Abstract
We investigated whether overexpression of catalase (CAT) in renal proximal tubular cells (RPTCs) could prevent the programming of hypertension and kidney disease in the offspring of dams with maternal diabetes. Male offspring of nondiabetic and diabetic dams from two transgenic (Tg) lines (Hoxb7-green fluorescent protein [GFP]-Tg [controls] and Hoxb7/CAT-GFP-Tg, which overexpress CAT in RPTCs) were studied from the prenatal period into adulthood. Nephrogenesis, systolic blood pressure, renal hyperfiltration, kidney injury, and reactive oxygen species (ROS) generation were assessed. Gene expression of transforming growth factor-β1 (TGF-β1), nuclear factor erythroid 2p45–related factor-2 (Nrf2), and heme oxygenase-1 (HO-1) was tested in both in vitro and in vivo studies. Renal dysmorphogenesis was observed in offspring of Hoxb7-GFP-Tg dams with severe maternal diabetes; the affected male offspring displayed higher renal ROS generation and developed hypertension and renal hyperfiltration as well as renal injury with heightened TGF-β1 expression in adulthood. These changes were ameliorated in male offspring of diabetic Hoxb7/CAT-GFP-Tg dams via the Nrf2–HO-1 defense system. CAT promoted Nrf2 nuclear translocation and HO-1 gene expression, seen in both in vitro and in vivo studies. In conclusion, CAT overexpression in the RPTCs ameliorated maternal diabetes–induced perinatal programming, mediated, at least in part, by triggering the Nrf2–HO-1 defense system.
Gestational diabetes occurs in 3–14% of pregnancies worldwide (www.diabetes.com), conferring substantial risk to the offspring. Infants of diabetic mothers are thus prone to developing a variety of diseases later in life, such as metabolic syndrome, hypertension, and chronic kidney disease (1,2). This phenomenon, in which intrauterine events are linked with later changes, is termed “perinatal programming,” but the mechanisms by which it occurs are incompletely delineated (3,4).
The broad spectrum of birth defects seen in the offspring of women with gestational diabetes and in animal models is thought to be associated with either increased reactive oxygen species (ROS) or diminished antioxidant defense systems (both enzymatic and nonenzymatic defense systems), leading to increased susceptibility to ROS-induced injury in multiple tissues, including the kidney (5–7). Studies to determine whether antioxidant supplementation or the provision of nonenzymatic antioxidants prevents these abnormalities are needed. To date, reports on the efficacy of antioxidant supplementation to pregnant women with or without diabetes are preliminary and controversial (8,9), as is the case in experimental models (7). Hence, the current study focuses on antioxidant enzymatic pathways, specifically the catalase (CAT)-nuclear factor erythroid 2p45-related factor-2 (Nrf2)–heme oxygenase-1 (HO-1) pathway.
The key initial step in the formation of all ROS is the conversion of oxygen to superoxide anion (O2•−). O2•− has a very short half-life and is rapidly converted to less-reactive hydrogen peroxide (H2O2) by superoxide dismutases and then reduced to H2O by CAT and glutathione peroxidase (10,11). In the kidneys, CAT is localized to the renal proximal tubular cells (RPTCs) (12–14). CAT has been postulated to be a key enzyme regulating H2O2 levels because cells overexpressing CAT are more resistant to H2O2 toxicity and oxidant-mediated injury (15,16), whereas overexpression of glutathione peroxidase alone is not protective against renal injury in diabetic mice (17).
Nrf2 is a transcriptional factor that acts as a key regulator of cellular antioxidant enzymes, including CAT, HO-1, superoxide dismutases, glutathione S-transferase, peroxidase, NAD(P)H quinone oxidoreductase, and thioredoxin, among others (18), by binding to the antioxidant response element to defend against oxidative stress (18,19). Under basal conditions, Nrf2 is bound within the cytoplasm to protein Kelch-like erythroid cell-derived protein with Cap‘n’collar homology-associated protein 1 (Keap1, an oxidative stress sensor) and then undergoes rapid ubiquitination, with subsequent proteasome-dependent degradation. Upon exposure of cells to oxidative stress, Nrf2 is released from Keap1 and translocates to the nucleus, where it subsequently guides expression of antioxidant stress genes to trigger the cellular antioxidant defense response (18,19).
