Abstract
We hypothesized that in vitro treatment of peripheral blood mononuclear cells (PB-MNCs) from diabetic patients with ephrin-B2/Fc (EFNB2) improves their proangiogenic therapeutic potential in diabetic ischemic experimental models. Diabetes was induced in nude athymic mice by streptozotocin injections. At 9 weeks after hyperglycemia, 105 PB-MNCs from diabetic patients, pretreated by EFNB2, were intravenously injected in diabetic mice with hindlimb ischemia. Two weeks later, the postischemic neovascularization was evaluated. The mechanisms involved were investigated by flow cytometry analysis and in vitro cell biological assays. Paw skin blood flow, angiographic score, and capillary density were significantly increased in ischemic leg of diabetic mice receiving EFNB2-activated diabetic PB-MNCs versus those receiving nontreated diabetic PB-MNCs. EFNB2 bound to PB-MNCs and increased the adhesion and transmigration of PB-MNCs. Finally, EFNB2-activated PB-MNCs raised the number of circulating vascular progenitor cells in diabetic nude mice and increased the ability of endogenous bone marrow MNCs to differentiate into cells with endothelial phenotype and enhanced their proangiogenic potential. Therefore, EFNB2 treatment of PB-MNCs abrogates the diabetes-induced stem/progenitor cell dysfunction and opens a new avenue for the clinical development of an innovative and accessible strategy in diabetic patients with critical ischemic diseases.
Lower extremity peripheral arterial disease (PAD) is a common syndrome that affects a large proportion of most adult populations worldwide. PAD is associated with significant morbidity and mortality and often results in critical limb ischemia (1,2). Approximately 30% of patients are not considered for vascular or endovascular procedures, with amputation often being the only option (3). In addition, leg amputation due to PAD with other cardiovascular risk factors gives rise to an acute mortality rate of ∼30% and a 5-year prognosis with survival rates of <30% (4,5). Hence, there is a great need for the development of therapeutic strategies to implement novel management procedures and therapeutic options.
Diabetes is the most significant risk factor for amputation in PAD patients (6–10). Indeed, there is considerable evidence to suggest that microvascular abnormality is a common hallmark in diabetes and that angiogenic response is altered in wound healing and ulcers in diabetic patients (6–11). In preclinical models of PAD, diabetic animals displayed attenuated perfusion recovery in response to ischemia (12–14), likely related to impaired release of endothelial progenitor cells (EPCs) and altered activity of growth factors (12,15–17). The concept of (stem) cell–based revascularization emerged in 1997 with the description of adult circulating EPCs by Asahara et al. (18). Animal experiments and clinical trials then proved that cell-based therapies increase blood perfusion in ischemic tissues (19). Different stem/progenitor cell types have been tested, including mesenchymal stem cells (20,21), adult bone marrow (BM) mononuclear cells (BM-MNCs) (22), umbilical cord blood–derived EPCs (uEPCs) (23,24), and peripheral blood–derived circulating EPCs (25). BM-MNCs are effective proangiogenic cells and are used in clinical studies in patients with ischemic disease (26); however, the collection of BM-MNCs is of concern in patients with severe disease. Indeed, in most previous trials, 500 mL BM was necessary and collected in anesthetized patients. Thus, in patients with critical limb ischemia, BM collection is difficult and almost impossible to repeat (27). In a similar manner, the uEPC-based therapy requires long and complex preparation processes and could cause immune problems.
Alternatively, extensive studies have also unraveled the therapeutic effectiveness of mobilized or injected peripheral blood (PB)-MNCs in patients with critical limb ischemia (28–31). It is noteworthy that PB-MNCs are easy to obtain and prepare. They are suitable for repeated autologous administration since similar clinical manipulations, such as blood apheresis and blood transfusion, have been widely applied over past decades and are well accepted by patients and clinicians. We previously reported that activation of the EphB4/ephrin-B2 (EphB4/EFNB2) system further enhanced uEPC proangiogenic potential (23). It is interesting that mature monocytes and lymphocytes express EFNB2 and its receptors (32,33). The in vitro stimulation by EFNB2 improves the proliferation and cytotoxic function of T cells and increases the adhesion and transmigration of monocytes (32,33), suggesting that an EFNB2-dependent signaling pathway is functional in PB-MNCs and is involved in the regulation of their activity. Therefore, we hypothesized that EFNB2 could activate PB-MNCs from diabetic patients and enhance their proangiogenic and therapeutic potential in ischemic tissues in diabetic animals. Here we show that PB-MNCs from diabetic patients, in vitro stimulated by EFNB2, acquire a marked proangiogenic therapeutic potential in a diabetic mouse model of hindlimb ischemia.
RESEARCH DESIGN AND METHODS
Cells.
Blood samples from consenting nonhospitalized diabetic patients, unscathed of any acute disease during systematic annual visit, and healthy donors were collected in heparinized tubes. The inclusion criteria were male sex, age ≥40 years, and type 2 diabetes. The patients with diabetes received oral antidiabetic drugs (gliclazide, metformin, acarbose, and sitagliptin), antihypertensive treatments (ACE inhibitor, diuretic, and calcium channel blocker), anticoagulant therapy, and/or atorvastatin. PB-MNCs were isolated by gradient density centrifugation using Pancoll (PAN Biotech, Aidenbach, Germany). Cells were then incubated with EFNB2 (R&D Systems, Minneapolis, MN) at 37°C. The uEPCs were isolated from umbilical cord blood, and human umbilical vein endothelial cells (HUVECs) were isolated by collagenase digestion. The uEPCs from the 10th to 15th passages and HUVECs from the 3rd to 5th passages were used (23).
Cellular assays
Cell adhesion assay.
Human PB-MNCs were labeled with a Vybrant CFDA SE Cell Tracer. PB-MNCs were seeded over the HUVEC monolayer and incubated at 37°C for 1 h. The adhered cells were evaluated using a Victor3 spectrofluorimeter (PerkinElmer, Turku, Finland). To determine signaling pathways involved in cell adhesion, PB-MNCs were preincubated with LY29004 (phosphatidylinositol [PI] 3-kinase inhibitor) or PD98059 (mitogen-activated protein kinase kinase [MEK]-1/2 inhibitor; Sigma-Aldrich, St. Louis, MO) before the incubation with EFNB2.
Cell transmigration assay.
