all 3 peptides a peptide concentration of 5 µg/ml was sufficient to saturate the binding sites of HLA-A2 molecules on T2 cells for T cell recognition. Therefore, we consistently used this peptide concentration for pre-loading T2 cells in ELISA and/or ELISPOT assays. The expanded T cells maintained antigen-specificity and secreted significant amounts of IFN-γ after stimulation with T2 cells pulsed with the corresponding peptides, but not with a control peptide (Figures 1A, B, C, D). ELISPOT assay further confirmed the presence of PSGR peptide-specific T cells in expanded T cells (Figures 1E, F, G)
Figure 1. PSGR-derived peptides induced peptide-specific T cells.
The recognition of T2 cells pre-loaded with titrated concentrations of peptides (0–20 µg/ml) by expanded PSGR peptide-specific T cells was tested by ELISA assay (A). The expanded PSGR3 T cells (B and E), PSGR4 T cells (C and F) and PSGR14 T cells (D and G) were respectively co-incubated with T2 cells (1×104 cells/well) alone in complete medium (CM), or with T2 cells pre-loaded with either a corresponding peptide (5 µg/mL) or a control peptide as a negative control. Cells were incubated for 18 −24 hours, the IFN-γ secretion in the supernatant was determined by ELISA assay (B, C and D). IFN-γ spot-forming cells (SFC) were enumerated by ELISPOT assay (E, F and G). Data are plotted as means ± SD. Results are representative of at least three independent experiments. *P<0.05, **P<0.01, ***P<0.001 versus controls (T2 cells alone or T2 cells pulsed with a control peptide).
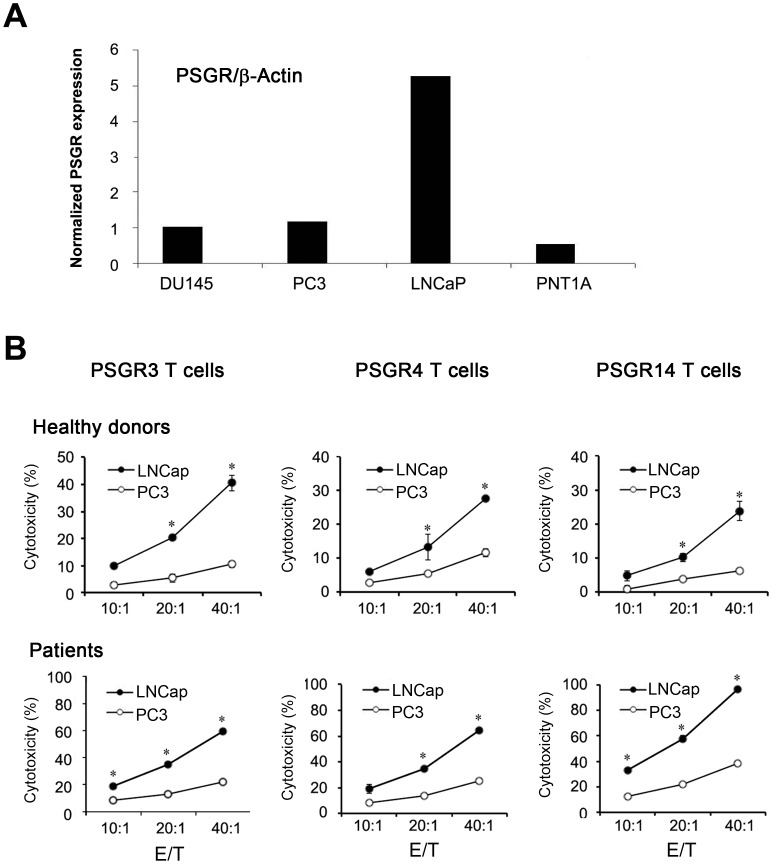
To determine whether PSGR-derived peptide-specific T cells were able to recognize and kill HLA-A2+, PSGR-expressing prostate cancer cells, we used an HLA-A2 negative PC3 cell line and an HLA-A2 positive LNCaP prostate cancer cell line. The expression of PSGR in these two cell lines was examined by RT-PCR. Consistent with a previous report [31], PSGR was highly expressed in LNCaP, but not in PC3, DU145 or a normal prostate cell line PNT1A (Figure 2 A). As shown in Figure 2B, PSGR3-, PSGR4-, or PSGR14-specific T cells from both healthy donors and patients could recognize and kill HLA-A2 positive, PSGR expressing LNCaP, but not HLA-A2 negative PC3 cells. These results suggest that PSGR-specific T cells recognize T cell epitopes that are endogenously processed and presented by prostate tumor cells.
