Abstract
The activation of glucose-6-phosphatase (G6Pase), a key enzyme of endogenous glucose production, is correlated with type 2 diabetes. Type 2 diabetes is characterized by sustained hyperglycemia leading to glucotoxicity. We investigated whether glucotoxicity mechanisms control the expression of the G6Pase catalytic unit (G6pc). We deciphered the transcriptional regulatory mechanisms of the G6pc promoter by glucotoxicity in a hepatoma cell line then in primary hepatocytes and in the liver of diabetic mice. High glucose exposure induced the production of reactive oxygen species (ROS) and, in parallel, induced G6pc promoter activity. In hepatocytes, glucose induced G6pc gene expression and glucose release. The decrease of ROS concentrations by antioxidants eliminated all the glucose-inductive effects. The induction of G6pc promoter activity by glucose was eliminated in the presence of small interfering RNA, targeting either the hypoxia-inducible factor (HIF)-1α or the CREB–binding protein (CBP). Glucose increased the interaction of HIF-1α with CBP and the recruitment of HIF-1 on the G6pc promoter. The same mechanism might occur in hyperglycemic mice. We deciphered a new regulatory mechanism induced by glucotoxicity. This mechanism leading to the induction of HIF-1 transcriptional activity may contribute to the increase of hepatic glucose production during type 2 diabetes.
Unrestrained hepatic glucose production (HGP) is a contributing factor to the postabsorptive hyperglycemia characteristic of type 2 diabetes in animals (1,2) and humans (3). In contrast, a decrease of HGP has been shown to improve overall glycemic control (4,5). The key enzyme of HGP is the glucose-6-phosphatase (G6Pase) complex, which is composed of a ubiquitous transporter of glucose-6-phosphate (G6PT) and a catalytic unit (G6PC) (6). G6PT is ubiquitously expressed, whereas G6PC only is expressed in the liver, kidney, and small intestine and confers on these tissues the capacity to release glucose into the blood (7).
G6pc overexpression in rat liver is sufficient to trigger hepatic and peripheral deregulations associated with diabetes (8). Consequently, G6pc regulation has been studied in view to targeting HGP to prevent or at least reduce hyperglycemia. G6pc gene expression is paradoxically increased by hyperglycemia in vivo (independently of the effect of insulin) (9) and high concentrations of glucose in vitro (10,11). Glucose controls G6pc gene expression both by inducting its transcription and by stabilizing its mRNA (12). Molecular mechanisms depend on intracellular glucose metabolites (12). Some of them (G6P and fructose 2,6-biphosphate) have been shown to induce G6pc gene expression by inducting the binding of carbohydrate response element–binding protein (ChREBP) to a distal region of the G6pc promoter (13). Moreover, O-glycosylation of Foxo-1 and CRTC2 have been implicated in the transcriptional regulation of the G6pc gene by hyperglycemia (14,15).
Intracellular glucose metabolism during hyperglycemia produces reactive oxygen species (ROS). ROS are the common inducers of five major pathways responsible for causing the major microvascular and cardiovascular complications associated with type 2 diabetes (16). ROS also prevent the degradation of hypoxia-inducible factor (HIF)-1α (17,18). HIF-1 is a key transcriptional mediator of the hypoxic response in eukaryotic cells, regulating the expression of genes involved in oxygen transport, glucose uptake, glycolysis, and angiogenesis (19). HIF-1 is a dimeric protein complex, composed of a constitutively stable subunit, HIF-1β (also called the aryl hydrocarbon nuclear translocator; ARNT) and of HIF-1α, whose transcriptional activity is sensitive to O2 levels. HIF-1α transcriptional activity is regulated by two O2-sensitive hydroxylases. In normoxia, prolyl-4-hydroxylase (PHD) provokes the prolyl-hydroxylation of HIF-1α, which leads to proteosomal targeting and degradation of the transcription factor (20). In parallel, the asparaginyl hydroxylase FIH-1 (factor inhibiting HIF-1) prevents the interaction of the COOH-terminal part of HIF-1α with coactivators (21). Even a small decrease in the O2 concentration inhibits PHDs and FIH, such that HIF-1α escapes degradation and heterodimerizes with HIF-1β and is thus able to recruit coactivators (20,22).
In view of these various findings, a possible mechanism of gene regulation by glucose is the induction of HIF-1α transcriptional activity by ROS induced by high glucose levels. Here, we report an investigation of whether the regulation of G6pc transcription by glucose depends on ROS production and is mediated by HIF-1α.
RESEARCH DESIGN AND METHODS
Reporter plasmids, expression vectors, and antibodies.
The −320/+60 rat G6pc promoter construct used has been described previously (23). The pRc/CMV-hHIF1α expression vector encoding the human HIF-1α protein was a generous gift from R. Wenger (24), and the pSVSport-ARNT expression vector encoding the human ARNT protein was a generous gift from V. Carrière. The pcDNA3-EGFP-Rac1-Q61 L expression vector encoding the human Rac1-Q61 L protein was a gift from G. Bokoch (plasmid 12981; Addgene, Cambridge, MA). The plasmids used for transfection were purified using NucleoBond Xtra columns (Macherey-Nagel, Hoerd, France).
The following commercially available antibodies were used for chromatin immunoprecipitation (ChIP) assay or Western blot assays: β-actin (clone AC-74; Sigma-Aldrich, St. Quentin Fallavier, France), CBP (Santa Cruz Biotechnology, Heidelberg, Germany), and HIF-1α (clone 1Hα67; Novus Biological, Cambridge, U.K.).
Cell culture and transfection.
All cell culture products were purchased from Invitrogen (Cergy Pontoise, France). HepG2 human hepatoma cells (ECACC 85011430) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 6% fetal bovine serum and 5 mmol/L glutamine, streptomycin (1 μg/mL), and penicillin (1 units/mL) at 37°C in a humidified 5% CO2/95% air atmosphere. For transient transfection of HepG2 cells, 200,000 cells were plated out in 35-mm wells in six-well cell culture plates. The complete medium was replaced the next day. One hour later, the cells were transfected as previously described (25) using ExGen500 (Euromedex, Souffelweyersheim, France), with 1 μg G6PC-B plasmid, 1 ng pCMV-RL (Promega) to correct for transfection efficiency, and 100 ng of the appropriate expression vector. The total amount of DNA (2 μg) was kept constant by addition of pBluescript SK+ plasmid. The small interfering RNA (siRNA) used were CBP (5′CGGCACAGCCUCUCAGUCAdTdT-3′) and HIF-1α (L-004018–00–0005; Dharmacon), whereas Alexa 488-siRNA (Invitrogen) was used as a control. The siRNA were transfected in HepG2 cells with the Lipofectamin RNAiMAX system, as described by the manufacturer (Invitrogen). Twenty-four hours after transfection, the medium was replaced with DMEM containing 5 or 25 mmol/L glucose without serum and containing antioxidant as indicated. The cultures were incubated for 16–18 h at 37°C. Renilla luciferase and firefly luciferase activities were determined as previously described (25). The Mann-Whitney test for unpaired data and the Kruskal-Wallis test were used for statistical analyses.
