Abstract
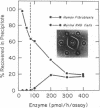
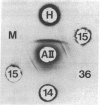
The structural gene encoding human alpha-L-iduronidase has been assigned to chromosome 22 by using immunologic, electrophoretic, and somatic cell hybridization techniques. Polyclonal rabbit antibodies raised against purified human low-uptake alpha-L-iduronidase were used to discriminate the human and murine isozymes by a sensitive immuno-precipitation assay. The human chromosome constitution of each clone was determined by cytogenetic and enzyme marker electrophoretic techniques. In 65 human (fibroblast)-mouse (RAG) somatic cell hybrids (from four independent fusions), the expression of human alpha-L-iduronidase was 100% concordant with the presence of human chromosome 22; the assignment was confirmed by the demonstration of the human enzyme in tertiary somatic cell hybrids containing only chromosome 22. Further verification of the gene assignment was made by detection of the human enzyme in tertiary chromosome 22 positive hybrids by Ouchterlony immunodiffusion and rocket immunoelectrophoretic experiments with polyclonal anti-human alpha-L-iduronidase antibodies that were monospecific for the human enzyme. Expression of human enzymatic activity in chromosome 22 positive hybrid lines was markedly reduced; for example, a tertiary hybrid (R-G21-J-15), which contained an average of 1.7 chromosome 22s per cell, only had about 15% of the activity detected in normal diploid fibroblasts. Immunologic studies suggested that the reduced expression was due to abnormal post-translational processing or aggregation (or both) of the human and murine isozymes in these hybrids. Regional assignment of the human structural gene to 22pter----q11 was accomplished by gene dosage studies using diploid human fibroblast lines that were partially monosomic or trisomic for chromosome 22.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach G., Friedman R., Weissmann B., Neufeld E. F. The defect in the Hurler and Scheie syndromes: deficiency of -L-iduronidase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2048–2051. doi: 10.1073/pnas.69.8.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R. W., Neufeld E. F. The Hurler corrective factor. Purification and some properties. J Biol Chem. 1971 Dec 25;246(24):7773–7779. [PubMed] [Google Scholar]
- Beratis N. G., LaBadie G. U., Hirschhorn K. Genetic heterogeneity in acid alpha-glucosidase deficiency. Am J Hum Genet. 1983 Jan;35(1):21–33. [PMC free article] [PubMed] [Google Scholar]
- Breg W. R. Quinacrine fluorescence for identifying metaphase chromosomes, with special reference to photomicrography. Stain Technol. 1972 Mar;47(2):87–93. doi: 10.3109/10520297209116456. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Mintz B. J., Leary A. C., Regina V. M., Gerald P. S. Human lysosomal genes: arylsulfatase A and beta-galactosidase. Biochem Genet. 1979 Dec;17(11-12):1031–1059. doi: 10.1007/BF00504344. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science. 1968 Nov 1;162(3853):570–572. doi: 10.1126/science.162.3853.570. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler and Hunter syndromes. II. Deficiency of specific factors involved in mucopolysaccharide degradation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):360–366. doi: 10.1073/pnas.64.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend K. K., Dorman B. P., Kucherlapati R. S., Ruddle F. H. Detection of interspecific translocations in mouse-human hybrids by alkaline Giemsa staining. Exp Cell Res. 1976 Apr;99(1):31–36. doi: 10.1016/0014-4827(76)90676-5. [DOI] [PubMed] [Google Scholar]
- George D. L., Francke U. Regional mapping of human genes for hexosaminidase B and diphtheria toxin sensitivity on chromosome 5 using mouse X human hybrid cells. Somatic Cell Genet. 1977 Nov;3(6):629–638. doi: 10.1007/BF01539070. [DOI] [PubMed] [Google Scholar]
- Haskins M. E., Jezyk P. F., Desnick R. J., McDonough S. K., Patterson D. F. Alpha-L-iduronidase deficiency in a cat: a model of mucopolysaccharidosis I. Pediatr Res. 1979 Nov;13(11):1294–1297. doi: 10.1203/00006450-197911000-00018. [DOI] [PubMed] [Google Scholar]
- Human gene mapping 6. Oslo conference (1981): Sixth International Workshop on Human Gene Mapping. Cytogenet Cell Genet. 1982;32(1-4):1–341. doi: 10.1159/000131680. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle F. H. Controlled production of proliferating somatic cell hybrids. J Cell Biol. 1970 Apr;45(1):74–82. doi: 10.1083/jcb.45.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Matalon R., Cifonelli J. A., Dorfman A. L-iduronidase in cultured human fibroblasts and liver. Biochem Biophys Res Commun. 1971 Jan 22;42(2):340–345. doi: 10.1016/0006-291x(71)90108-2. [DOI] [PubMed] [Google Scholar]
