Abstract
We aimed to evaluate insulin secretion and insulin sensitivity in adults born preterm and their children. Subjects were adults born both preterm and at term, with their children aged 5–10 years born at term. Insulin sensitivity and secretion were assessed using hyperglycemic clamps in adults and frequently sampled intravenous glucose tolerance tests using Bergman minimal model in children. In total, 52 adults aged 34–38 years participated (31 born preterm, mean gestational age 33.3 weeks). Adults born preterm were less insulin sensitive than those born at term (19.0 ± 2.5 vs. 36.3 ± 5.2 mg ⋅ kg−1 ⋅ min−1mU ⋅ L; P < 0.05) with compensatory increased first-phase insulin secretion (56.1 ± 8.5 vs. 25.3 ± 3.7 mU/L; P < 0.001) but similar disposition index indicating appropriate insulin secretion. These differences were independent of sex and remained when subjects born <32 weeks' gestation were excluded from analyses. In total, 61 children were studied (37 of preterm parents, mean age 7.9 ± 0.3 years). Children of parents born preterm had similar insulin sensitivity to children of parents born at term, but a correlation between parental and offspring insulin sensitivity was noted only among children of parents born preterm. In conclusion, adults born preterm have insulin resistance in midadulthood, but this was not associated with insulin resistance in their children.
Preterm birth is associated with abnormalities in glucose homeostasis (1,2). Most studies show a 30–40% reduction in insulin sensitivity in children and young adults born very preterm (<32 weeks' gestation) in comparison with those born at term (1–3). While there is some evidence suggesting later preterm survivors (32–36 weeks' gestation) also have impairments in insulin sensitivity, data using gold standard assessments of insulin sensitivity are lacking (4). Since later preterm survivors constitute up to 10% of all live births in the U.S., as opposed to very preterm subjects who account for only 1.5% (5,6), the potential public health impact of metabolic perturbations in those born at 32–36 weeks is considerable.
In addition, there are few data on β-cell function in children or adults born preterm. Impairments in both insulin sensitivity and secretion are required for the development of diabetes (7), but it remains unclear whether those born preterm have impaired insulin secretory capacity as well as impaired insulin sensitivity and, therefore, are more likely to develop diabetes.
Furthermore, there is growing evidence from animal and human models that environmental insults may result in phenotypic changes in subsequent generations (8,9). Although there is good evidence that exposure to maternal diabetes during pregnancy increases the risk of diabetes in the offspring (10) and that parental preterm birth increases the risk of preterm birth of the offspring (11), there are no data on possible consequences of parental preterm birth on metabolism of their offspring born at term.
We hypothesized that adults born preterm, including those born between 32 and 36 weeks' gestation, have abnormalities in insulin sensitivity and insulin secretion and that these abnormalities also occur in their offspring born at term.
RESEARCH DESIGN AND METHODS
Ethics approval was obtained from the Multiregion Ethics Committee, Wellington, New Zealand. Two groups of subjects were recruited for this study: 1) adults aged 34–38 years and 2) their offspring aged 5–10 years born at term (37–42 weeks' gestation). The adults (F1) were the offspring of mothers (F0) recruited to the Auckland Steroid Trial between 1969 and 1974 (12). That trial randomized 1,142 mothers at risk for preterm delivery (<37 weeks' gestation by last menstrual period, confirmed by neonatal exam, as per best practice at the time) (12,13) to receive either antenatal betamethasone or placebo. We attempted to trace their surviving children (F1), then aged 30 years, of whom one-third had been born at term, between 2003 and 2005 (13). From that cohort, we recruited adults for this study if they were healthy, born from singleton pregnancies, had children aged 5–10 years, and lived in the greater Auckland area. Adults were excluded if they had chronic illness or used medication known to affect insulin sensitivity. Their children (F2) were excluded if they were born preterm (<37 completed weeks' gestation) or small for gestational age (SGA; birth weight <10th percentile), had a first-degree relative with diabetes, or had clinical signs of puberty (defined as Tanner stage 2 breast development in girls and testicular volume >3 mL in boys) or adrenarche (defined as presence of pubic or axillary hair). The investigators performing the studies were blinded to the perinatal characteristics of the participants.