Nrf2 is highly expressed in the kidney (19), and it is thought that the Nrf2–HO-1 defense system is renoprotective and that its induction might even improve kidney function in renal fibrosis (20), diabetic nephropathy (21), and ischemic acute kidney injury (22), as well as in the progression of focal glomerulosclerosis (23,24). Moreover, HO-1 induction has been considered as a useful target for the development of antihypertensive drugs, since HO-1 or its metabolites can attenuate the development of hypertension and lower blood pressure (BP) in models of established hypertension (25).
Previously, we reported that a high-glucose milieu ex vivo or severe maternal diabetes in utero (defined as maternal blood glucose concentration ∼30 mmol/L) induces ROS generation, which impairs nephrogenesis, resulting in offspring with relatively smaller kidneys and nascent nephron deficiency due to excessive apoptosis (through activation of the nuclear factor-κB [NF-κB] and p53 pathways) (26–28). Moreover, we have shown that severe maternal diabetes is linked to low birth weight in offspring (mean decrease = 20%), which then manifests hypertension, glucose intolerance, and kidney injury in adulthood, as well as with heightened ROS generation (26,29). Taken together, our prior data suggest that an imbalance between ROS production and antioxidative capacity can lead to a state of “oxidative stress” that is intimately associated with perinatal programming of hypertension and kidney disease.
In the present studies, we investigated whether overexpression of CAT in RPTCs could prevent the perinatal programming of hypertension and kidney injury in male offspring of diabetic dams and examined the potential underlying mechanisms both in vivo and in vitro.
RESEARCH DESIGN AND METHODS
Animal models.
We used both Hoxb7-green fluorescent protein (GFP)–transgenic (Tg) (Hoxb7-GFP-Tg) and Hoxb7/CAT-GFP-Tg (Hoxb7/CAT-GFP-Tg) murine lines (both in C57/BL6 background); both lines are fertile, with a normal phenotype at birth and during adult life. Hoxb7-GFP-Tg mice (GFP expression specifically in ureteric bud [UB] driven by Hoxb7 promoter), provided by Dr. Frank Costantini (Columbia University Medical Center, New York, NY) (30,31), were engineered to allow UB branching morphogenesis to be visualized in real time in vivo as reported previously (28). CAT-Tg mice (e.g., rat CAT gene overexpressing specifically in RPTCs driven by kidney-specific, androgen-regulated protein [KAP] promoter) were obtained from Dr. John S.D. Chan (CRCHUM) (32–34). High levels of androgens have been reported in the fetal and maternal circulation in both humans (35–38) and mice (39), rendering the KAP2 promoter a feasible way to direct CAT transgene expression during nephrogenesis (which occurs in mice both in the prenatal and postnatal periods). We thus created hybrid Hoxb7/CAT-GFP-Tg mice by cross-breeding CAT-Tg mice with Hoxb7-GFP-Tg mice; the resultant hybrid mice permit the visualization of CAT impact on nephrogenesis (e.g., UB branching morphogenesis), which we examined both in the presence and absence of maternal diabetes in vivo.
Induction of maternal diabetes.
We have successfully used a single intraperitoneal injection of 150 mg/kg body weight (BW) of streptozotocin (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada) at embryonic day 13 (E13) to create an in vivo murine model of maternal diabetes (26–29). We studied the male offspring from nondiabetic (control) and diabetic dams of both Hoxb7-GFP-Tg and Hoxb7/CAT-GFP-Tg.
Animal care.
Animal care and the procedures used were approved by the Institutional Animal Care Committee of the CRCHUM. Mice were housed under standard humidity and lighting conditions (12-h light-dark cycles) with free access to standard mouse chow and water.