Animals.
The animals were housed in the animal facility of Lyon 1 University (Animaleries Lyon Est Conventionnelle and Specific Pathogen Free) in controlled-temperature (22°C) conditions, with a 12-h light/12-h dark cycle. Rat and mice had free access to water and standard rodent chow diet (Safe, Augy, France). A high-fat high-sucrose (HFHS) diet (containing 36% fat and 17% sucrose) was produced by the Unité de Préparation des Aliments Expérimentaux (Institut National de la Recherche Agronomique, Jouy-en Josas, France) and administered to the mice for 16 weeks, as previously described (26). All the procedures were performed in accordance with the principles and guidelines established by the European Convention for the Protection of Laboratory Animals. All the experiments were approved by the regional animal care committee (CREEA [Comités Régionaux d’Ethique en matière d’Expérimentaion Animale] CNRS, Rhône-Alpes Auvergne, France).
Primary culture of hepatocytes.
Hepatocytes were isolated from the livers of fed rats as previously described (27). In brief, rat livers were perfused with Hank’s balanced salt solution (5.4 mmol/L KCl, 0.45 mmol/L KH2PO4, 138 mmol/L NaCl, 4.2 mmol/L NaHCO3, 0.34 mmol/L Na2HPO4, 5.5 mmol/L glucose, 50 mmol/L HEPES, and 0.5 mmol/L EGTA, pH 7.4). The livers were washed at a rate of 5 mL/min using the portal vein, and collagenase (0.025%) was added after 5 min. Cell viability was assessed with the trypan blue exclusion test and was always higher than 80%. Hepatocytes were seeded at a density of 2 × 106 cells per well in six-well cell culture plates in medium M199 with Earle salts (Invitrogen), supplemented with 10 μg/mL streptomycin, 100 units/mL penicillin, 2.4 mmol/L glutamine, and 1% fetal bovine serum (Invitrogen). After cell attachment (6 h), the hepatocytes were then incubated for 16–18 h at 37°C in DMEM (in the presence of 5 or 25 mmol/L glucose) without serum. Hepatocytes were incubated for 4 h at 37°C in DMEM without glucose, but with 1 mmol/L pyruvate and 10 mmol/L lactate. The glucose content of the supernatant was measured by the glucose oxidase method.
Determination of intracellular ROS generation.
Intracellular ROS generation was assessed using 6-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, diacetoxymethylester (H2-DCFDA) as a probe (Invitrogen) (28). The probe was hydroxylated to 6-carboxy-2’,7’-dichlorodihydrofluorescein (H2-DCF) after entering the cells, serving as a fluorescent redox-sensitive dye. ROS products (H2O2 and other peroxides) cause oxidation of H2-DCF, yielding the fluorescent product DCF. In our study, after 16 h in 5 and in 25 mmol/L glucose medium, cells were incubated with 2 μmol/L H2-DCFDA for 30 min and washed twice with PBS. The cells then were resuspended in water to disrupt cell membranes, and fluorescence (excitation 493 nm, emission 527 nm) and protein content were measured. The intensity of fluorescence is expressed in arbitrary units per milligram of protein.
Western blot.
Whole-cell extracts were lysed by incubation in a denaturating buffer at 4°C for 20 min (50 mmol/L HEPES, 150 mmol/L NaCl, 10 mmol/L EDTA, 10 mmol/L Na4P2O7, 100 mmol/L NaF, 2 mmol/L vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 g/mL aprotinin, 10 g/mL leupeptin, and 1% Triton, pH 7). Aliquots of 40 μg of the resulting whole-cell extracts were separated by 6% SDS-PAGE and transferred to polyvinylidene fluoride immobilon membranes (Millipore, St.-Quentin en Yveline, France). The membranes were probed with anti–HIF-1α antibodies (1:1,000) in TBS/0.2% Tween /5% milk or with anti-CBP antibodies (1:1,000) in TBS/0.2% Tween /2% milk and with goat secondary anti-mouse IgG linked to peroxidase (Biorad, Marnes-la-Coquette, France). Bound antibodies were revealed using a specichrom-chemiluminescence system with enhanced chemiluminescent hyperfilms (Amersham, Saclay, France). Membranes were stripped with Reblot plus strong solution (Millipore) and probed for β-actin antibodies as controls.
Immunoprecipitation assay.
HepG2 cells and primary rat hepatocytes were lysed in a nondenaturating lysis buffer (50 mmol/L Tris, pH 7.4; 300 mmol/L NaCl; 5 mmol/L EDTA; and 1% Triton) supplemented with a protease inhibitor cocktail (Sigma-Aldrich). A total of 3 mg protein from whole-cell extracts were precleared with 15 μL of a solution of protein A-sepharose (6 mg/mL PBS; Sigma-Aldrich) supplemented with 0.05% (wt/vol) bovine serum albumin for 30 min at 4°C on a rotating wheel. Protein complexes were immunoprecipitated for 16–18 h at 4°C while rotating with 2 μg of CBP antibody, or β-actin antibody as a negative control, in the presence of 15 μL of a solution of protein A-sepharose and 0.2% (wt/vol) bovine serum albumin. The samples were centrifuged at 14,000g for 30 s at 4°C, and the pelleted beads were washed three times for 5 min at 4°C with washing buffer (50 mmol/L Tris, pH 7.4; 300 mmol/L NaCl; 5 mmol/L EDTA; and 0.1% Triton) supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and once with PBS. Immunoprecipitated proteins were eluted from beads by denaturation and analyzed by Western blot.
mRNA extraction and quantification.
Total RNA was isolated from primary rat hepatocytes after treatment using Qiagen RNeasy columns (Qiagen, Courtaboeuf, France). Reverse transcription and real-time PCR were performed as previously described (29). The rat ribosomal gene L19 was used as a reference. The sequences of the specific primers used are available on request.
ChIP.
We performed immunoprecipitation of chromatin from FAO cells to study the endogenous rat G6pc promoter instead of chromatin from HepG2 cells, which contain the human promoter. After treating rat hepatoma FAO cells and primary rat hepatocytes for 16 h in culture medium containing 5 or 25 mmol/L glucose, the cells were fixed in 1% formaldehyde in PBS at room temperature for 5 min. Fixation was stopped by the addition of glycine directly to the medium to a final concentration of 125 mmol/L and further incubation of 10 min. Approximately 0.5 g mouse liver was sampled and fixed as previously described (30). Sheared chromatin fragments of 200–500 bp were prepared as previously described using the enzymatic shearing kit (Active Motif, Rixensart, Belgium) (30). Each immunoprecipitation was performed with ~50 μg chromatin as previously described (30). PCR amplification was performed using primers specific for the −174/+44 bp region of G6pc (forward: 5′TTTGCTATTTTACGTAAATCACCCT-3′; reverse: 5′-GTACCTCAGGAAGCTGCCA-3′) or for the −3,056/-3,108 bp region of Glut1 (31) (forward: 5′-ATTTCTAGGGCCTTGGGTCC-3′; reverse: 5′CCGGCCTGATGCGTGTCA-3′).