All subjects were assessed after an overnight fast. Women were tested within 10 days following menses. In adults, glucose metabolism was assessed using hyperglycemic clamps (14). Parameters calculated were glucose disposal (M), first-phase insulin response, second-phase insulin response (I), and insulin sensitivity (SI, glucose metabolized per unit of plasma insulin = M/I × 100) (15).
In children, glucose metabolism was assessed using a modified frequently sampled intravenous glucose tolerance test (16) and Bergman minimal model software (17). Values derived included the insulin sensitivity index, acute insulin response (AIR), glucose effectiveness (Sg), and glucose disposal index (Kg) (1). Weight, height, and blood pressure were recorded in all subjects. For the children studied, reported weight and height of the parent who did not participate were also recorded.
Physical activity was assessed by questionnaire reporting weekly frequency, duration, and intensity of exercise and was graded as 0 (<30 min for at least 4 days/week), 1 (30–60 min for at least 4 days/week), or 2 (>60 min for at least 4 days/week).
Food diaries were collected for 2 weekdays and 1 weekend day for each participant. Nutritional intake was estimated using standard household measures, and food labels where appropriate. Records were entered into Foodworks software (v5.0, Xyris Software, Brisbane, Queensland, Australia) by a trained investigator, and the calculated mean daily caloric intake was used in the analysis.
Assays.
Whole blood glucose concentrations in adults were measured using a YSI 2300 STAT PLUS glucose analyzer (YSI Inc., Yellow Springs, Ohio), which had a precision of ± 2%. Plasma glucose concentrations in children were measured with a Hitachi 902 autoanalyzer (Hitachi High-Technologies Corporation, Tokyo, Japan), while plasma insulin concentrations were measured using the IMX system (Abbott Laboratories, Abbott Park, IL). Interassay coefficient of variation was 1.5% for glucose and 4.5% for insulin.
Statistical analyses.
General linear regression models were used to investigate the effect of preterm birth on glucose metabolism. Square root transformations were used on both insulin sensitivity and AIR to better satisfy assumptions of normality. Preterm birth, sex, age, BMI, and antenatal steroid exposure were controlled for in the analyses. To compare the participants with the larger cohort of nonparticipants, linear regression models were used for continuous variables, logistic regression for binary or ordinal variables, and generalized models for categorical variables.