Isolation of metanephroi and counting of UB tips.
E15 embryos were dissected aseptically from both timed-pregnant Hoxb7-GFP-Tg and Hoxb7/CAT-GFP-Tg mice with or without diabetes. The E15 metanephroi were isolated under sterile conditions, and quantitative assessment of the number of UB tips in each group was performed as reported previously (28).
Physiological studies.
Blood glucose levels were measured with a Side-Kick Glucose Analyzer (model 1500; InterScience, Markham, ON, Canada) in the morning after a 4-h fast, as reported previously (26,27,29). Mean systolic BP (SBP) was monitored by the tail-cuff method with the Visitech BP-2000 Blood Pressure Analysis System for Mice (Visitech System Inc., Apex, NC), as reported elsewhere (29,33,34). The animals were acclimated to BP measurement (2-week period of pretraining starting at 6 weeks of age, followed by actual measurement of SBP three times per week from 8 until 18 weeks of age). Although the technique of tail-cuff measurement is generally considered less sensitive than telemetry, we judged that our SBP data are valid, based on the substantial numbers of animals used and the fact that the animals were well acclimated and used to the measurement in our longitudinal studies, thus minimizing stress.
Urine samples, collected from mice individually housed in metabolic cages, were assayed for albumin and creatinine (ELISA; Albuwell and Creatinine Companion; Exocell Inc., Philadelphia, PA) as reported previously (29,33,34). All animals were killed at 20 weeks of age under CO2, and the kidneys were removed immediately. BW and kidney weight (KW) were rapidly recorded. The left kidney was used for renal morphology and immunohistochemistry (IHC) (29,33,34). The right kidney cortex was reserved for ROS generation and gene expression experiments as previously reported (29,33,34).
Measurement of glomerular filtration rate.
As reported previously (40), we estimated the glomerular filtration rate (GFR) in 20-week-old male animals by the fluorescein isothiocyanate–inulin (FITC-inulin) method, as described by Qi et al. (41) and recommended by the Diabetes Complications Consortium (www.diacomp.org).
Renal morphology, mean glomerular volume, and nephron number.
Kidney morphology was assessed with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) staining. As in previous reports (26,27,29), mean glomerular volume (Vg) was determined using PAS-stained images with the aid of an image analysis software system (Motics Images Plus 2.0; Motic, Richmond, BC, Canada) (42); quantification of neonatal nephron number was adapted from the Bertram method, using serial sections (43).
ROS generation.
Freshly isolated renal cortex was used immediately for ROS measurement by the lucigenin method, as described elsewhere (26,27,32,33). ROS production was normalized by protein concentration and expressed as relative light units per microgram protein.
Real-time quantitative PCR.
Total RNA extracted from freshly isolated renal cortex was assayed for gene expression by real-time quantitative PCR (RT-qPCR), as reported previously (27,29,40). The Fast SYBR Green Mastermix Kit and the 7500 Fast Real-Time PCR System (Applied Biosystems; Life Technologies, Foster City, CA) were used for this purpose (26,27,32,33).
Immunohistochemistry.
IHC was performed by the standard avidin-biotin-peroxidase complex method (ABC Staining System; Santa Cruz Biotechnologies, Santa Cruz, CA), as described elsewhere (26,27,29). Polyclonal anti-CAT antibody was purchased from Sigma-Aldrich Canada; polyclonal anti-Nrf2 antibody was purchased from Abcam (Cambridge, MA); and transforming growth factor-β1 (TGF-β1) and HO-1 antibodies were purchased from Santa Cruz Biotechnology.
Immortalized RPTCs.
The immortalized RPTC (IRPTC) line reported previously (44) was used for our studies in vitro. This in vitro setting is useful for studies of the effect of high glucose (25 mmol/L d-glucose) on both Nrf2 and HO-1 gene expression, as well as Nrf2 nuclear translocation with or without the administration of CAT (250 units). The cells incubated in low-glucose (5 mmol/L d-glucose) medium with 20 mmol/L mannitol, to compensate for the same osmolality as high glucose, serve as the control.