RESULTS
The induction of G6pc promoter activity by glucose depends on ROS.
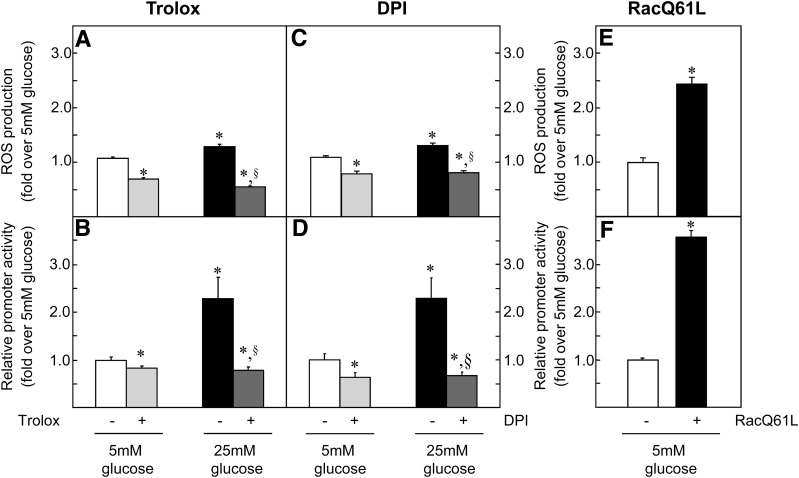
Glucose regulation of G6pc expression depends on glucose metabolism (12). As glucose metabolism leads to ROS production (16), we investigated the involvement of ROS in the induction of G6pc promoter activity. ROS can be produced by mitochondrial enzyme complexes and nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase). Trolox (an analog of vitamin E) was used as a potent antioxidant to decrease ROS concentrations regardless of the production site. Diphenyleneiodonium chloride was used to specifically decrease ROS production by NADPH oxidase. High glucose exposure increased ROS concentrations by 20% (Fig. 1A and C), whereas the inclusion of both antioxidants in the culture medium was sufficient to completely eliminate this induction (Fig. 1A and C). In parallel, high glucose exposure induced G6pc promoter activity twofold (Fig. 1B and D), whereas both antioxidants completely eliminated induction by glucose (Fig. 1B and D). Antioxidants also decreased both ROS concentrations and G6pc promoter activity under low-glucose conditions (Fig. 1A–D). These results suggest that glucose could induce G6pc promoter activity by the generation of ROS. The decrease of ROS concentrations by diphenyleneiodonium chloride (Fig. 1B) suggested that ROS were produced by NADPH oxidase. NADPH oxidase activation occurs via the assembly of the cytosolic regulatory proteins p47phox, p67phox, and Rac with the membrane-associated flavocytochrome b558 (cyt b558) (32). The overexpression of a constitutively active form of Rac (RacQ61 L) stabilizes NADPH oxidase (33) and increases ROS production in HT29 cells (34). We therefore used this mutant protein to mimic an increase in ROS production by NADPH oxidase. In HepG2 cells, the overexpression of RacQ61 L induced ROS concentrations 2.5-fold (Fig. 1E) and G6pc promoter activity 3-fold (Fig. 1F). In brief, our results strongly implicate ROS in the regulation of G6pc promoter activity by glucose.
FIG. 1.
Glucose induces G6pc promoter activity via induction of ROS. Transfected HepG2 cells were treated for 16–18 h with 5 mmol/L (mM) (□) and 25 mmol/L (■) glucose. Where indicated, cells were treated with 10−4 mol/L Trolox (A and C, gray bars) and 5 μmol/L dpi (B and D, gray bars). A and B: Induction of the production of ROS by glucose in HepG2 cells. The amount of ROS in the presence of 5 mmol/L glucose was defined as 1. C and D: Relative promoter activity of the −320/+60 bp fragment of the rat G6pc promoter in transiently transfected HepG2 cells. Promoter activity in the presence of 5 mmol/L glucose was arbitrarily defined as 1. E and F: HepG2 ROS levels (E) and relative promoter activity of the −320/+60 bp fragment of the rat G6pc promoter in transiently transfected HepG2 cells (F) in the absence (□) and in the presence (■) of RacQ61 L. Reported values are means of three experiments performed in duplicate ± SEM. *Significantly different from the values obtained in the presence of 5 mmol/L glucose (P < 0.01). §Significantly different from the values obtained in the presence of 25 mmol/L glucose (P < 0.01).
HIF-1α is involved in the induction of the G6pc promoter activity by glucose.
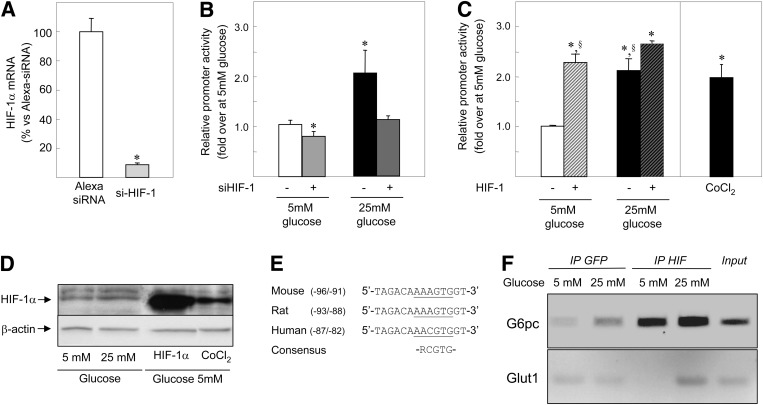
ROS and glucose are involved in the stabilization of HIF-1α protein during hypoxia (35,36). Moreover, cytosolic ROS produced by NADPH oxidase are crucial in regulating the HIF-dependent pathway under nonhypoxic stimuli (37). The transfection of HIF-1α siRNA resulted in a strong reduction of HIF-1α mRNA levels (Fig. 2A) and completely eliminated the induction of G6pc promoter activity by glucose (Fig. 2B). The transfection of HIF-1α siRNA also decreased G6pc promoter activity by 20% under low-glucose conditions (Fig. 2B). These results show that HIF-1α is involved in G6pc transcriptional regulation by glucose. We tested whether HIF-1α alone was sufficient to induce G6pc promoter activity by increasing HIF-1α protein concentration either by HIF-1α overexpression or by chemical hypoxia (CoCl2 treatment). These conditions did indeed increase HIF-1α protein abundance in HepG2 cells (Fig. 2D). Endogenous HIF-1β availability might be a limiting step in HIF-1 transcriptional activity. To prevent this eventuality, cells were transfected with both HIF-1α and HIF-1β expression vectors (HIF-1 dimer). Overproduction of HIF-1β alone had no effect on G6pc promoter activity (data not shown). Treatment with 25 mmol/L glucose, or with CoCl2, or the overexpression of both HIF-1 subunits induced G6pc promoter activity to the same extent (~2- to 2.5-fold) (Fig. 2C). Finally, we assessed whether HIF-1 controls G6pc transcription by binding on its promoter. The proximal region of the rat G6pc promoter exhibited a putative HIF-1 responsive element (HRE) with a high degree of sequence conservation among species (Fig. 2E). ChIP assays demonstrated the binding of HIF-1α to the −174/+44 region of the endogenous G6pc promoter regardless of glucose concentrations (Fig. 2E). In addition, high glucose exposure induced HIF-1α binding to the HRE of the known HIF-1 target Glut1 (Fig. 2F). In brief, the results presented in Fig. 2 suggest that the induction of HIF-1 transcriptional activity by glucose might be a general mechanism of gene regulation in hepatoma cells.