Figure 2. PSGR-derived peptide-specific T cells recognized HLA-A2 positive PSGR-expressing LNCaP prostate cancer cells.
The expression of PSGR mRNA in different cell lines was determined by RT-PCR (A). PSGR derived peptide-specific T cells were tested for cytotoxicity against both PC3 and LNCaP by the LDH assay (B). Data from B are plotted as means ± SD. Results are representative of three independent experiments. *P<0.05, versus control.
T Cells Recognize PSGR-derived Peptides in an HLA-I Restricted Manner
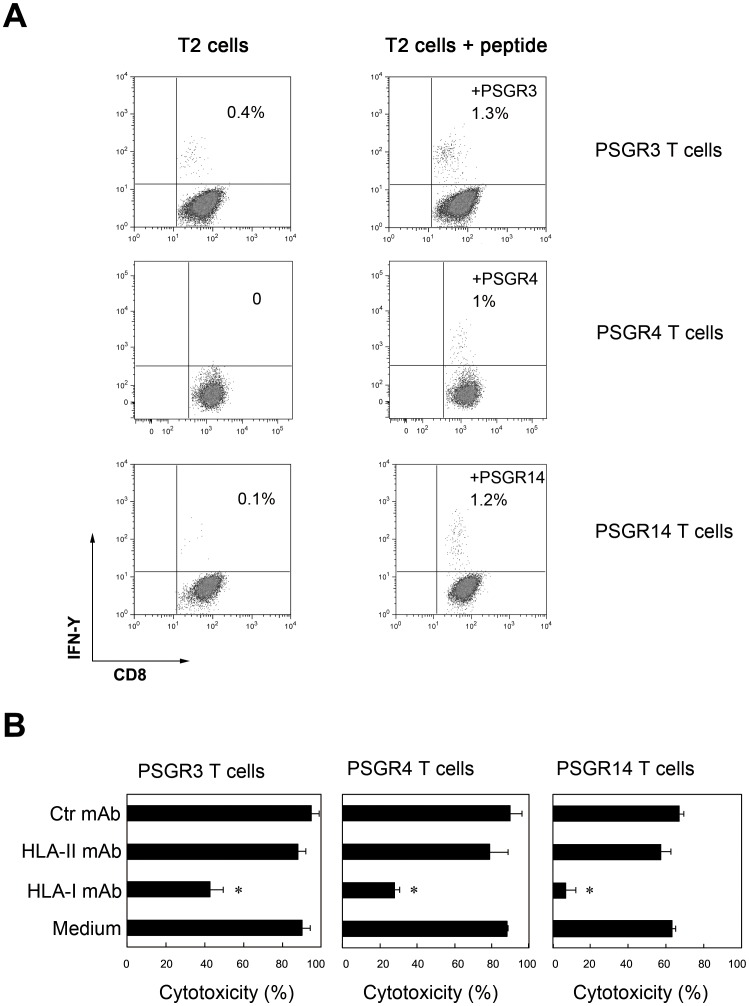
To test whether the responses induced by PSGR-derived peptides are dependent on CD8+ T cells, we co-cultured PSGR-derived peptide-specific T cells with T2 cells pulsed with or without corresponding peptides in the presence of GolgiStop for 4 h. Staining for CD8 molecules and intracellular IFN-γ was subsequently performed. Only CD8+ T cells were found to produce IFN-γ in response to T2 cells pulsed with corresponding peptides (Figure 3A), while CD4+ T cells did not produce IFN-γ (data not shown).
Figure 3. PSGR-derived peptide-induced T cell responses were CD8+ T cell dependent and restricted by HLA-I.