Nuclear protein and cytosolic protein extracts were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Burlington, ON, Canada), as reported previously (26,27). Anti–histone H3 (3H1) rabbit mAb was purchased from Cell Signaling Technology, Inc. (Boston, MA). Western blot (WB) and immunofluorescence (IF) staining on both Nrf2 and HO-1 in IRPTCs were performed as reported previously (45).
Statistical analysis.
Statistical significance between the experimental groups was analyzed by one-way ANOVA, followed by the Bonferroni test using Graphpad Software, Prism 5.0 (http://www.graphpad.com/prism/prism.htm). A probability level of P ≤ 0.05 was considered to be statistically significant (26,27,29).
RESULTS
Hybrid Hoxb7/CAT-Tg mouse generation.
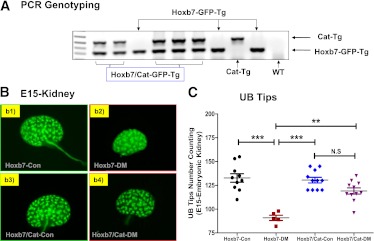
The success of generating hybrid Hoxb7/CAT-Tg mice was confirmed by PCR genotyping (Fig. 1A), as well as GFP live image in E15 metanephroi (Fig. 1B). Once these animals were obtained, we compared UB branching morphogenesis in E15 metanephroi after 2 days of streptozotocin administration (e.g., maternal blood glucose concentration: nondiabetic dams [Hoxb7-GFP-Tg, 9.45 ± 1.69 mmol/L; Hoxb7/CAT-GFP-Tg, 9.68 ± 1.01 mmol/L] vs. diabetic dams [Hoxb7-GFP-Tg, 28.6 ± 2.14 mmol/L; Hoxb7/CAT-GFP-Tg, 27.9 ± 2.0 mmol/L]). As compared with the E15 metanephroi isolated from nondiabetic Hoxb7-GFP-Tg animals, the E15 metanephroi from diabetic Hoxb7-GFP-Tg mice displayed smaller size (Fig. 1B) with a smaller number of UB tips (Fig. 1C), and those UB branching impairments appear to be ameliorated by diabetic Hoxb7/CAT-GFP-Tg mice (Fig. 1B and C).
FIG. 1.
Characterization of Hoxb7-GFP-Tg and Hoxb7/CAT-GFP-Tg mice. A: PCR genotyping. B: E15 metanephroi isolation either from Hoxb7-GFP-Tg dam (nondiabetic, Hoxb7-Con [b1]; diabetic, Hoxb7-DM [b2]) or Hoxb7/CAT-GFP-Tg dam (nondiabetic, Hoxb7/CAT-Con [b3]; diabetic, Hoxb7/CAT-DM [b4]). Original magnification ×4. C: The number of E15 metanephroi UB tips. ●, Hoxb7-Con; ■, Hoxb7-DM; ♦, Hoxb7/CAT-Con; ▼, Hoxb7/CAT-DM. **P ≤ 0.01 and ***P ≤ 0.001. NS, nonsignificant. (A high-quality color representation of this figure is available in the online issue.)
Neonatal kidney outcomes in offspring.