HUVECs were preseeded onto the upper side of the gelatin-coated FluoroBlok inserts (3 µm; BD Biosciences, Franklin Lakes, NJ). Labeled PB-MNCs were added onto the HUVEC monolayer and incubated for 1 h. The cells that had transmigrated through the HUVEC monolayer to the lower surface of the filters were evaluated.
Capillary-like tube formation assay on Matrigel.
HUVECs and diabetic PB-MNCs were seeded on Matrigel (Ibidi, Martinsried, Germany). After 24 h, capillary-like structures were quantified using HistoLab software (Microvision, Evry, France).
Model of diabetes and surgical procedure.
Experiments were performed in accordance with the animal protection legislation for animal studies in experimentation and all other applicable laws and regulations in force in France (in accordance with the European Community guidelines for the care and use of laboratory animals, no. 07430). Male adult athymic nude mice and C57Bl6 mice (aged 6–7 weeks; Harlan, Indianapolis, IN) were used. For diabetes induction, the nude mice received successive intraperitoneal injections of streptozotocin (40 mg/kg/day; Sigma-Aldrich) during 7 days. Surgical intervention was performed to create a unilateral hindlimb ischemia under sterile conditions at 9 weeks after constant hyperglycemia was observed in animals (34). In anesthetized mice, the femoral artery was gently dissected and separated from the vein using a microscope. The ligature was performed just above the origin of the circumflexa femoris lateralis. Five hours after the ligature of femoral artery, PBS, PB-MNCs, or uEPCs were injected into the tail vein. The PB-MNC optimal stimulation conditions used for injection were determined by in vitro cell adhesion assay.
Evaluation of postischemic revascularization
Laser Doppler flowmetry.
At 14 days postligature, animals were anesthetized by injection of pentobarbital (10 mg/kg i.p.). Mice were next placed on a heating platform, and their temperature was kept at 37 ± 0.5°C to minimize the influence of body temperature on paw blood perfusion. The evaluation was then performed using a laser Doppler flowmetry (Moor Instruments, Devon, U.K.).
Microangiography.
Vessel density was determined by high-definition microangiography (2100; Kodak Dental Systems, Atlanta, GA). A longitudinal laparotomy was performed and a polyethylene catheter (PE-10) was inserted into abdominal aorta through which a contrast medium (barium sulfate, 1.67 g/mL saline) was injected. Microangiography of hindlimb was then assessed and images were acquired by a digital X-ray transducer. Vascular density of each hindlimb was calculated as a percentage of pixels per image in the quantification area occupied by arteries using Primed Software (Paris, France).
Capillary density.
For immunohistochemical analysis, ischemic and nonischemic gastrocnemius muscles were sectioned at 7-µm thickness with cryostat at three levels separated by a distance of 300 µm. The sections were incubated with fluorescein isothiocyanate (FITC)-conjugated isolectin B4 (10 µg/mL; Sigma-Aldrich). Using a fluorescence microscope, six images (two images per level) were acquired for each muscle sample. HistoLab software was used to quantify capillary density.
The results for each animal were expressed as a ratio of right (ischemic) side versus left (nonischemic) side.
Flow cytometry analysis.
EFNB2 was biotinylated using a Micro Biotinilation Buffer and Desalting Set (Thermo Fisher Scientific, Rockford, IL). PB-MNCs isolated from diabetic patients and healthy volunteers were incubated with 15 µg/mL biotinylated EFNB2 for 30 min at 37°C. Bound biotinylated EFNB2 was detected by phycoethrin (PE)-streptavidin incubation. To determine the subpopulations of PB-MNCs interacting with EFNB2, cells were coimmunolabeled with a mixture of appropriate antibody (BD Biosciences), including V450-anti-human CD3 antibody, PerCP-Cy5.5-anti-human CD4 antibody, FITC-anti-human CD8 antibody, APC-anti-human CD19 antibody, APC-H7-anti-human CD14 antibody, V500-anti-human-CD45 antibody, PE-Cy7-anti-human CD34 antibody, FITC-anti-mouse CD34 antibody, and PE-anti-mouse VEGFR2 antibody. The cells were then examined by BD Biosciences LSRII flow cytometry. The percentage of positive cells was analyzed using FlowJo software (TreeStar, Ashland, OR).
BM-MNC differentiation assay.
Mouse BM was obtained by flushing tibia and femur. Low-density MNCs were then isolated by centrifugation on a Ficoll gradient (31). BM-MNCs (2 × 106 per mL), isolated from nondiabetic or diabetic nude mice, were plated on 11-mm cell culture dishes coated with gelatin (0.1%) and rat plasma vitronectin. BM-MNCs were maintained in complete EGM2 medium for 7 days. Adherent cells were then incubated in EGM2 containing 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine–labeled acetylated LDL (Dil-LDL; Harbor Bio-Products, Norwood, MA) at 37°C for 1 h. Cells were fixed in 2% paraformaldehyde and incubated with FITC-labeled BS-1 lectin (Sigma-Aldrich). Endothelial cell phenotype was revealed with double-positive staining for both Dil-LDL and BS-1 lectin. Cell numbers were counted per well by using epifluorescence microscopy. Results were expressed as percentages of total adherent cell numbers (35).
RT-PCR.
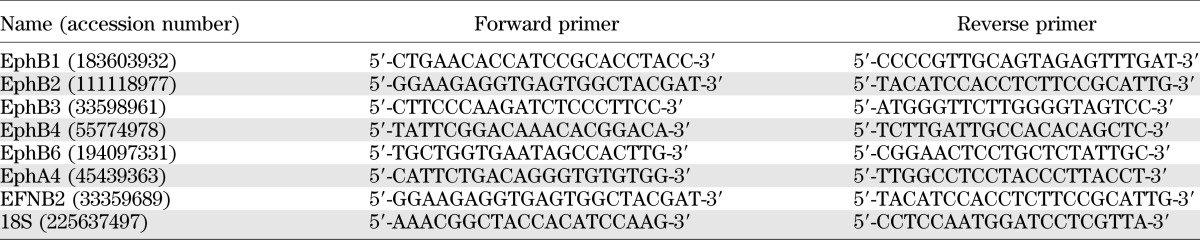
Total RNA was isolated using the AllPrep DNA/RNA Mini Kit (QIAGEN, Hilden, Germany). Total RNA was reverse transcribed, and the generated cDNA was further amplified for 35 cycles (Roche Diagnostics, Mannheim, Germany). The primers used for PCR are listed in Table 1. The mRNA levels were normalized to 18S ribosomal RNA.