FIG. 2.
HIF-1 transcriptional activity is involved in the induction of G6pc promoter activity by glucose. A: HIF-1α mRNA levels were analyzed from HepG2 cells transfected with an Alexa 488-siRNA (□) as a control and siRNA targeting HIF-1α (gray bar). The amount of mRNA in the presence of control siRNA was arbitrarily defined as 100. Reported values are the means of three experiments performed in duplicate ± SEM. *Significantly different from the 5 mmol/L (mM) glucose condition (P < 0.01). B: HepG2 cells were transiently transfected with the −320/+60B construct and with control siRNA in the presence of 5 mmol/L glucose (□), in the presence of 25 mmol/L glucose (■), with siHIF-1α in the presence of 5 mmol/L glucose (light gray bar), and in the presence of 25 mmol/L glucose (dark gray bar). Promoter activity in the presence of 5 mmol/L glucose was defined as 1. Reported values are means of three experiments performed in duplicate ± SEM. *Significantly different from the 5 mmol/L glucose condition (P < 0.01). C: HepG2 cells were transiently transfected with the −320/+60B construct in the presence of 5 mmol/L glucose (□), in the presence of 25 mmol/L glucose and 200 μmol/L CoCl2 (■) and in the presence of plasmids expressing the HIF-1 dimer proteins as indicated (▨). Promoter activity in the presence of 5 mmol/L glucose was defined as 1. Reported values are means of three experiments performed in duplicate ± SEM. *Significantly different from the 5 mmol/L glucose condition (P < 0.01). §Significantly different from the 25 mmol/L glucose + HIF-1 condition (P < 0.01). D: HIF-1α expression was analyzed by Western blotting with whole-cell extracts from HepG2 cells treated for 16–18 h with 5 or 25 mmol/L glucose and treated for 2 h with 5 mmol/L glucose and 200 μmol/L CoCl2 and from HepG2 cells transfected with a plasmid expressing HIF-1α. Total protein content was assessed by probing for β-tubulin. E: Sequences of the proximal promoter region of the mouse, rat, and human G6pc promoters and the consensus HRE were aligned using ClustalW2. Underlined text corresponds to the putative HRE. F: Chromatin from FAO cells treated for 16 h with 5 or 25 mmol/L glucose were immunoprecipitated with antibodies against HIF-1α and against GFP as a control. The images show representative electrophoresis of PCR fragments amplified from immunoprecipitated chromatin using primer pairs specific to the G6pc promoter (first lane) and to the Glut1 promoter (second lane). The input lane shows the result with samples not subjected to immunoprecipitation.
ROS produced by glucose induce HIF-1α interaction with CBP.
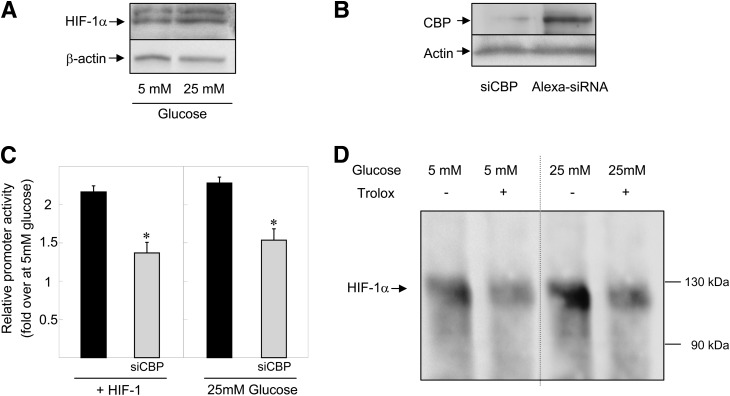
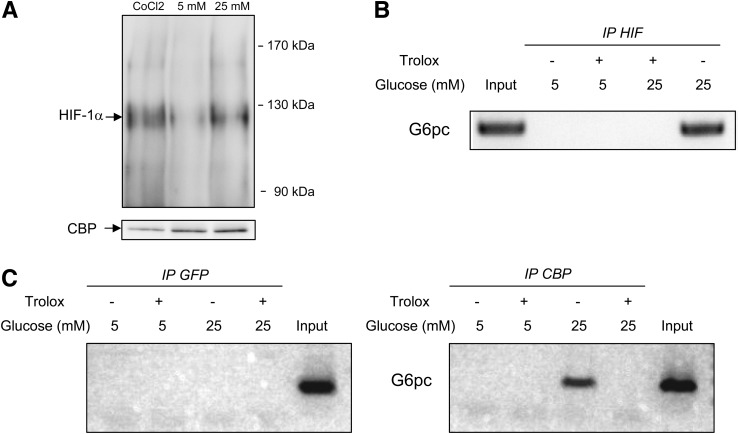
The transcriptional activity of HIF-1α depends on the stabilization of the protein and on its capacity to interact with cofactors, such as CBP (38). High glucose exposure did not increase HIF-1α protein abundance (Fig. 3A), whereas it had an additive effect with HIF-1 on G6pc promoter activity (Fig. 2C). These results suggest that glucose did not increase HIF-1 transcriptional activity by inducing HIF-1α protein stabilization. We then studied whether CBP was involved in the induction of G6pc promoter activity by binding to HIF-1α after high exposure to glucose. First, the decrease of CBP protein production by siRNA (Fig. 3B) substantially decreased induction of G6pc promoter activity (Fig. 3C). Second, immunoprecipitation of lysates from cells overexpressing HIF-1α with CBP antibodies revealed that high exposure to glucose increased the amount of HIF-1α associated with CBP (Fig. 3D, line 3). The inclusion of Trolox in the culture medium completely arrested induction by glucose (Fig. 3D, line 4). Trolox also decreased the interaction between HIF-1α and CBP under low-glucose conditions (Fig. 3D, line 2). In summary, the results presented in Fig. 3 suggest that the increase in the interaction of HIF-1α with CBP induced by ROS was responsible for the induction of HIF-1 transcriptional activity by glucose.
FIG. 3.