To investigate the effect of parental preterm birth on offspring metabolism, linear mixed models were used, with the parent included as a random factor to allow for the clustering of children in a family. Models also included the child’s sex, age, and BMI and the parent’s insulin sensitivity, sex, and preterm birth. Data are expressed as mean ± SEM.
RESULTS
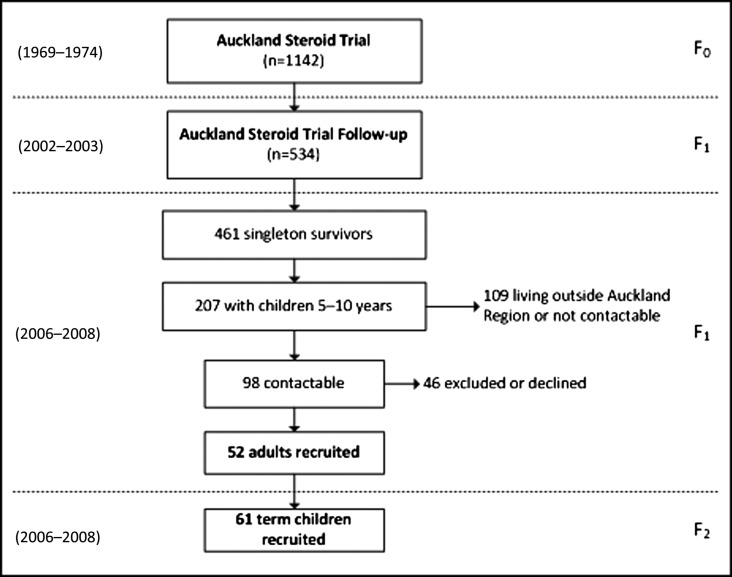
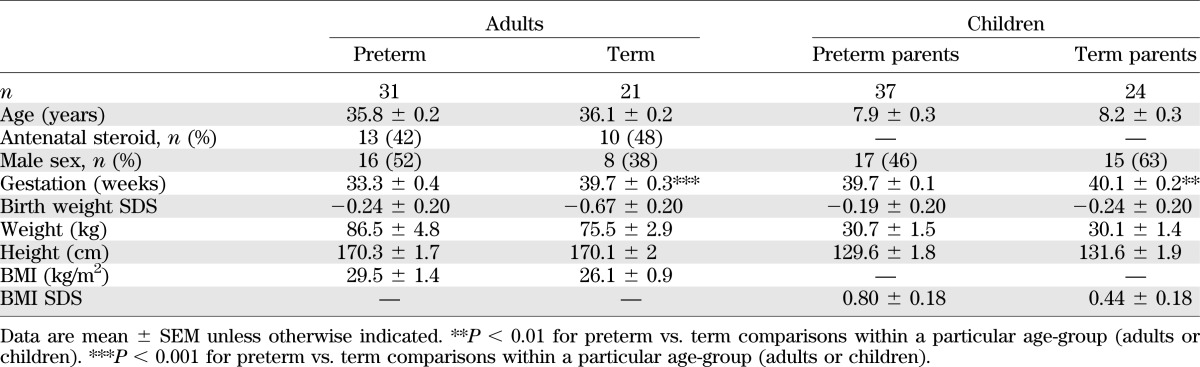
Of the 534 adult (F1) survivors previously traced at 30 years, 461 were born from singleton pregnancies, 207 had children aged 5–10 years, and 127 were living in the Auckland region (Fig. 1). Of this group, 98 were contactable, 19 were excluded as a result of chronic illness, and 27 declined to participate (Fig. 1). A total of 52 adults aged 34–38 years were studied, of whom 31 were born preterm (Table 1). Among preterm subjects, mean gestational age was 33.3 weeks (Table 1), with 8 born <32 weeks and the remaining 23 born 32–36 weeks. When compared with the 409 singleton adults from the original survivor cohort who did not participate in this study, there were no differences in demographics, anthropometry, blood pressure, fasting glucose, or insulin levels at age 30 years (data not shown).
FIG. 1.
Summary of the recruitment process. F0, F1, and F2 identify the particular generation covered by each study, while numbers in parentheses on the left indicate the years when the studies were carried out.
TABLE 1.
Baseline characteristics of adults and their children
From the adult cohort, 45 parents agreed to have their children assessed (27 parents born preterm and 18 parents born at term). Of these, 1 girl with a parent born preterm and 1 with a parent born at term were excluded because of evidence of puberty. A total of 61 children aged 5.2–10.6 years were studied, of whom 17 had fathers and 20 had mothers born preterm (Table 1). Children of parents born preterm were on average born 0.4 weeks earlier than those of parents born at term (P < 0.01) (Table 1), even though children born preterm had been excluded from the study.
Adults.
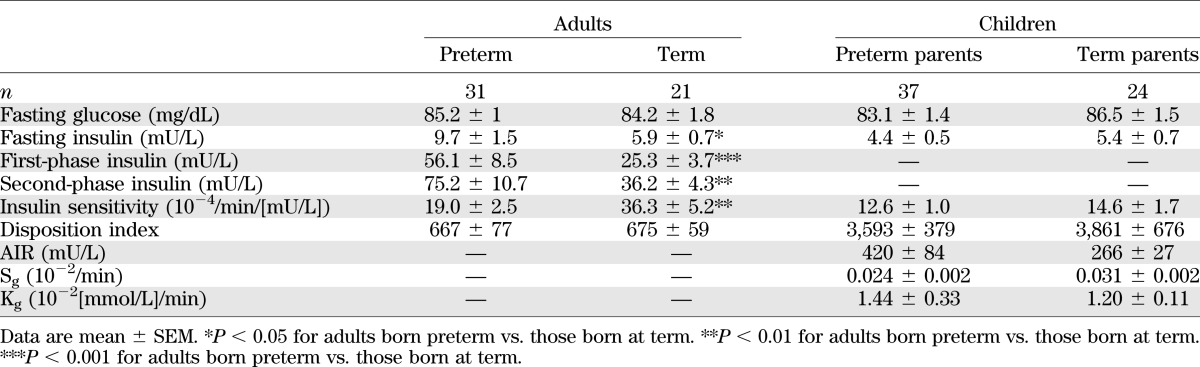
Adults born preterm had similar fasting plasma glucose concentrations but higher insulin concentrations than those born at term (P < 0.05) (Table 2). The unadjusted insulin sensitivity was 47% lower in adults born preterm (P < 0.01) (Table 2) and remained 29% lower after adjustment for sex, age, BMI, and antenatal steroid exposure (19.6 vs. 27.6 10−4/min/(mU/L); P < 0.05). Male sex (P < 0.001) and increased BMI (P < 0.001) also were associated with lower insulin sensitivity.