PSGR-derived peptide-specific T cells were co-cultured with T2 cells pulsed with or without a given peptide in the presence of GolgiStop in a 48-well plate for 4 hrs at 37°C. Cells were stained with anti-CD8 and anti-IFN-γ, then analyzed on a FACScalibur machine (A). PSGR-derived peptide-specific T cells were co-incubated with LNCaP cells alone in medium, or with LNCaP cells in the presence of either anti-HLA-I mAb (W6/32), HLA-II mAb or a control mAb (anti-CD19 mAb). After 4 hours of incubation, the cytotoxicity against LNCaP was determined by the LDH assay (B). Data from B are plotted as means ± SD. Results are representative of three independent experiments. *P<0.05, versus controls.
To determine whether the recognition of LNCaP cells by PSGR-derived peptide-specific T cells is HLA-I restricted, we co-cultured LNCaP cells with PSGR-derived peptide-specific T cells in the presence of either anti-HLA-I mAb (W6/32) or control mAbs (HLA-II mAb or anti-CD19 mAb). As shown in Figure 3B, the cytotoxicity of these peptide-specific T cells was completely inhibited by the addition of anti-HLA-I mAb, but not by anti-HLA- II (HLA-DR) or a control mAb (anti-CD19), which suggests that the recognition of LNCaP cells by PSGR-derived peptide-specific T cells is HLA-I restricted.
Discussion
It is well established that CD8+ T cells play a critical role in controlling tumor development and progression. Peptide epitopes derived from tumor-associated antigens (TAAs) can be recognized as antigens by T cells in the context of MHC-I molecules [32], [33]. Identification of TAAs and their peptides that are recognized by T cells are essential for the development of effective cancer vaccines.
The aim of the current study was to identify HLA-A2 binding PSGR-derived epitopes recognized by CD8+ T cells in PBMCs of healthy subjects and prostate cancer patients. Three different computer-based prediction algorithms including BIMAS, SYFPEITHI, and Rankpep were used to scan the PSGR protein sequence for HLA-A2 binding peptides based on the HLA-A2 binding motif. Only peptides that were predicted successfully by at least 2 out of 3 of the different computer-based prediction algorithms were included. Twenty-one 9mer or 10mer peptides were selected in this study according to this criterion. All these peptides were tested for their ability to stimulate PBMCs from either healthy subjects or prostate cancer patients to release IFN-γ. Of 21 peptides, three peptides frequently induced specific T cell responses in PBMCs obtained from either healthy subjects or cancer patients, and these peptide-specific T cells also recognized HLA-A2+ PSGR-expressing LNCaP cells, suggesting that these peptides are naturally processed by prostate cancer cells.
PSGR is a prostate tissue-specific gene with homology to the G protein-coupled odorant receptor gene family and it is specifically expressed in human prostate tissues [23]–[25]. The expression of PSGR is significantly higher in human prostate intraepithelial neoplasia and prostate tumors than normal tissues [25]. Intriguingly, although PSGR has been considered to be a novel target for prostate cancer immunotherapy, T cell epitopes derived from PSGR have not been identified. This is, to our knowledge, the first report to identify and characterize PSGR-derived epitopes recognized by CD8+ T cells. The identification of PSGR-derived epitopes recognized by T cells further validates PSGR as a promising target for the development of cancer vaccines.
Most TAAs are self-antigens [34], therefore, self-tolerance may occur in an attempt to protect the individual from the development of autoimmunity. This is considered to be a major obstacle in the induction of TAA-specific T cells capable of eradicating tumors in vivo. However, in our study, although PSGR is expressed in normal prostate tissue, immune tolerance against PSGR can be broken, since T cell responses against PSGR-derived epitopes were frequently detectable in PBMCs from either healthy subjects or prostate cancer patients.
A vast number of immunotherapy clinical trials based on vaccinations with tumor lysates, TAA proteins, TAA peptides and RNA or DNA encoding TAA have already been conducted. However, most of these trials have not achieved desirable results. One reason is that expression of these TAAs is heterogeneous among tumors from different patients and can vary even among metastases obtained from one patient [35], [36], thus immune escape may occur when the immunotherapeutic approach is only based on one TAA. To avoid immune escape, vaccine-based immunotherapeutic strategies that target several tumor antigens are essential for the development of successful cancer vaccines. Thus identification of additional prostate specific tumor antigens, such as PSGR, for T-cell-based immunotherapy is still needed, despite that a number of prostate specific tumor antigens including PSA [12], [13], PSCA [16], PSMA [17]–[19], PAP [20], Prostein [14], [15] and trp-p8 [21], have been identified in the last few years.