CBP is involved in the induction of G6pc promoter activity by ROS through its interaction with HIF-1α. A: HIF-1α expression was analyzed by Western blotting with whole-cell extracts from HepG2 cells treated for 16–18 h with 5 or 25 mmol/L (mM) glucose. Total protein content was assessed by probing for β-actin. B: CBP expression was analyzed by Western blotting using whole-cell extracts from HepG2 cells transfected with siRNA targeting CBP or with control siRNA. Total protein content was assessed by probing for β-actin. C: HepG2 cells were transiently transfected with the −320/+60B construct in the presence of 25 mmol/L glucose or with plasmids expressing the HIF-1 dimer proteins and in the presence of control siRNA (■) and siCBP (gray bars). Promoter activity in the presence of 5 mmol/L glucose was arbitrarily defined as 1. Reported values are means of three experiments performed in duplicate ± SEM. *Significantly different from the Alexa 488-siRNA condition (P < 0.01). D: CBP protein was immunoprecipitated from whole-cell extracts from HepG2 cells transfected with a plasmid expressing HIF-1α and treated for 16–18 h with 5 mmol/L (first lane), 5 mmol/L glucose and 10−4 mol/L Trolox (second lane), 25 mmol/L glucose (third lane), and 25 mmol/L glucose and 10−4 mol/L Trolox (fourth lane). The image shows a representative Western blot probed for HIF-1α proteins associated with CBP.
Glucose induces G6pc expression by a mechanism dependent on ROS and HIF-1 in primary hepatocytes.
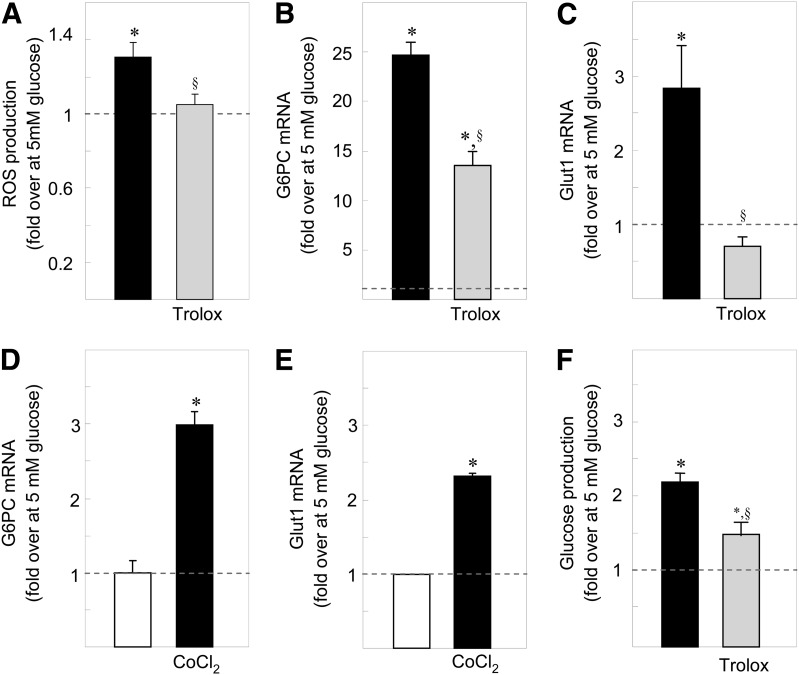
In primary rat hepatocytes, high glucose exposure increased ROS concentrations (Fig. 4A) and G6pc and Glut1 mRNA levels (Fig. 4B and C). The presence of antioxidant prevented both the production of ROS and the induction of Glut1 mRNA levels by glucose (Fig. 4A and C) and decreased G6pc mRNA levels by 50% (Fig. 4B). As in HepG2 cells, chemical hypoxia also induced G6pc and Glut1 mRNA levels (Fig. 4D and E). The G6Pase enzymatic complex is composed of the catalytic unit G6PC and of a G6P transporter (G6PT). Because the latter might be a rate-limiting step in G6P hydrolysis, we checked that glucose induces G6pt gene expression as previously described (39). High glucose exposure induced G6PT mRNA levels in primary hepatocytes (1.83 ± 0.22-fold induction 25 vs. 5 mmol/L glucose; P < 0.01). Moreover, high glucose exposure increased glucose production from primary hepatocytes, whereas antioxidants reduced this induction (Fig. 4F). G6pc gene regulations thus translated into a physiological release of glucose.
FIG. 4.
The induction of G6pc expression by glucose occurs in primary hepatocytes and is dependent on ROS. Primary rat hepatocytes were treated for 16–18 h with 25 mmol/L glucose (■) and with 25 mmol/L glucose and 10−6 mol/L Trolox (gray bars). A: Induction of the production of ROS by glucose in primary hepatocytes. The amount of ROS in the presence of 5 mmol/L glucose was defined as 1. B and C: Induction of G6pc mRNA (B) or Glut1 mRNA (C) by glucose in primary rat hepatocytes. The amount of mRNA in the presence of 5 mmol/L glucose was arbitrarily defined as 1. D and E: Induction of G6pc mRNA (D) and Glut1 mRNA (E) by chemical hypoxia in primary rat hepatocytes. The amount of mRNA in the presence of 5 mmol/L glucose was arbitrarily defined as 1. F: Induction of glucose release by primary rat hepatocytes. The amount of glucose produced in the presence of 5 mmol/L glucose (28.03 ± 1.43 nmol/h/millon of cells) was arbitrarily defined as 1. Reported values are means of three experiments performed in duplicate ± SEM. *Significantly different from the values obtained in the presence of 5 mmol/L glucose (P < 0.01). §Significantly different from the values obtained in the presence of 25 mmol/L glucose (P < 0.01).
We then assessed whether gene regulation by glucose in primary hepatocytes involves the same mechanism deciphered in HepG2 cells. Immunoprecipitation of hepatocyte protein lysates with CBP antibodies revealed that a small amount of HIF-1α was associated with CBP under low-glucose conditions (Fig. 5A). However, high glucose exposure and chemical hypoxia increased the amount of HIF-1α associated with CBP (Fig. 5A). ChIP assays with chromatin from primary hepatocytes indicated that high glucose exposure induced the binding of the complex HIF-1α/CBP (Fig. 5B and C) to the proximal part of the G6pc promoter. This binding was prevented by antioxidants (Fig. 5C). Our results thus suggest that ROS produced by glucose induced 1) the interaction of HIF-1α with CBP and 2) their binding to the G6pc promoter, permitting the induction of G6pc expression by HIF-1 (Fig. 6).
FIG. 5.
Induction of G6pc expression by ROS involves HIF-1α and CBP. Primary rat hepatocytes were treated for 16–18 h with 5 and 25 mmol/L glucose. Where indicated, cells were treated for 16–18 h with 10−6 mol/L Trolox and for 2 h with 200 μmol/L CoCl2. A: The CBP protein was immunoprecipitated from whole-cell extracts from primary rat hepatocytes. The image shows a representative Western blot probed for HIF-1α proteins associated with CBP. B and C: Chromatin from hepatocytes was immunoprecipitated with antibodies against GFP as a control (C), against HIF-1α (B), and against CBP (C). The images show representative electrophoresis of PCR fragments amplified from immunoprecipitated chromatin using primer pairs specific to the G6pc promoter. The input lane shows the result with samples not subjected to immunoprecipitation.