TABLE 2.
Parameters of glucose metabolism in adults and their children
Adults born preterm had a compensatory increase in both first- (P < 0.001) and second-phase insulin secretion (P < 0.01) (Table 2). Insulin secretion also increased with increasing BMI (data not shown; P < 0.001). However, there was no evidence of a defect in β-cell function, with the disposition index (insulin sensitivity × second-phase insulin secretion) similar in adults born preterm and at term (Table 2). All the observed metabolic differences were unchanged when the eight subjects born <32 weeks' gestation were excluded from the analyses, indicating that the reduction in insulin sensitivity was not confined to those born <32 weeks' gestation.
There were no differences between adults born preterm and at term in intensity and duration of physical activity or mean caloric intake (data not shown).
Children.
Parameters of glucose metabolism were similar in children of parents born preterm and those of parents born at term (Table 2). It is not surprising that increasing BMI was associated with a reduction in insulin sensitivity (P < 0.05) and an increase in AIR (P < 0.001). Insulin sensitivity in children was positively correlated with parental insulin sensitivity if the parent was born preterm (r = 0.33, P < 0.05) but not if the parent was born at term (P = 0.70). Physical activity levels and mean caloric intake were similar between groups (data not shown).
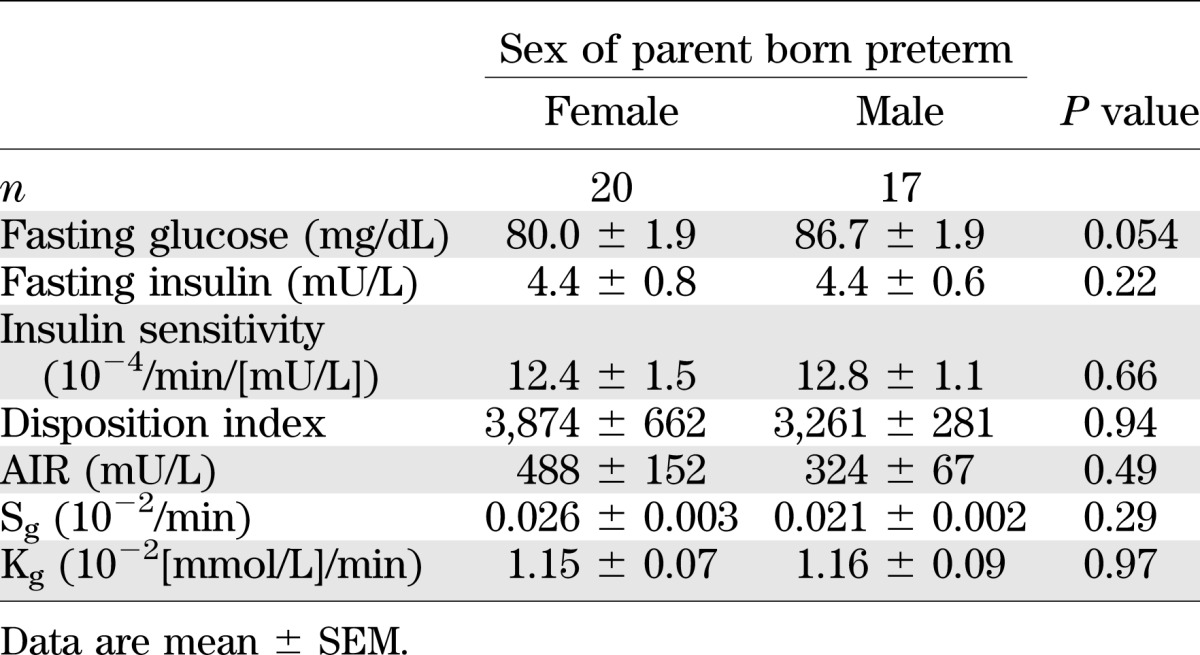
Children whose mothers were born preterm had lower fasting plasma glucose concentrations than those whose fathers were born preterm (P = 0.05), but the sex of the preterm parent did not affect other parameters of glucose metabolism (Table 3).