The FDA has recently approved a cancer vaccine, Sipuleucel-T, for the treatment of patients with advanced prostate cancer based on a phase III study [8]. Sipuleucel-T is prepared from autologous PBMCs containing antigen presenting cells that are incubated with a recombinant protein composed of a PAP linked to granulocyte-macrophage colony-stimulating factor (GM-CSF). Sipuleucel-T presumably works in part by augmenting PAP-specific CD8+ T cell responses, further demonstrating the importance of tumor antigen-specific CD8+ T cells induced by cancer vaccines. So far, Sipuleucel-T is the first cellular immunotherapeutic agent approved by the FDA to be used for the treatment of cancer patients. The FDA approval of Sipuleucel-T as a therapeutic cancer vaccine not only validates the efficacy of cancer immunotherapy, but also provides a strong impetus in the field of cancer immunology [37]. Therefore, identification and development of more novel TAAs including PSGR and peptide derivates recognized by CTLs is definitely essential to facilitate the development of effective cancer vaccines against prostate cancers as well as other types of cancers in the future.
Furthermore, the epitopes recognized by CD8+ T cells may be used as diagnostic tools to monitor peptide-specific CD8+ T cells in individuals during the course of immunization, thus identifying optimal time frames for immunization during treatment, including whether subsequent immunizations are needed in individuals when anti-tumor immunity declines.
In summary, we have identified three novel PSGR-derived CTL epitopes. Since PSGR expression is strongly up-regulated in human prostate cancers, PSGR-derived peptides may serve as diagnostic tools or immunotherapeutic targets of anticancer vaccines alone or in combination with other epitopes that are derived from other prostate-specific antigens.
Acknowledgments
We would like to thank Drs. Adebusola Alagbala Ajibade and Audrea M. Burns for the critical reading of this manuscript and Hui An for assistance in figure preparation.
Funding Statement
This work was in part supported by grants from National Cancer Institute, National Institute of Health and Cancer Research Institute, and The Methodist Hospital Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA: a cancer journal for clinicians 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Joniau S, Abrahamsson PA, Bellmunt J, Figdor C, Hamdy F, et al. (2012) Current vaccination strategies for prostate cancer. European urology 61: 290–306. [DOI] [PubMed] [Google Scholar]
- 3.Drake CG, Antonarakis ES (2012) Current status of immunological approaches for the treatment of prostate cancer. Current opinion in urology. [DOI] [PMC free article] [PubMed]
- 4. Di Lorenzo G, Buonerba C, Kantoff PW (2011) Immunotherapy for the treatment of prostate cancer. Nature reviews Clinical oncology 8: 551–561. [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg SA (2011) Cell transfer immunotherapy for metastatic solid cancer–what clinicians need to know. Nature reviews Clinical oncology 8: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lesterhuis WJ, Haanen JB, Punt CJ (2011) Cancer immunotherapy–revisited. Nature reviews Drug discovery 10: 591–600. [DOI] [PubMed] [Google Scholar]
- 7. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 9. Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, et al. (2011) gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. The New England journal of medicine 364: 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T (2012) Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, et al. (2008) Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundwall A, Lilja H (1987) Molecular cloning of human prostate specific antigen cDNA. FEBS letters 214: 317–322. [DOI] [PubMed] [Google Scholar]
- 13. Oesterling JE (1991) Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. The Journal of urology 145: 907–923. [DOI] [PubMed] [Google Scholar]
- 14. Xu J, Kalos M, Stolk JA, Zasloff EJ, Zhang X, et al. (2001) Identification and characterization of prostein, a novel prostate-specific protein. Cancer research 61: 1563–1568. [PubMed] [Google Scholar]
- 15. Kalos M, Askaa J, Hylander BL, Repasky EA, Cai F, et al. (2004) Prostein expression is highly restricted to normal and malignant prostate tissues. The Prostate 60: 246–256. [DOI] [PubMed] [Google Scholar]