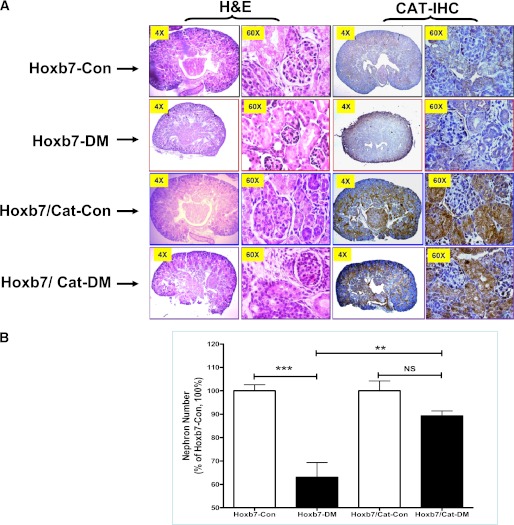
Neonatal renal morphology was reviewed by H&E staining and CAT IHC. CAT IHC revealed that CAT is highly expressed in RPTCs in the neonatal kidneys of Hoxb7/CAT-GFP-Tg compared with Hoxb7-GFP-Tg mice (Fig. 2A). As compared with the offspring of nondiabetic Hoxb7-GFP-Tg dams, the neonates of diabetic Hoxb7-GFP-Tg dams had smaller kidneys with small glomeruli (Fig. 2A), as well as fewer nephrons (Fig. 2B). This dysnephrogenesis appeared to be attenuated in the neonatal offspring of Hoxb7/CAT-GFP-Tg diabetic dams (Fig. 2A and B). Also, there is no significant difference in litter size and sex distribution among the four groups of animals (Supplementary Fig. 1).
FIG. 2.
A: Neonatal renal morphology reviewed by H&E staining and CAT expression (CAT-IHC). Neonatal offspring (nondiabetic: Hoxb7-Con [black frame] and Hoxb7/CAT-Con [blue frame] vs. diabetic: Hoxb7-DM [red frame] and Hoxb7/CAT-DM [purple frame]). Original magnification ×4 and ×60. B: Quantification of neonatal nephron number. □, nondiabetic offspring (Hoxb7-Con, n = 9; Hoxb7/CAT-Con, n = 8); ■, diabetic offspring (Hoxb7-DM, n = 7; Hoxb7/CAT-DM, n = 9). The y-axis shows the percentage of nephron number compared with the Hoxb7-GFP-Tg control animal (Hoxb7-Con, 100%). **P ≤ 0.01 and ***P ≤ 0.001. NS, nonsignificant. (A high-quality digital representation of this figure is available in the online issue.)
Physical and biochemical measurements in the male offspring in adulthood.
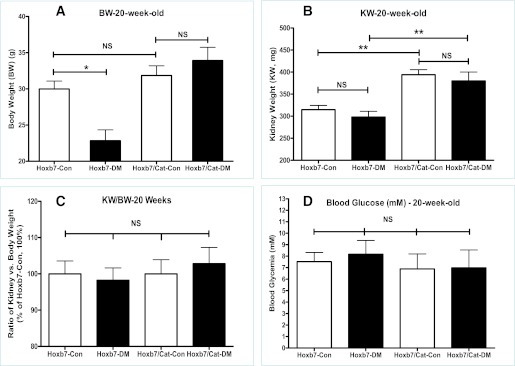
Figure 3 displays the physical and biochemical findings in the male offspring at 20 weeks of age. The offspring of Hoxb7-GFP-Tg diabetic dams were significantly smaller and lighter as compared with the offspring of nondiabetic Hoxb7-GFP-Tg dams (BW in Hoxb7-GFP-Tg offspring: nondiabetic [Hoxb7-control (Con), 27.98 ± 1.095 g, n = 16] vs. diabetic [Hoxb7–diabetes (DM), 22.84 ± 1.506 g, n = 12]; P ≤ 0.05). In contrast, there were no significant differences between the BWs of the offspring from nondiabetic and diabetic Hoxb7/CAT-GFP-Tg dams (Hoxb7/CAT-GFP-Tg offspring: nondiabetic [Hoxb7/CAT-Con: 31.845 ± 1.35 g, n = 13] vs. diabetic [Hoxb7/CAT-DM: 33.92 ± 1.82 g, n = 14]) (Fig. 3A). Although the 20-week-old male Hoxb7/CAT-GFP-Tg offspring had significantly bigger kidneys, as compared with those of Hoxb7-GFP-Tg mice (Fig. 3B) (KW: Hoxb7-Con, 314.6 ± 37.96 mg [n = 15]; Hoxb7-DM, 298 ± 41.31 mg [n = 10]; Hoxb7/CAT-Con, 394 ± 26.08 mg [n = 11]; Hoxb7/CAT-DM, 380 ± 5.07 mg [n = 12]), the KW to BW ratio among all groups of 20-week-old animals, however, did not differ significantly (Fig. 3C), as well as the fasting blood glucose levels (in mmol/L) (Fig. 3D).