TABLE 1.
Primers used for real-time RT-PCR
Western blot.
Cell extracts of PB-MNCs were prepared in 50 mmol/L Tris, pH 8, 100 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100, 0.5% Nonidet P-40, 1 mmol/L phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL aprotinin. Proteins were separated by electrophoresis and transferred to nitrocellulose membranes. Membranes were incubated with the appropriate primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The signal was revealed with the Pierce Enhanced Chemiluminescence System.
Data analysis and statistics.
The results are expressed as mean ± SEM. The comparisons were performed using a Student t test for two experimental groups and a one-way ANOVA followed by post hoc Bonferroni complementary analysis for more than two experimental groups. P < 0.05 was considered significant.
RESULTS
EphB2 and EphB4 are expressed in PB-MNCs.
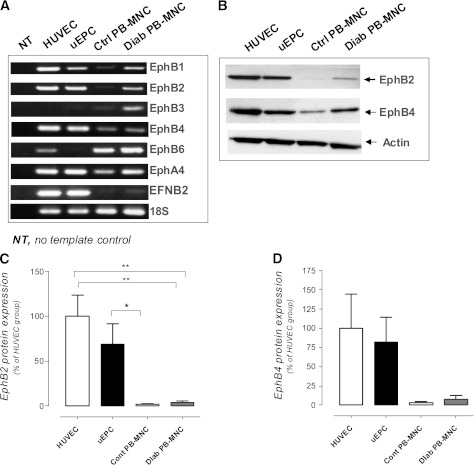
RT-PCR analysis demonstrated that as in cultured HUVECs and in vitro expanded uEPCs (positive controls), PB-MNCs of both healthy volunteers and diabetic patients expressed mRNAs encoding for all Eph receptors known to interact with EFNB2 (32,33). However, EphB4 mRNA levels were ∼100-fold higher, and those of EphB1, EphB2, and EphA4 were ∼10-fold higher in HUVECs and uEPCs compared with PB-MNCs. In contrast, mRNA encodings for EphB3 and EphB6 were more abundantly expressed in PB-MNCs than in HUVECs and uEPCs. The expression of EFNB2 mRNA was barely detectable in PB-MNCs, whereas it was strongly expressed in both HUVECs and uEPCs (Fig. 1A). Western blot analysis evidenced expression of EphB2 and EphB4 proteins only in human PB-MNCs from diabetic patients and healthy volunteers (Fig. 1B–D).
FIG. 1.
A and B: Expression of EFNB2 receptor mRNA and protein levels in PB-MNCs and cultured HUVECs or uEPCs. Quantification of protein content of EphB2 (C) and EphB4 (D). Ctrl and Cont, control; Diab, diabetic. *P < 0.05; **P < 0.01.
Optimal conditions for PB-MNC pretreatment.
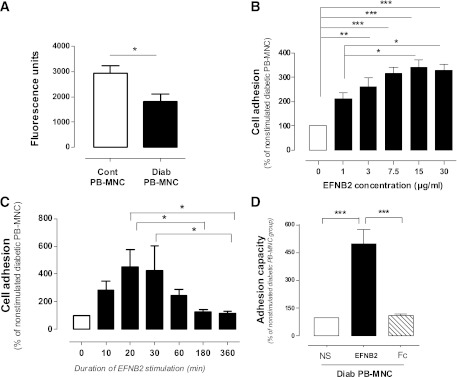
We next sought to determine the optimal concentration and duration of stimulation of human PB-MNCs with EFNB2 by using an in vitro cell adhesion assay. The nonstimulated PB-MNCs derived from diabetic patients showed less adhesion ability than those from healthy donors (P < 0.05) (Fig. 2A). EFNB2 pretreatment significantly increased the adhesion of human diabetic PB-MNCs in a dose-dependent manner, with a maximal effect observed at 15 μg/mL (Fig. 2B). Moreover, the EFNB2 pretreatment modified the adhesion ability of diabetic PB-MNCs in a time-dependent manner. It is noteworthy that the treatment for 30 min led to the highest adhesion of human diabetic PB-MNCs (Fig. 2C). We also showed that EFNB2 pretreatment exerted a similar effect on the adhesion ability of control PB-MNCs (Supplementary Fig. 1A and B). The pretreatment of diabetic PB-MNCs with isolated IgG1/Fc fragment, representing the COOH-terminal half of EFNB2 chimera, did not change the adhesion ability of diabetic PB-MNCs to the HUVEC layer (Fig. 2D). Therefore, in all the following experiments, PB-MNCs were stimulated with EFNB2 at 15 μg/mL for 30 min.
FIG. 2.
Dose- and time-dependent effects of EFNB2 treatment on PB-MNC adhesion. A: In vitro adhesion ability of nonstimulated PB-MNCs from healthy donors (n = 15) and diabetic patients (n = 9). Dose-related effects (n = 9) (B) and time-related effects (n = 8) (C) of pretreatment with EFNB2 on the in vitro adhesion ability of PB-MNCs from diabetic patients. D: Effect of IgG1/Fc fragment on the adhesion ability of PB-MNCs from diabetic patients (n = 7). Cont, control; Diab, diabetic; NS, nonstimulated. *P < 0.05; **P < 0.01; ***P < 0.001.
Optimal cell number for PB-MNC–based therapy.