- 16. Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, et al. (2000) Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 19: 1288–1296. [DOI] [PubMed] [Google Scholar]
- 17. Kawakami M, Nakayama J (1997) Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer research 57: 2321–2324. [PubMed] [Google Scholar]
- 18. Su SL, Huang IP, Fair WR, Powell CT, Heston WD (1995) Alternatively spliced variants of prostate-specific membrane antigen RNA: ratio of expression as a potential measurement of progression. Cancer research 55: 1441–1443. [PubMed] [Google Scholar]
- 19. Israeli RS, Powell CT, Fair WR, Heston WD (1993) Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer research 53: 227–230. [PubMed] [Google Scholar]
- 20. Solin T, Kontturi M, Pohlmann R, Vihko P (1990) Gene expression and prostate specificity of human prostatic acid phosphatase (PAP): evaluation by RNA blot analyses. Biochimica et biophysica acta 1048: 72–77. [DOI] [PubMed] [Google Scholar]
- 21. Tsavaler L, Shapero MH, Morkowski S, Laus R (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer research 61: 3760–3769. [PubMed] [Google Scholar]
- 22. Kiessling A, Fussel S, Wehner R, Bachmann M, Wirth MP, et al. (2008) Advances in specific immunotherapy for prostate cancer. European urology 53: 694–708. [DOI] [PubMed] [Google Scholar]
- 23. Xu LL, Stackhouse BG, Florence K, Zhang W, Shanmugam N, et al. (2000) PSGR, a novel prostate-specific gene with homology to a G protein-coupled receptor, is overexpressed in prostate cancer. Cancer research 60: 6568–6572. [PubMed] [Google Scholar]
- 24. Yuan TT, Toy P, McClary JA, Lin RJ, Miyamoto NG, et al. (2001) Cloning and genetic characterization of an evolutionarily conserved human olfactory receptor that is differentially expressed across species. Gene 278: 41–51. [DOI] [PubMed] [Google Scholar]
- 25. Weng J, Wang J, Cai Y, Stafford LJ, Mitchell D, et al. (2005) Increased expression of prostate-specific G-protein-coupled receptor in human prostate intraepithelial neoplasia and prostate cancers. International journal of cancer Journal international du cancer 113: 811–818. [DOI] [PubMed] [Google Scholar]
- 26. Hammond AS, Klein MR, Corrah T, Fox A, Jaye A, et al. (2005) Mycobacterium tuberculosis genome-wide screen exposes multiple CD8 T cell epitopes. Clinical and experimental immunology 140: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang M, Lamberth K, Harndahl M, Roder G, Stryhn A, et al. (2007) CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine 25: 2823–2831. [DOI] [PubMed] [Google Scholar]
- 28. Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF (2001) CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proceedings of the National Academy of Sciences of the United States of America 98: 3964–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA (2003) Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. Journal of immunotherapy 26: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weng J, Ma W, Mitchell D, Zhang J, Liu M (2005) Regulation of human prostate-specific G-protein coupled receptor, PSGR, by two distinct promoters and growth factors. Journal of cellular biochemistry 96: 1034–1048. [DOI] [PubMed] [Google Scholar]
- 31. Carlsson B, Forsberg O, Bengtsson M, Totterman TH, Essand M (2007) Characterization of human prostate and breast cancer cell lines for experimental T cell-based immunotherapy. The Prostate 67: 389–395. [DOI] [PubMed] [Google Scholar]
- 32. Van den Eynde BJ, Boon T (1997) Tumor antigens recognized by T lymphocytes. International journal of clinical & laboratory research 27: 81–86. [DOI] [PubMed] [Google Scholar]
- 33. Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A (1994) Tumor antigens recognized by T lymphocytes. Annual review of immunology 12: 337–365. [DOI] [PubMed] [Google Scholar]
- 34. Novellino L, Castelli C, Parmiani G (2005) A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer immunology, immunotherapy : CII 54: 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cormier JN, Hijazi YM, Abati A, Fetsch P, Bettinotti M, et al. (1998) Heterogeneous expression of melanoma-associated antigens and HLA-A2 in metastatic melanoma in vivo. International journal of cancer Journal international du cancer 75: 517–524. [DOI] [PubMed] [Google Scholar]
- 36. Albino AP, Lloyd KO, Houghton AN, Oettgen HF, Old LJ (1981) Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. The Journal of experimental medicine 154: 1764–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]