FIG. 6.
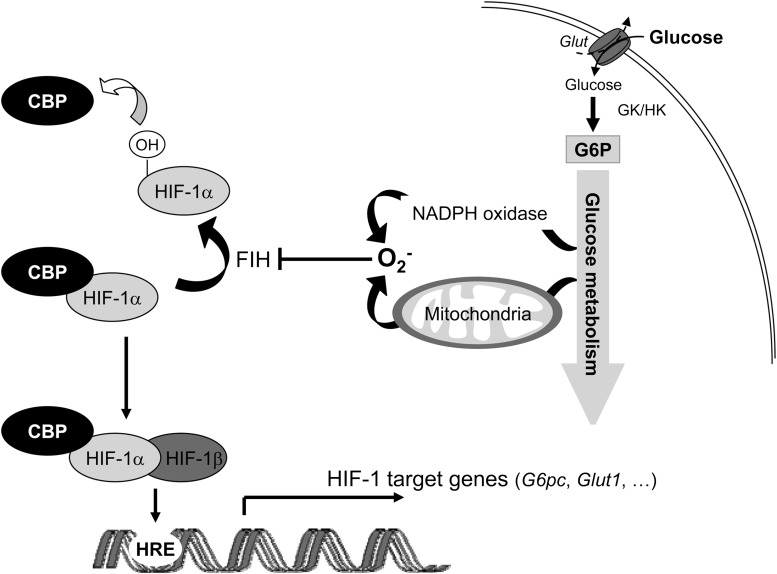
Diagram of the molecular mechanism of G6pc gene regulation by glucose and HIF-1α. High glucose exposure induces an increase in intracellular glucose metabolism, leading to the production of ROS by NADPH oxidase or mitochondria. ROS then inhibit FIH-1 activity. The inhibition of FIH-1 activity decreases the hydroxylation of proline residues in the C-TAD domain of HIF-1α (38). HIF-1α can therefore interact with CBP. Then the HIF-1β/HIF-1α/CBP complex binds to G6pc promoter and to promoters of other HIF-1 target genes.
Induction of G6pc expression by HIF-1 occurs in type 2 diabetes.
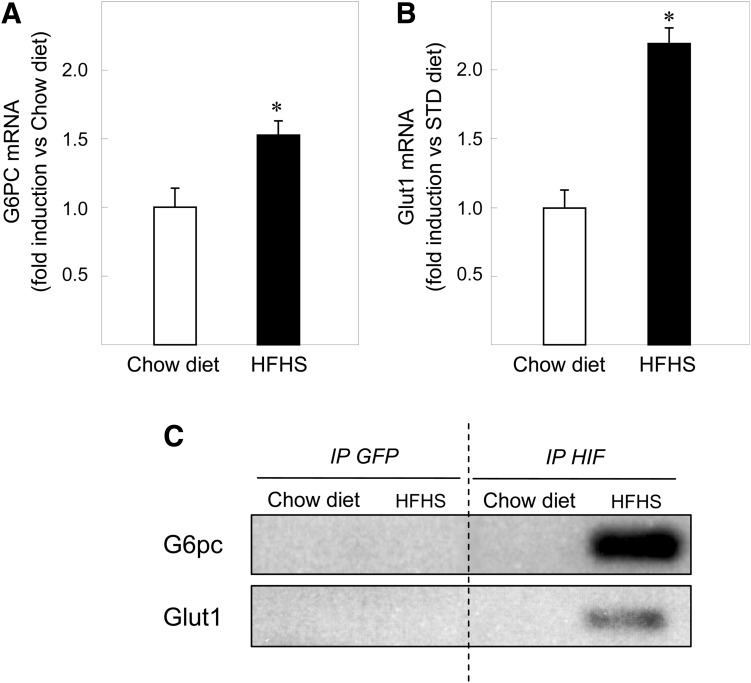
Sustained hyperglycemia is a major feature of type 2 diabetes. Mice fed for >11 weeks with an HFHS have a postabsorptive glucose levels of 223.3 ± 26.9 mg/dL vs. 142.2 ± 16.5 mg/dL with a standard chow diet (26) and are used as a model of type 2 diabetes. In the liver of mice fed for 11 weeks on an HFHS diet, G6pc mRNA levels increased 1.5-fold and Glut1 mRNA levels increased 2.2-fold, compared with mRNA levels of mice fed with a chow diet (Fig. 7A). In parallel, HIF-1α was not bound to the G6pc and Glut1 promoters in the liver of mice fed with a chow diet, whereas HIF-1α was bound to both promoters in the liver of mice on an HFHS diet (Fig. 7B). These results suggest that the regulation of gene expression by HIF-1α might take place in an animal model of hyperglycemia.
FIG. 7.
HIF-1 transcriptional activity might contribute to elevated G6pc expression levels in diabetic mice. Induction of G6pc mRNA (A) and Glut1 mRNA (B) in the liver of mice fed with an HFHS diet (■). The amount of mRNA in the liver of mice fed with a chow diet was arbitrarily defined as 1 (□). Reported values are means of three experiments performed in duplicate ± SEM. *Significantly different from the values obtained in the liver of mice fed with a chow diet (P < 0.01). C: Liver chromatin from mice fed with an HFHS diet or with a chow diet was immunoprecipitated with antibodies against HIF-1α and against GFP as a control. The images show representative electrophoresis of PCR fragments amplified from immunoprecipitated chromatin using primer pairs specific for the G6pc promoter (first lane) and for the Glut1 promoter HRE (second lane). The input lane shows the result with samples not subjected to immunoprecipitation.
DISCUSSION
As a member of the G6Pase complex, the G6pc gene plays a key role in blood glucose homeostasis. The induction of G6pc expression by glucose probably contributes to the elevation of HGP, a contributing factor for the development of type 2 diabetes (8,14). Type 2 diabetes is associated with vascular complications mainly attributed to glucose-induced ROS production (16). In this study, we deciphered the induction of G6pc promoter activity in hepatocytes by glucotoxicity. We found that decreasing ROS concentrations by antioxidant treatment prevented the induction of G6pc promoter activity by glucose. Induction of G6pc promoter activity by glucose depended on HIF-1α and CBP in both hepatoma cells and primary hepatocytes. Glucose increased the amount of ROS, which increased the association of HIF-1α with CBP and the recruitment of both proteins to the proximal part of the G6pc promoter but also to Glut1 HRE (Fig. 6). These findings lead to the description of a novel molecular mechanism of gene regulation by glucotoxicity, involving HIF-1α and dependent on CBP.