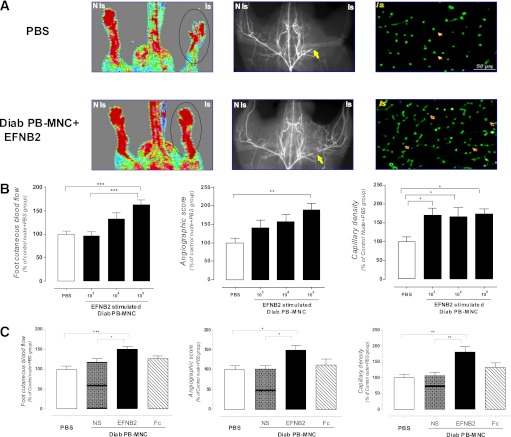
We next investigated the effects of EFNB2-pretreated human diabetic PB-MNCs on postischemic revascularization. Intravenous injection of EFNB2-stimulated diabetic PB-MNCs markedly improved the neovascularization in a cell number–dependent manner when compared with PBS. The maximal effect was observed after treatment of 105 EFNB2-stimulated diabetic PB-MNCs: foot skin blood flow, angiographic score, and capillary density were significantly increased by 63 ± 8, 89 ± 17, and 74 ± 14% in mice ischemic limb (P < 0.001) (Fig. 3A and B). At this dose, the nonstimulated diabetic PB-MNCs did not modify these parameters in ischemic limb (Fig. 3C). Moreover, stimulation of diabetic PB-MNCs with isolated IgG1/Fc fragment did not affect the proangiogenic potential of diabetic PB-MNCs (Fig. 3C). Subsequently, 105 pretreated PB-MNCs were used in all our in vivo experiments.
FIG. 3.
Dose-dependent effects of EFNB2 stimulation on PB-MNC proangiogenic effects. A: Representative images of foot cutaneous blood flow (left), microangiography (middle), and capillary density (right) of nondiabetic ischemic nude mice receiving PBS and 105 EFNB2-pretreated PB-MNCs from diabetic patients. The circled portions of images show the area of laser Doppler blood flow measurement. The arrows show the location of arterial ligation in the microarteriograms (middle) and capillaries in the histological sections (right). B: Quantitative evaluation of foot cutaneous blood flow, microangiography, and capillary density of nondiabetic ischemic nude mice treated with PBS, 103, 104, or 105 EFNB2-pretreated PB-MNCs from diabetic patients (n = 10–14 per group). C: Quantification of foot cutaneous blood flow, microangiography, and capillary density of nondiabetic ischemic nude mice receiving PBS, 105 nonstimulated diabetic PB-MNCs, 105 EFNB2-pretreated diabetic PB-MNCs, or 105 IgG1/Fc-treated diabetic PB-MNCs (n = 12–14 per group). Diab, diabetic; NS, nonstimulated; Is, ischemic limb; N Is, nonischemic limb. *P < 0.05; **P < 0.01; ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)
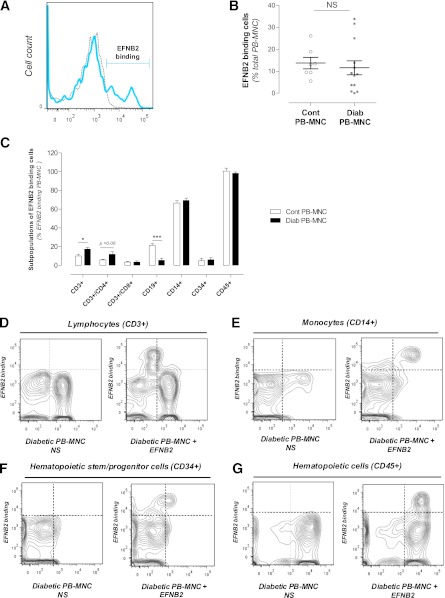
EFNB2 binding to subpopulations of PB-MNCs.
We next assessed the EFNB2 ability to bind to PB-MNCs by flow cytometry analysis. EFNB2 interacted with 11.6 ± 3.2% of human diabetic PB-MNCs (n = 13) and 13.8 ± 2.6% of human control PB-MNCs (n = 7) (Fig. 4A and B). Among these EFNB2-interacted diabetic cells, 69.3 ± 2.5% were monocytes (CD14+), 17.3 ± 1.8% were T cells (CD3+), 5.5 ± 1.9% were B cells (CD19+), and 6.0 ± 2.08% were hematopoietic stem/progenitor cells (CD34+/CD45+) (Fig. 4C–G). The percentage of EFNB2 interacting with B cells was significantly increased compared with that of diabetic B cells (P < 0.001) (Fig. 4C). In contrast, the number of CD3+ T cells in the diabetic group was significantly increased compared with that of the control group (P < 0.05) (Fig. 4C). This increase seems to be the result of an upregulation in CD3+/CD4+ lymphocytes since their percentage was enhanced, albeit not significantly (5.8 ± 0.9 vs. 11.8 ± 2.6% for control and diabetic groups, respectively, P = 0.06) (Fig. 4C). No additional differences were observed for the other subpopulations of PB-MNCs (Fig. 4C).
FIG. 4.
EFNB2 binding to subpopulations of diabetic PB-MNCs. A: Representative images of flow cytometry analysis of EFNB2 binding to nonstimulated PB-MNCs (gray) and stimulated PB-MNCs (blue) from diabetic patients. B: Percentage of control and diabetic PB-MNCs that interacted with EFNB2 (n = 7 and n = 13 for control and diabetic groups, respectively). C: Quantitative evaluation of different subpopulations in EFNB2-treated diabetic PB-MNCs (n = 7 and n = 8 for control and diabetic groups, respectively). Representative scattergram of lymphocyte (CD3+) (D), monocyte (CD14+) (E), hematopoietic stem/progenitor cell (CD34+) (F), and total hematopoietic cell (CD45+) (G) contents in nonstimulated PB-MNCs and stimulated PB-MNCs from diabetic patients. Cont, control; Diab, diabetic; NS, nonstimulated. *P < 0.05; ***P < 0.001.
Finally, EFNB2 pretreatment did not change the activity of platelets, as determined by flow cytometry analysis of CD62P levels (data not shown).
Signaling pathways involved in EFNB2-induced PB-MNC activation.
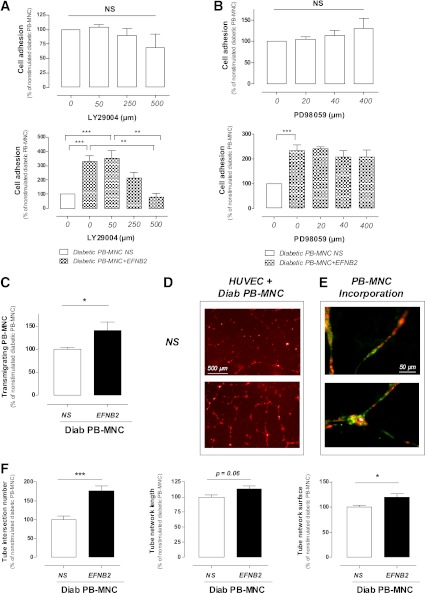
To study the pathways involved in EFNB2-mediated signaling, we preincubated human diabetic PB-MNCs with specific inhibitors of PI 3-kinase (LY29004) or MEK-1/2 (PD98059) for 30 min before the EFNB2 stimulation. LY29004 and PD98059 did not significantly modify the adhesion of nonstimulated diabetic PB-MNCs. Preincubation of diabetic PB-MNCs with LY29004 but not PD98059 dose-dependently abolished the increase of diabetic PB-MNC adhesion induced by EFNB2 (P < 0.001) (Fig. 5A and B). These results suggest that the PI 3-kinase–dependent pathway is involved in EFNB2-mediated effects on human diabetic PB-MNCs.