- Meera Khan P., Wijnen L. M., Pearson P. L. Assignment of the mitochondrial aconitase gene (ACONM) to human chromosome 22. Cytogenet Cell Genet. 1978;22(1-6):212–214. doi: 10.1159/000130938. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Neufeld E. F. Maturation of alpha-L-iduronidase in cultured human fibroblasts. J Biol Chem. 1981 Mar 25;256(6):3044–3048. [PubMed] [Google Scholar]
- Paigen K. Acid hydrolases as models of genetic control. Annu Rev Genet. 1979;13:417–466. doi: 10.1146/annurev.ge.13.120179.002221. [DOI] [PubMed] [Google Scholar]
- Povey S., Slaughter C. A., Wilson D. E., Gormley I. P., Buckton K. E., Perry P., Bobrow M. Evidence for the assignment of the loci AK1, AK3 and ACONs to chromosome 9 in man. Ann Hum Genet. 1976 May;39(4):413–422. doi: 10.1111/j.1469-1809.1976.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Weissmann B., Neufeld E. F. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. doi: 10.1073/pnas.76.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Schuchman E. H., Guzman N. A., Desnick R. J. Human alpha-L-iduronidase. I. Purification and properties of the high uptake (higher molecular weight) and the low uptake (processed) forms. J Biol Chem. 1984 Mar 10;259(5):3132–3140. [PubMed] [Google Scholar]
- Seabright M. The use of proteolytic enzymes for the mapping of structural rearrangements in the chromosomes of man. Chromosoma. 1972;36(2):204–210. doi: 10.1007/BF00285214. [DOI] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Devine E. A., Smith M., Arredondo-Vega F., Desnick R. J. Assignment of the gene for acid beta-glucosidase to human chromosome 1. Am J Hum Genet. 1981 Jul;33(4):564–575. [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. J., Hall C. W., Leder I. G., Neufeld E. F. The relationship of alpha-L-iduronidase and Hurler corrective factor. Arch Biochem Biophys. 1976 Jan;172(1):156–161. doi: 10.1016/0003-9861(76)90061-8. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Shimizu Y., Ruddle F. H. Assignment of the human mitochondrial NAD-linked malate dehydrogenase gene to the p22 leads to qter region of chromosome 7. Cytogenet Cell Genet. 1978;22(1-6):441–445. doi: 10.1159/000130992. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Scrafford-Wolff L. R., Brown J. A., Meisler M. H. GM1-gangliosidosis: chromosome 3 assignment of the beta-galactosidase-A gene (beta GALA). Somatic Cell Genet. 1979 Mar;5(2):147–158. doi: 10.1007/BF01539157. [DOI] [PubMed] [Google Scholar]
- Shull R. M., Munger R. J., Spellacy E., Hall C. W., Constantopoulos G., Neufeld E. F. Canine alpha-L-iduronidase deficiency. A model of mucopolysaccharidosis I. Am J Pathol. 1982 Nov;109(2):244–248. [PMC free article] [PubMed] [Google Scholar]
- Smith M., Gold P., Freedman S. O., Shuster J. Studies of the linkage relationship of beta-2-microglobulin in man-mouse somatic cell hybrids. Ann Hum Genet. 1975 Jul;39(1):21–31. doi: 10.1111/j.1469-1809.1975.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Mohandas T., Sparkes M. C., Shulkin J. D. Aconitase (E.C. 4.2.1.3) mitochondrial locus (ACONM) mapped to human chromosome 22. Cytogenet Cell Genet. 1978;22(1-6):226–227. doi: 10.1159/000130942. [DOI] [PubMed] [Google Scholar]
- Steiner A. W., Rome L. H. Assay and purification of a solubilized membrane receptor that binds the lysosomal enzyme alpha-L-iduronidase. Arch Biochem Biophys. 1982 Apr 1;214(2):681–687. doi: 10.1016/0003-9861(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Thorpe S. R., Fiddler M. B., Desnick R. J. Enzyme therapy IV. A method for determining the in vivo fate of bovine beta-glucuronidase in beta-glucuronidase deficient mice. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1464–1470. doi: 10.1016/s0006-291x(74)80448-1. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Sparkes R. S., Bertolotti R. Expression of differentiated functions in hepatoma cell hybrids: IX extinction and reexpression of liver-specific enzymes in rat hepatoma-Chinese hamster fibroblast hybrids. Somatic Cell Genet. 1975 Jan;1(1):27–40. doi: 10.1007/BF01538730. [DOI] [PubMed] [Google Scholar]
- Weissmann B. Synthetic substrates for alpha-L-iduronidase. Methods Enzymol. 1978;50:141–150. doi: 10.1016/0076-6879(78)50012-8. [DOI] [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegouwen H., Los W., Wurzer-Figurelli E., Doppert B., Vervloet M., Meera Khan P. Enzyme electrophoresis on cellulose acetate gel. II. Zymogram patterns in man-Chinese hamster somatic cell hybrids. Humangenetik. 1974;25(3):189–201. doi: 10.1007/BF00281426. [DOI] [PubMed] [Google Scholar]