Increased ROS production is a major cause of micro- and macrovascular complications leading to disability and death in patients with type 2 diabetes (16,40). The inhibition of ROS production by antioxidants led to a 50% decrease of induction of G6pc expression by glucose in primary hepatocytes (Figs. 4A and B), whereas it completely eliminated the induction of G6pc promoter activity by glucose in HepG2 cells (Figs. 1B and D). This suggests that ROS and an additional mechanism are involved in the regulation of the G6pc gene by glucose in primary hepatocytes. ChREBP has been implicated in the induction of G6pc expression by glucose through a distal binding site (−3702/−3686 [13]). In our study, this binding site was absent from the −320/+60 promoter construct transfected in HepG2 cells but was present in the endogenous gene of primary rat hepatocytes. We therefore postulate that the glucose-induced G6pc gene transcription mechanism may depend only on ROS in HepG2 cells and at least on ROS and ChREBP in primary hepatocytes. On the contrary, the inhibition of ROS production by glucose completely eliminated the induction of Glut1 expression in hepatocytes (Fig. 4C), suggesting that glucose may control Glut1 by acting mainly on ROS.
O-glycosylation of CRTC2 and Foxo1 have been implicated in the glucose regulation of the G6pc gene in animal models of type 2 diabetes (14,15,41). During type 2 diabetes, ROS produced under conditions of hyperglycemia induce glutamine:fructose-6-phosphate amidotransferase-1 (GFAT), leading to protein O-glycosylation (16). HIF-1α controls the expression of GFAT and therefore also may be linked to the O-glycosylation pathway (42). However, under our experimental conditions, we did not measure any induction of GFAT mRNA levels after high exposure to glucose (Supplementary Fig. 1A). In addition, the inhibition of GFAT activity by 6-diazo-5-oxonorleucine (DON) had no effect on the induction of G6pc mRNA levels by glucose (Supplementary Fig. 1B). We therefore surmise that the induction of G6pc gene expression by glucose through HIF-1α does not depend on the O-glycosylation of transcription factors.
HIF-1α protein is continuously synthesized and degraded under normoxia. However, some hormones (such as insulin), cytokines, and growth factors control the amount of HIF-1α protein in normoxia (43). Functional HIF-1α also can be stabilized by ROS (37). Full transcriptional activity of HIF-1α requires the inhibition of both PHD2 and FIH-1 (38). Hydroxylation of proline residues in HIF-1α by PHD2 leads to HIF-1α proteosomal degradation, whereas hydroxylation of asparagine residues in HIF-1α by FIH-1 blocks its interaction with the coactivators p300 and CBP. CBP is required for full induction of G6pc promoter activity by protein kinase A (30,44). We demonstrated here that CBP was required for the transactivation of the G6pc promoter by glucose (Figs. 3 and 5). High glucose exposure had no effect on HIF-1α protein levels (Fig. 3A) but increased the interaction between CBP and HIF-1α (Figs. 3D and 5A). Moreover, HIF-1 and high glucose exposure has an additive effect on G6pc promoter activity (Fig. 2C). Thus, ROS produced by glucose probably inhibit FIH-1 activity and have no effect on PHD2. As previously described in several cancer cell lines (45,46), the HIF-1α protein was detected in HepG2 cells under low-glucose conditions. In HepG2 cells treated under low-glucose conditions, antioxidants decreased ROS production, G6pc promoter activity, and the interaction between HIF-1α and CBP. This suggests that the molecular mechanism of G6pc regulation by ROS, HIF-1α, and CBP takes place even at physiological glucose concentration in hepatoma cells.
HIF-1α also is involved in the transcriptional regulation of other key genes of gluconeogenesis. HIF-1α controls G6pt transcription in hypoxic mesenchymal cells (47) and Pck1 (phosphoenolpyruvate carboxykinase) gene expression in hepatoma cells (48). It therefore is likely that HIF-1α plays a role in the unrestrained glucose production of type 2 diabetes. Moreover, the regulation of gluconeogenic genes by HIF-1α may play a role in another pathological situation. Indeed, the induction of gluconeogenesis induced after partial hepatectomy is impaired in mice lacking hepatic HIF-1α (49).
To conclude, we described a novel gene regulation mechanism induced by glucose, involving HIF-1α associated with CBP and depending on ROS production. We demonstrated that this regulatory mechanism is not restricted to the G6pc gene but can be generalized to other HIF-1 target genes. Glucose-induced ROS now are considered to be important regulators of glucose signaling, particularly in obese and diabetic states (50). Better understanding of glucose regulation mediated by HIF-1α and CBP may thus contribute greatly to the characterization of these metabolic diseases.
ACKNOWLEDGMENTS
This work was funded in part by a grant from the Société Francophone du Diabetes and a grant from Institut Nestlé. The high-fat high-sucrose diet was produced by the Unité de Préparation des Aliments Expérimentaux (UE0300) of the Institut National de la Recherche Agronomique (INRA), Jouy-en-Josas, France.
No potential conflicts of interest relevant to this article were reported.
A.G.-S. collected data, designed the study, and wrote the manuscript. M.S., J.C., and C.Z. collected data. F.R. and G.M. contributed to the design of the study, contributed to the discussion, and reviewed and edited the manuscript. A.G.-S. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Professional native English speakers (Keith Hodson, Accent Europe, Ecully, France) edited the manuscript.
The authors thank INSERM for funding the work and positions (C.Z.) and the CNRS (F.R. and G.M.), INRA (A.G.-S.), and the Ministère Français de l'Enseignement Supérieur et de la Recherche (M.S. and J.C.) for funding the positions.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0986/-/DC1.