FIG. 5.
Effects of EFNB2 stimulation on diabetic PB-MNCs. A: Effects of specific inhibitor of PI 3-kinase signaling pathway (LY29004) on the adhesion of untreated (upper panel) (n = 4–10 per group) or pretreated (lower panel) (n = 5–10 per group) PB-MNCs from diabetic patients. B: Effects of specific inhibitor of MEK-1/2 signaling pathway (PD98059) on the adhesion ability of control (upper panel) (n = 5 per group) or pretreated (lower panel) (n = 5 per group) PB-MNCs from diabetic patients. C: Role of EFNB2 stimulation on the migration of PB-MNCs from diabetic patients (n = 12 per group). D: Representative images of capillary-like tube formation in cocultures of HUVECs and nonpretreated (upper panel) or pretreated (lower panel) diabetic PB-MNCs. E: Representative images of incorporated EFNB2-pretreated diabetic PB-MNCs (green) in the HUVEC network (red). F: Quantitative analysis of HUVEC network formation: intersection number, length, and surface (n = 9). NS, nonstimulated; Diab, diabetic. *P < 0.05; **P < 0.01; ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)
EFNB2 pretreatment increases PB-MNC transmigration capacity.
We next investigated whether EFNB2 pretreatment could affect PB-MNC transmigration capacity. We observed that EFNB2 stimulation increased the migration rate of human diabetic PB-MNCs by 40 ± 18% (P < 0.001) (Fig. 5C). In addition, transcriptome analysis demonstrated that EFNB2 pretreatment rapidly (1 h and 4 h after pretreatment) increased the expression of proinflammatory/proangiogenic factors, such as interleukin (IL)-1, IL-6, and monocyte chemotactic protein-1 in human diabetic PB-MNCs (n = 5; data not shown).
EFNB2-pretreated PB-MNCs stimulate in vitro capillary-like tube formation.
The effect of EFNB2 pretreatment on the ability of PB-MNCs to form capillary-like structures was also assessed in an in vitro three-dimensional Matrigel assay. Nonstimulated diabetic PB-MNCs had no effect on HUVEC capillary-like tube formation (data not shown). EFNB2-stimulated diabetic PB-MNCs markedly increased capillary-like tube formation compared with nonstimulated diabetic PB-MNCs (Fig. 5D). Tube intersection number, tube network length, and tube network surface were increased by 77 ± 13, 13 ± 5, and 20 ± 7%, respectively, in cocultures of HUVECs and EFNB2-stimulated diabetic PB-MNCs (P < 0.001, P = 0.06, and P < 0.05, respectively) (Fig. 5F). In addition, we showed that EFNB2-stimulated diabetic PB-MNCs could incorporate into the capillary-like network formed by HUVECs (Fig. 5E).
Moreover, 14 days after transplantation of human diabetic PB-MNCs in nude mice, human DNA was detected in 83% of ischemic tibias muscles. In contrast, the human DNA was not found in liver, spleen, heart, and lung tissues of mice (Supplementary Fig. 2). These results suggest that EFNB2 pretreatment enhances the angiogenic property of human diabetic PB-MNCs, at least partly, through their ability to incorporate vascular structure.
PB-MNC–based cell therapy in limb ischemia associated with diabetes.
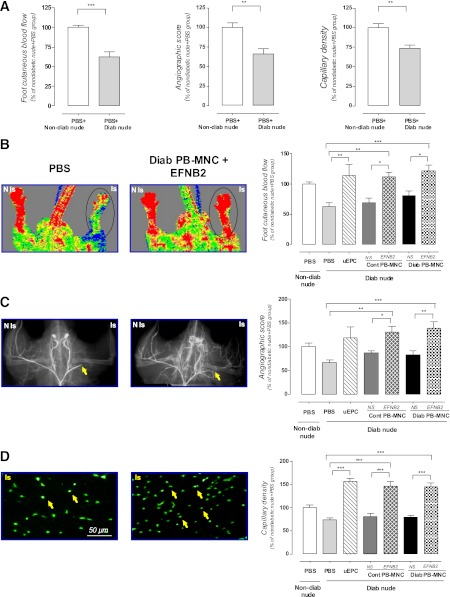
Indexes of postischemic neovascularization in diabetic nude athymic mice decreased by 37 ± 6 (foot skin blood flow), 34 ± 6 (angiographic score), and 26 ± 4% (capillary density) when compared with nondiabetic animals (P < 0.01) (Fig. 6A). As a positive control, we showed that 105 uEPCs significantly restored vascular network formation and perfusion in the ischemic limb of diabetic mice (113 ± 17, 118 ± 24, and 156 ± 8% vs. nondiabetic nude mice for foot skin blood flow, angiographic score, and capillary density, respectively, P < 0.001) (Fig. 6B–D). At 14 days after the onset of ischemia, untreated 105 PB-MNCs from diabetic patients or from healthy donors had no effect on the neovascularization process in diabetic nude mice. It is interesting that the EFNB2-pretreated diabetic PB-MNCs increased the foot skin blood flow, angiographic score, and capillary density in the ischemic hindlimb of diabetic animals compared with PBS or nonstimulated PB-MNCs (122 ± 10, 139 ± 14, and 145 ± 7% vs. nondiabetic nude group, respectively, P < 0.001) (Fig. 6B–D). In a similar manner, the EFNB2-stimulated PB-MNCs extracted from healthy donors significantly enhanced the postischemic neovascularization in ischemic lower extremity of diabetic animals (112 ± 7, 131 ± 13, and 146 ± 11% vs. nondiabetic nude group for foot skin blood flow, angiographic score, and capillary density, respectively, P < 0.001) (Fig. 6B–D).