TABLE 3.
Parameters of glucose metabolism in term children who were born of a male or female parent born preterm
DISCUSSION
This study confirms that reduced insulin sensitivity is present in midadulthood in survivors of preterm birth, even among those born moderately preterm (32–36 weeks' gestation). This observation, together with a number of previous cross-sectional studies from childhood until early adulthood, suggests that an early impairment in insulin sensitivity occurs in these subjects and persists throughout life. We report a reduction in insulin sensitivity of similar magnitude (30–40%) to previous reports of children and adults born preterm in comparison with term control subjects, after adjustment for fat mass and other variables (1–3). Of concern is the much larger reduction (47%) in unadjusted insulin sensitivity between the term and preterm groups in this study. This predominantly reflects the interaction between insulin sensitivity and BMI in the preterm group. Because BMI likely reflects fat mass, it highlights the known effect of fat mass in magnifying underlying insulin resistance, consequently increasing the risk of later adult disease. Adults born preterm appear to be at greater risk of increased adiposity, and this will likely increase the risk of later adult sequelae (18).
Impairments in both insulin secretion and insulin sensitivity contribute to the development of type 2 diabetes. Veening et al. (19) showed that SGA subjects had normal β-cell function, but this had not been formally assessed in preterm subjects before. Our findings were similar in adults born preterm; although insulin sensitivity was reduced, β-cell function and glucose disposal were normal. Therefore, in contrast to many animal studies where lower birth weight is associated with impaired β-cell function in adulthood (20), humans of lower birth weight due to either small size for gestation or preterm birth appear to have an isolated reduction in insulin sensitivity with an appropriate compensatory increase in insulin secretion.
While very preterm birth (<32 weeks) is associated with reduced insulin sensitivity in both children and adults (1,2), it is less clear whether this is also true after only moderately preterm birth. Our previous report of data on 458 subjects aged 30 years (357 born preterm) using three insulin measurements during an oral glucose tolerance test (4) shows that insulin area under the curve was elevated in subjects born up to 35 weeks' gestation but progressively decreased with increasing gestational age beyond 35 weeks. Although an indirect measure of insulin sensitivity, these results suggest that reduced insulin sensitivity occurred after preterm birth across the whole range of preterm gestations. This current study using a gold standard measure of insulin sensitivity has confirmed these findings.
A number of animal models of intrauterine growth restriction show effects on glucose metabolism in the offspring that persist in subsequent generations (21,22). Human studies (such as those on the 1944 Dutch famine) also show intergenerational effects, but not in glucose metabolism (23). While we hypothesized that insulin sensitivity and/or β-cell function would be altered in the offspring of parents born preterm, as observed following in utero insults at a similar developmental stage, our data did not support this (1,24). It is interesting that we found a correlation between the child’s insulin sensitivity and that of the parent only when the parent was born preterm. This suggests that there may be an underlying reduction in insulin sensitivity in the offspring of parents born preterm that may become apparent only with an increased sample size or in older children. Subtle differences in glucose metabolism, especially among the children, may not have been identified in this study because of the limited number of participants, reflecting the difficulty in recruiting offspring of adults born preterm. Nonetheless, while our study of insulin sensitivity in the children of parents born preterm was limited by small sample size, this investigation did have adequate power to detect a 5 × 10−4/min/(mU/L) difference in insulin sensitivity (or a difference of ∼40%) between groups. This difference is similar to those seen in their parents or in cohorts with extreme prematurity or SGA (1,24). Future longitudinal studies will be needed to better define the health relevance of preterm birth for subsequent generations.
In conclusion, adults born even moderately preterm (32–36 weeks' gestation) have an isolated reduction in insulin sensitivity but normal β-cell function. There was no evidence of impaired glucose metabolism in their children. Reduced insulin sensitivity is strongly associated with diseases such as type 2 diabetes, hypertension, dyslipidemia, ischemic heart disease, and stroke. Because the rate of preterm birth is rising, with the majority born only moderately preterm, the associated reduced insulin sensitivity in adults born preterm is likely to pose a substantial public health burden in the future.