REFERENCES
- 1.Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 1999;117:132–139 [DOI] [PubMed] [Google Scholar]
- 2.Mithieux G, Bady I, Gautier A, Croset M, Rajas F, Zitoun C. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 2004;286:E370–E375 [DOI] [PubMed] [Google Scholar]
- 3.Clore JN, Stillman J, Sugerman H. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes 2000;49:969–974 [DOI] [PubMed] [Google Scholar]
- 4.Erion DM, Yonemitsu S, Nie Y, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci USA 2009;106:11288–11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Valadés AG, Méndez-Lucas A, Vidal-Alabró A, et al. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes 2008;57:2199–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J 2002;362:513–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mithieux G. New data and concepts on glutamine and glucose metabolism in the gut. Curr Opin Clin Nutr Metab Care 2001;4:267–271 [DOI] [PubMed] [Google Scholar]
- 8.Trinh KY, O’Doherty RM, Anderson P, Lange AJ, Newgard CB. Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J Biol Chem 1998;273:31615–31620 [DOI] [PubMed] [Google Scholar]
- 9.Massillon D, Barzilai N, Chen W, Hu M, Rossetti L. Glucose regulates in vivo glucose-6-phosphatase gene expression in the liver of diabetic rats. J Biol Chem 1996;271:9871–9874 [DOI] [PubMed] [Google Scholar]
- 10.Chatelain F, Pégorier JP, Minassian C, et al. Development and regulation of glucose-6-phosphatase gene expression in rat liver, intestine, and kidney: in vivo and in vitro studies in cultured fetal hepatocytes. Diabetes 1998;47:882–889 [DOI] [PubMed] [Google Scholar]
- 11.Argaud D, Kirby TL, Newgard CB, Lange AJ. Stimulation of glucose-6-phosphatase gene expression by glucose and fructose-2,6-bisphosphate. J Biol Chem 1997;272:12854–12861 [DOI] [PubMed] [Google Scholar]
- 12.Massillon D. Regulation of the glucose-6-phosphatase gene by glucose occurs by transcriptional and post-transcriptional mechanisms: differential effect of glucose and xylitol. J Biol Chem 2001;276:4055–4062 [DOI] [PubMed] [Google Scholar]
- 13.Arden C, Tudhope SJ, Petrie JL, et al. Fructose 2,6-bisphosphate is essential for glucose-regulated gene transcription of glucose-6-phosphatase and other ChREBP target genes in hepatocytes. Biochem J 2012;443:111–123 [DOI] [PubMed] [Google Scholar]
- 14.Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science 2008;319:1402–1405 [DOI] [PubMed] [Google Scholar]
- 15.Housley MP, Rodgers JT, Udeshi ND, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem 2008;283:16283–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 17.Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ 2008;15:660–666 [DOI] [PubMed] [Google Scholar]
- 18.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol 2007;27:5737–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol 2000;203:1253–1263 [DOI] [PubMed] [Google Scholar]
- 20.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472 [DOI] [PubMed] [Google Scholar]
- 21.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 2002;16:1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999;399:271–275 [DOI] [PubMed] [Google Scholar]
- 23.Rajas F, Gautier A, Bady I, Montano S, Mithieux G. Polyunsaturated fatty acyl coenzyme A suppress the glucose-6-phosphatase promoter activity by modulating the DNA binding of hepatocyte nuclear factor 4 alpha. J Biol Chem 2002;277:15736–15744 [DOI] [PubMed] [Google Scholar]
- 24.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem 1997;272:20055–20062 [DOI] [PubMed] [Google Scholar]
- 25.Gautier-Stein A, Domon-Dell C, Calon A, et al. Differential regulation of the glucose-6-phosphatase TATA box by intestine-specific homeodomain proteins CDX1 and CDX2. Nucleic Acids Res 2003;31:5238–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gout J, Sarafian D, Mutel E, et al. Metabolic and melanocortin gene expression alterations in male offspring of obese mice. Mol Cell Endocrinol 2010;319:99–108 [DOI] [PubMed] [Google Scholar]
- 27.Dentin R, Pégorier JP, Benhamed F, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem 2004;279:20314–20326 [DOI] [PubMed] [Google Scholar]
- 28.Carrière A, Carmona MC, Fernandez Y, et al. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem 2004;279:40462–40469 [DOI] [PubMed] [Google Scholar]
- 29.Pillot B, Soty M, Gautier-Stein A, Zitoun C, Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology 2009;150:616–624 [DOI] [PubMed] [Google Scholar]
- 30.Gautier-Stein A, Zitoun C, Lalli E, Mithieux G, Rajas F. Transcriptional regulation of the glucose-6-phosphatase gene by cAMP/vasoactive intestinal peptide in the intestine. Role of HNF4alpha, CREM, HNF1alpha, and C/EBPalpha. J Biol Chem 2006;281:31268–31278 [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 2001;276:9519–9525 [DOI] [PubMed] [Google Scholar]
- 32.Mizrahi A, Berdichevsky Y, Ugolev Y, et al. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. J Leukoc Biol 2006;79:881–895 [DOI] [PubMed] [Google Scholar]
- 33.Miyano K, Fukuda H, Ebisu K, Tamura M. Remarkable stabilization of neutrophil NADPH oxidase using RacQ61L and a p67phox-p47phox fusion protein. Biochemistry 2003;42:184–190 [DOI] [PubMed] [Google Scholar]
- 34.Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell 2008;19:2984–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 1998;95:11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osada-Oka M, Hashiba Y, Akiba S, Imaoka S, Sato T. Glucose is necessary for stabilization of hypoxia-inducible factor-1alpha under hypoxia: contribution of the pentose phosphate pathway to this stabilization. FEBS Lett 2010;584:3073–3079 [DOI] [PubMed] [Google Scholar]
- 37.Pouysségur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem 2006;387:1337–1346 [DOI] [PubMed] [Google Scholar]
- 38.Brahimi-Horn MC, Pouysségur J. HIF at a glance. J Cell Sci 2009;122:1055–1057 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, van de Werve G. Distinct hormone stimulation and counteraction by insulin of the expression of the two components of glucose 6-phosphatase in HepG2 cells. Biochem Biophys Res Commun 2000;272:41–44 [DOI] [PubMed] [Google Scholar]
- 40.Wiernsperger NF. Oxidative stress: the special case of diabetes. Biofactors 2003;19:11–18 [DOI] [PubMed] [Google Scholar]
- 41.Koo SH, Flechner L, Qi L, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 2005;437:1109–1111 [DOI] [PubMed] [Google Scholar]
- 42.Manzari B, Kudlow JE, Fardin P, et al. Induction of macrophage glutamine: fructose-6-phosphate amidotransferase expression by hypoxia and by picolinic acid. Int J Immunopathol Pharmacol 2007;20:47–58 [DOI] [PubMed] [Google Scholar]
- 43.Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta 2005;1755:107–120 [DOI] [PubMed] [Google Scholar]
- 44.Gautier-Stein A, Mithieux G, Rajas F. A distal region involving hepatocyte nuclear factor 4alpha and CAAT/enhancer binding protein markedly potentiates the protein kinase A stimulation of the glucose-6-phosphatase promoter. Mol Endocrinol 2005;19:163–174 [DOI] [PubMed] [Google Scholar]
- 45.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 2002;277:23111–23115 [DOI] [PubMed] [Google Scholar]
- 46.Dayan F, Bilton RL, Laferrière J, et al. Activation of HIF-1alpha in exponentially growing cells via hypoxic stimulation is independent of the Akt/mTOR pathway. J Cell Physiol 2009;218:167–174 [DOI] [PubMed] [Google Scholar]
- 47.Lord-Dufour S, Copland IB, Levros LC, Jr, et al. Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1alpha: Targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells. Stem Cells 2009;27:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JH, Park MJ, Kim KW, et al. Molecular mechanism of hypoxia-mediated hepatic gluconeogenesis by transcriptional regulation. FEBS Lett 2005;579:2795–2801 [DOI] [PubMed] [Google Scholar]
- 49.Tajima T, Goda N, Fujiki N, et al. HIF-1alpha is necessary to support gluconeogenesis during liver regeneration. Biochem Biophys Res Commun 2009;387:789–794 [DOI] [PubMed] [Google Scholar]
- 50.Colombani AL, Carneiro L, Benani A, et al. Enhanced hypothalamic glucose sensing in obesity: alteration of redox signaling. Diabetes 2009;58:2189–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]