FIG. 6.
Proangiogenic therapeutic strategy based on EFNB2-pretreated diabetic PB-MNCs for diabetes-associated ischemic pathology. A: Postischemic neovascularization in nondiabetic (n = 6) and diabetic (n = 8) animals receiving PBS. Representative images and quantitative evaluation of foot cutaneous blood flow (B), microangiography (C), and capillary density (D) of diabetic ischemic nude mice treated with or without PBS, uEPCs, control PB-MNCs, and diabetic PB-MNCs prestimulated or not with EFNB2 (n = 5–12 per group). The circled portions of images (B) show the area of laser Doppler blood flow measurement. The arrows show the location of arterial ligation in the microarteriograms (C) and capillaries in the histological sections (D). Cont, control; Diab, diabetic; NS, nonstimulated; Is, ischemic limb; N Is, nonischemic limb. *P < 0.05; **P < 0.01; ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)
Endogenous circulating stem/progenitor cell number in host animals receiving human PB-MNCs.
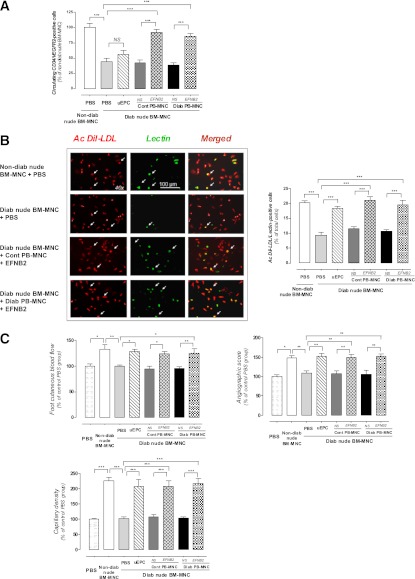
We next attempted to determine the cellular and molecular mechanisms involved in EFNB2-treated PB-MNC–related effects. At 14 days postischemia, we first determined the number of circulating vascular stem/progenitor cell numbers in nude mice treated with human PB-MNCs. The number of CD34+/VEGFR2+–circulating cells was significantly decreased by 56% in diabetic nude mice when compared with nondiabetic animals (CD34+/VEGFR2+: 0.0096 ± 0.0007% of total cells examined for nondiabetic animals, P < 0.001) (Fig. 7A). Administration of untreated human control and diabetic PB-MNCs, as well as human uEPCs, did not significantly affect the number of circulating vascular stem/progenitor cell in diabetic nude mice. In contrast, the number of CD34+/VEGFR2+–circulating MNCs almost returned to control levels in diabetic nude mice receiving EFNB2-stimulated PB-MNCs from healthy donors (92 ± 4%, P < 0.001) and from diabetic patients (86 ± 5%, P < 0.001) (Fig. 7A).
FIG. 7.
Host effects induced by transplantation of EFNB2-prestimulated PB-MNCs from diabetic patients. A: Quantitative evaluation of circulating CD34+/VEGFR2+ cell number of ischemic nude mice receiving PBS or diabetic ischemic nude mice treated with or without PBS, uEPCs, control PB-MNCs, and diabetic PB-MNCs prestimulated or not with EFNB2 (n = 5–12 per group). B: Representative images and quantitative assessment of BM-MNC differentiation into cells with endothelial phenotype. BM-MNCs were isolated from nondiabetic ischemic nude mice or diabetic ischemic nude mice treated with or without PBS, uEPCs, control PB-MNCs, and diabetic PB-MNCs prestimulated or not with EFNB2 (n = 5–12 per group). C: Quantitative evaluation of foot cutaneous blood flow, microangiography, and capillary density in ischemic C57Bl/6 mice transplanted with BM-MNCs extracted from nondiabetic ischemic nude mice or from diabetic ischemic nude mice treated with or without PBS, uEPCs, or nonpretreated or EFNB2-pretreated PB-MNCs from control and diabetic patients (n = 3–7 per group). Cont, control; Diab, diabetic; NS, nonstimulated; Ac, adherent cell. *P < 0.05; **P < 0.01; ***P < 0.001. (A high-quality digital representation of this figure is available in the online issue.)
Differentiation capacity of BM-MNCs derived from host animals transplanted with human PB-MNCs.
We then studied the effect of human PB-MNCs on the in vitro capacity of endogenous BM-MNCs derived from nude mice to differentiate into cells with endothelial phenotype. This differentiation potential was altered in diabetic mice (9.4 ± 1.1 vs. 20.2 ± 0.7% in nondiabetic nude, P < 0.001). The differentiation capacity of BM-MNCs of diabetic nude mice receiving nonstimulated control or diabetic PB-MNCs was similar to that of BM-MNCs isolated from PBS-receiving mice. Conversely, administration of EFNB2-stimulated PB-MNCs from healthy donors to diabetic animals significantly increased the differentiation capacity of endogenous BM-MNCs (P < 0.001) (Fig. 7B).
In vivo proangiogenic potential of BM-MNCs extracted from host animals treated with human PB-MNCs.
To evaluate the effects of transplanted human PB-MNCs on the in vivo proangiogenic potential of BM-MNCs from host animals, BM-MNCs were isolated from treated animals and intravenously injected into C57Bl6 mice with a hindlimb ischemia. BM-MNCs of nondiabetic mice significantly enhanced the postischemic neovascularization compared with PBS (133 ± 9, 149 ± 5, and 227 ± 11% vs. PBS group for foot skin blood flow, angiographic score, and capillary density, respectively, P < 0.001) (Fig. 7C). However, BM-MNCs isolated from diabetic mice treated with PBS or with nonstimulated control or diabetic human PB-MNCs did not significantly promote revascularization. It is interesting that the foot skin blood flow, angiographic score, and capillary density were increased by 25 ± 8, 52 ± 6, and 127 ± 11%, respectively, in C57Bl6 mice injected with BM-MNCs isolated from diabetic mice transplanted with EFNB2-treated human diabetic PB-MNCs (P < 0.001) (Fig. 7C). In a similar manner, uEPCs and EFNB2-treated human control PB-MNCs totally restored the in vivo proangiogenic potential of BM-MNCs isolated from diabetic animals (P < 0.001).