ACKNOWLEDGMENTS
This research was supported by grants from the National Research Centre for Growth and Development, the Health Research Council of New Zealand, and the Australasian Paediatric Endocrine Group.
No potential conflicts of interest relevant to this article were reported.
S.M. conceived and designed the study, collected and compiled data, and wrote the manuscript. W.S.C. and P.L.H. conceived and designed the study and wrote the manuscript. J.G.B.D. performed statistical analyses and wrote the manuscript. S.R.D. conceived and designed the study and collected and compiled data. J.E.H. and C.J. conceived and designed the study. E.R. performed statistical analyses. J.B. collected and compiled data. All authors contributed to discussion and reviewed the manuscript. P.L.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med 2004;351:2179–2186 [DOI] [PubMed] [Google Scholar]
- 2.Hovi P, Andersson S, Eriksson JG, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med 2007;356:2053–2063 [DOI] [PubMed] [Google Scholar]
- 3.Rotteveel J, van Weissenbruch MM, Delemarre-Van de Waal HA. Decreased insulin sensitivity in small for gestational age males treated with GH and preterm untreated males: a study in young adults. Eur J Endocrinol 2008;158:899–904 [DOI] [PubMed] [Google Scholar]
- 4.Dalziel SR, Parag V, Rodgers A, Harding JE. Cardiovascular risk factors at age 30 following pre-term birth. Int J Epidemiol 2007;36:907–915 [DOI] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. Natl Vital Stat Rep 2003;52:1–113 [PubMed] [Google Scholar]
- 6.Steer P. The epidemiology of preterm labor—a global perspective. J Perinat Med 2005;33:273–276 [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989;38:1512–1527 [DOI] [PubMed] [Google Scholar]
- 8.Stewart RJ, Preece RF, Sheppard HG. Twelve generations of marginal protein deficiency. Br J Nutr 1975;33:233–253 [DOI] [PubMed] [Google Scholar]
- 9.Zambrano E, Martínez-Samayoa PM, Bautista CJ, et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 2005;566:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plagemann A. Maternal diabetes and perinatal programming. Early Hum Dev 2011;87:743–747 [DOI] [PubMed] [Google Scholar]
- 11.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol 2008;167:474–479 [DOI] [PubMed] [Google Scholar]
- 12.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515–525 [PubMed] [Google Scholar]
- 13.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005;365:1856–1862 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 16.Cutfield WS, Bergman RN, Menon RK, Sperling MA. The modified minimal model: application to measurement of insulin sensitivity in children. J Clin Endocrinol Metab 1990;70:1644–1650 [DOI] [PubMed] [Google Scholar]
- 17.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–122 [DOI] [PubMed] [Google Scholar]
- 18.Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, Wit JM, Dekker FW, Dutch POPS-19 Collaborative Study Group Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr 2005;81:480–487 [DOI] [PubMed] [Google Scholar]
- 19.Veening MA, van Weissenbruch MM, Heine RJ, Delemarre-van de Waal HA. β-cell capacity and insulin sensitivity in prepubertal children born small for gestational age: influence of body size during childhood. Diabetes 2003;52:1756–1760 [DOI] [PubMed] [Google Scholar]
- 20.Gatford KL, Mohammad SNB, Harland ML, et al. Impaired β-cell function and inadequate compensatory increases in β-cell mass after intrauterine growth restriction in sheep. Endocrinology 2008;149:5118–5127 [DOI] [PubMed] [Google Scholar]
- 21.Martin JF, Johnston CS, Han C-T, Benyshek DC. Nutritional origins of insulin resistance: a rat model for diabetes-prone human populations. J Nutr 2000;130:741–744 [DOI] [PubMed] [Google Scholar]
- 22.Blondeau B, Avril I, Duchene B, Bréant B. Endocrine pancreas development is altered in foetuses from rats previously showing intra-uterine growth retardation in response to malnutrition. Diabetologia 2002;45:394–401 [DOI] [PubMed] [Google Scholar]
- 23.Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DIW, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 2008;115:1243–1249 [DOI] [PubMed] [Google Scholar]
- 24.Hofman PL, Cutfield WS, Robinson EM, et al. Insulin resistance in short children with intrauterine growth retardation. J Clin Endocrinol Metab 1997;82:402–406 [DOI] [PubMed] [Google Scholar]