DISCUSSION
Our major finding is that acute EFNB2 treatment of human diabetic PB-MNCs potentiates their in vitro and in vivo proangiogenic capacity. Incubation with EFNB2 increases adhesion, transmigration, paracrine activity, and proangiogenic properties of human diabetic PB-MNCs. After administration, activated human diabetic PB-MNCs also enhance the endogenous BM-MNC differentiation ability and mobilization/homing of vascular stem/progenitor cells in diabetic host animals.
An accessible and safe source of stem/progenitor cells is a prerequisite for the future development of efficient cell-based therapeutic strategies able to limit the important burden of the disease. A significant advantage of PB-MNCs over BM-MNCs and uEPCs is the relative ease of isolation and preparation procedures. Therefore, we aimed to develop a procedure enabling enhancement of the proangiogenic potential of human PB-MNCs from diabetic patients. In accordance with previous studies (32,33), the expression of EphB2 and EphB4 in human diabetic PB-MNCs suggests that EFNB2 could interact with human diabetic PB-MNCs. In this line, we demonstrated that EFNB2 binds to monocytes, lymphocytes, and hematopoietic stem/progenitor cells within the PB-MNC subpopulation. It has been reported that EFNB2 activates inflammatory cells, including monocytes and lymphocytes (32,33), which play a crucial role in the modulation of postischemic revascularization (36,37). Thus, EFNB2 could activate the human diabetic PB-MNCs and subsequently modulate the postischemic vascular neogenesis.
In most clinical settings, the natural adaptive responses to a compromised perfusion are insufficient to block the progression of ischemic diseases since certain cardiovascular risk factors, including diabetes, adversely affect postnatal vasculogenesis and revascularization (12,35,38,39). Our present results confirm that diabetes alters the process of postischemic angiogenesis in nude mice (12,40). More interesting, we demonstrated that EFNB2-stimulated PB-MNCs from diabetic patients could also restore vascular network formation in ischemic hindlimb of diabetic animals. This proangiogenic effect is comparable with that of human uEPCs, suggesting that EFNB2 treatment of diabetic PB-MNCs could be an effective proangiogenic therapeutic strategy in the treatment of ischemic disease associated with diabetes. We previously reported that the activation of the EphB4/EFNB2 system further enhanced uEPC proangiogenic potential in nondiabetic nude mice. Indeed, EphB4 activation enhanced P selectin glycoprotein ligand-1 expression and improved adhesion and incorporation of uEPCs (23). In the current study, we extended these previous findings and revealed that the EFNB2 pretreatment dose-dependently raised adhesion of diabetic PB-MNCs to the HUVEC monolayer through the PI 3-kinase signaling pathways. In addition, the transmigration of diabetic PB-MNCs was markedly enhanced by EFNB2. These results are also in agreement with previous studies showing the preferential adhesion of monocytes to endothelial cells overexpressing EFNB2 and the involvement of EFNB2 in monocyte transmigration through interaction of EFNB2 with EphB4 (32,33). Because EphB2 and EphB4 are expressed in diabetic PB-MNCs, EFNB2 may likely activate PB-MNCs through these two receptors. Further experiments are needed to confirm this hypothesis.
We next found that acute EFNB2 pretreatment significantly raised the gene expression of proinflammatory/proangiogenic factors, including IL-1, IL-6, and monocyte chemotactic protein-1 in human diabetic PB-MNCs (20,34,41). In addition, EFNB2 pretreatment increased the ability of PB-MNCs to incorporate into HUVEC-formed capillary-like tubes. It is interesting that cell adhesion, cell transmigration, proinflammatory/proangiogenic factor secretion, endothelial tube formation, and activation of PI 3-kinase–dependent signaling are essential for postischemic vascular neogenesis (42). We also showed that at 14 days postischemia, human DNA could be detected in >80% of ischemic muscle samples of animals receiving EFNB2-stimulated human diabetic PB-MNCs, suggesting that activated diabetic PB-MNCs could infiltrate into the ischemic tissues.
Finally, a previous study indicates that after human uEPC transplantation, BM-derived stem or progenitor cells were increased in the peripheral circulation and incorporated into the site of neovascularization and myocardial repair (41), indicating that exogenous stem/progenitor cell administration induces humoral effects, which are sustained by host tissues and play a crucial role in repairing tissue injury. Therefore, we assessed endogenous BM-MNC differentiation capacity and the numbers of circulating vascular stem/progenitor cells in host animals treated with EFNB2–PB-MNC. The differentiation capacity of BM-MNCs into cells with endothelial phenotype was significantly impaired, and the number of circulating CD34+ and VEGFR2+ cells were hampered in patients with diabetes (15,16). Of note, EFNB2-treated human diabetic PB-MNC and human uEPC administration restored the differentiation potential of BM-MNCs from diabetic mice into cells with endothelial phenotype. In addition, EFNB2-activated human diabetic PB-MNCs raised the circulating levels of CD34+/VEGFR2+ MNCs. Moreover, BM-MNCs isolated from nude diabetic mice receiving stimulated human diabetic PB-MNCs or uEPCs had a more pronounced in vivo effect on postischemic revascularization than that of BM-MNCs isolated from animals treated with nonstimulated diabetic PB-MNCs or PBS. Therefore, part of the beneficial effects of EFNB2-pretreated diabetic PB-MNCs can be attributed primarily to host cells. Indeed, transplantation of stimulated PB-MNCs further mobilizes endogenous BM-derived stem and progenitor cells into peripheral circulation, recruiting them into the ischemic limb and thereby providing an additional favorable milieu for neovascularization and repair or regeneration of ischemic tissues.
Taken together, our results suggest that EFNB2 treatment counteracts diabetes-induced stem/progenitor cell dysfunctions and opens the way for the clinical development of an innovative and very accessible strategy based on the intravenous autologous transplantation of PB-MNCs in patients with critical limb ischemia.
ACKNOWLEDGMENTS
This study was supported by generous financial support from the French Blood and Vessels Institute, Société pour le Développement de la Recherche Cardiovasculaire, and Naturalia & Biologia, Paris, France. D.B.-Y. was supported by a grant from the Ile-de-France CODDIM program. J.-S.S. was a recipient of a Contrat d’Interface from Assistance Publique-Hôpitaux de Paris.
No potential conflicts of interest relevant to this article were reported.
D.B.-Y. and B.I.L. conducted experimental work, analyzed data, and wrote the manuscript. C.L.-D. and T.M.-R. researched and analyzed data and wrote the manuscript. C.S.M., D.A., P.H., J.-O.C., Y.W., J.V., M.V., J.-J.M., and P.-J.G. contributed to experimental work and reviewed the manuscript. J.-S.S. contributed to the design of the experiment and discussion and reviewed the manuscript. B.I.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the American Heart Association 2011 Scientific Sessions, Orlando, Florida, 12–16 November 2011.
The authors would like to thank Coralie Guerin, Paris Cardiovascular Research Center (INSERM U970), for her expert technical help. The authors are grateful to the Department of Internal Medicine B and the Department of Maternity of Lariboisière Hospital, the Etablissement Français du Sang, and the Department of Internal Medicine and Arterial Hypertension of Avicenne Hospital for providing blood samples.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1768/-/DC1.
REFERENCES
- 1.Criqui MH, Denenberg JO, Langer RD, Fronek A. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc Med 1997;2:221–226 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317–1324 [DOI] [PubMed] [Google Scholar]
- 3.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost 2010;103:696–709 [DOI] [PubMed] [Google Scholar]
- 4.Adam DJ, Beard JD, Cleveland T, et al. BASIL trial participants Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925–1934 [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT. Critical limb ischemia and stem cell research: anchoring hope with informed adverse event reporting. Circulation 2006;114:2581–2583 [DOI] [PubMed] [Google Scholar]
- 6.Al-Delaimy WK, Merchant AT, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med 2004;116:236–240 [DOI] [PubMed] [Google Scholar]
- 7.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757–1763 [DOI] [PubMed] [Google Scholar]
- 9.Hueb W, Gersh BJ, Costa F, et al. Impact of diabetes on five-year outcomes of patients with multivessel coronary artery disease. Ann Thorac Surg 2007;83:93–99 [DOI] [PubMed] [Google Scholar]
- 10.Levy BI, Schiffrin EL, Mourad JJ, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008;118:968–976 [DOI] [PubMed] [Google Scholar]
- 11.Lévy BI. Commentary on viewpoint: the human cutaneous circulation as a model of generalized microvascular function [comment on: J Appl Physiol 2008;105:370–372]. J Appl Physiol 2008;105:380–; author reply 389. [DOI] [PubMed] [Google Scholar]
- 12.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res 2007;101:948–956 [DOI] [PubMed] [Google Scholar]
- 13.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol 1999;154:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roguin A, Nitecki S, Rubinstein I, et al. Vascular endothelial growth factor (VEGF) fails to improve blood flow and to promote collateralization in a diabetic mouse ischemic hindlimb model. Cardiovasc Diabetol 2003;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 16.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–2786 [DOI] [PubMed] [Google Scholar]
- 17.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res 2003;93:1159–1169 [DOI] [PubMed] [Google Scholar]
- 18.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 19.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature 2008;451:937–942 [DOI] [PubMed] [Google Scholar]
- 20.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004;109:1543–1549 [DOI] [PubMed] [Google Scholar]
- 21.van der Bogt KE, Sheikh AY, Schrepfer S, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation 2008;118(Suppl.):S121–S129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aranguren XL, McCue JD, Hendrickx B, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest 2008;118:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foubert P, Silvestre JS, Souttou B, et al. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest 2007;117:1527–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pesce M, Orlandi A, Iachininoto MG, et al. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res 2003;93:e51–e62 [DOI] [PubMed] [Google Scholar]
- 25.Yamahara K, Sone M, Itoh H, et al. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells.PLoS One 2008;3:e1666. Erratum in PLoS ONE 2009;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reffelmann T, Könemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol 2009;53:305–308 [DOI] [PubMed] [Google Scholar]
- 27.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002;360:427–435 [DOI] [PubMed] [Google Scholar]
- 28.Horie T, Onodera R, Akamastu M, et al. Japan Study Group of Peripheral Vascular Regeneration Cell Therapy (JPRCT) Long-term clinical outcomes for patients with lower limb ischemia implanted with G-CSF-mobilized autologous peripheral blood mononuclear cells. Atherosclerosis 2010;208:461–466 [DOI] [PubMed] [Google Scholar]
- 29.Onodera R, Teramukai S, Tanaka S, et al. BMMNC Follow-Up Study Investigators. M-PBMNC Follow-Up Study Investigators Bone marrow mononuclear cells versus G-CSF-mobilized peripheral blood mononuclear cells for treatment of lower limb ASO: pooled analysis for long-term prognosis. Bone Marrow Transplant 2011;46:278–284 [DOI] [PubMed] [Google Scholar]
- 30.Ozturk A, Kucukardali Y, Tangi F, et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J Diabetes Complications 2012;26:29–33 [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhang N, Li M, et al. Therapeutic angiogenesis of bone marrow mononuclear cells (MNCs) and peripheral blood MNCs: transplantation for ischemic hindlimb. Ann Vasc Surg 2008;22:238–247 [DOI] [PubMed] [Google Scholar]
- 32.Pfaff D, Héroult M, Riedel M, et al. Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J Cell Sci 2008;121:3842–3850 [DOI] [PubMed] [Google Scholar]
- 33.Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol 2003;171:106–114 [DOI] [PubMed] [Google Scholar]
- 34.You D, Waeckel L, Ebrahimian TG, et al. Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation 2006;114:328–338 [DOI] [PubMed] [Google Scholar]
- 35.You D, Cochain C, Loinard C, et al. Hypertension impairs postnatal vasculogenesis: role of antihypertensive agents. Hypertension 2008;51:1537–1544 [DOI] [PubMed] [Google Scholar]
- 36.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest 1998;101:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zouggari Y, Ait-Oufella H, Waeckel L, et al. Regulatory T cells modulate postischemic neovascularization [corrected in: Circulation 2009;121:e31]. Circulation 2009;120:1415–1425 [DOI] [PubMed] [Google Scholar]
- 38.Robich MP, Osipov RM, Nezafat R, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation 2010;122(Suppl. 11):S142–S149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 2004;110:1148–1155 [DOI] [PubMed] [Google Scholar]
- 40.Ebrahimian TG, Heymes C, You D, et al. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 2006;169:719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med 2007;204:3257–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res 2000;86:4–5 [DOI] [PubMed] [Google Scholar]