FIG. 3.
Physical parameters in the male offspring at 20 weeks of age. A: BW (g). B: KW (mg). C: Ratio of KW vs. BW. D: Fasting blood glucose concentration (mmol/L). The y-axis shows the percentage of value compared with Hoxb7-Con (100%). □, nondiabetic offspring (Hoxb7-Con; Hoxb7/CAT-Con); ■, diabetic offspring (Hoxb7-DM; Hoxb7/CAT-DM). *P ≤ 0.05 and **P ≤ 0.01. NS, nonsignificant.
Mean SBP in adulthood.
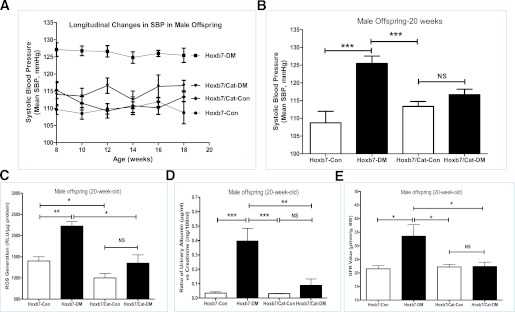
SBP as monitored by tail cuff is shown in Fig. 4A and B from 8 to 20 weeks of age. Longitudinal studies (Fig. 4A) revealed that the male offspring of diabetic Hoxb7-GFP-Tg dams have significantly higher SBP over the follow-up period, as compared with the control offspring. CAT overexpression in RPTCs seems to prevent maternal diabetes–induced perinatal programming of hypertension. Figure 4B summarized the SBP in the male offspring at 20 weeks (Hoxb7-Con, 108.74 ± 3.21 mmHg [n = 23]; Hoxb7-DM, 125.47 ± 2.08 mmHg [n = 21]; Hoxb7/CAT-Con, 113.35 ± 1.40 mmHg [n = 22]; Hoxb7/CAT-DM, 116.65 ± 1.52 mmHg [n = 23]).
FIG. 4.
Mean SBP, ROS generation, and renal function measurement in male offspring at 20 weeks of age. A: Longitudinal changes in mean SBP in the male offspring from 8 to 20 weeks of age. B: Mean SBP in the male offspring at 20 weeks of age. C: ROS generation. ROS production was normalized with protein concentration and expressed as relative light units (RLU) per microgram protein. D: Ratio of urinary albumin (μg/mL)/creatinine (mg/100 mL) (ACR) measurement. E: GFR measurement. □, nondiabetic offspring (Hoxb7-Con; Hoxb7/CAT-Con); ■, diabetic offspring (Hoxb7-DM; Hoxb7/CAT-DM). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. NS, nonsignificant.
ROS generation and renal function assay in adulthood.
The offspring of nondiabetic Hoxb7-GFP-Tg dams at 20 weeks of age have significantly augmented ROS generation in their freshly isolated renal cortex as compared with the offspring of diabetic Hoxb7-GFP-Tg dams (Fig. 4C). The offspring of diabetic dams exhibited significantly increased urinary albumin/creatinine ratio (ACR) (Fig. 4D) (Hoxb7-Con, 0.026 ± 0.024 [n = 12], vs. Hoxb7-DM, 0.38 ± 0.34 [n = 15]; P ≤ 0.001) and GFR (Fig. 4E) (Hoxb7-Con, 21.50 ± 2.0 [n = 6], vs. Hoxb7-DM, 36.1 ± 5.2 [n = 6]; P ≤ 0.01).
In contrast, offspring with overexpression of CAT did not have an increase in renal ROS (Fig. 4C), ACR (Fig. 4D), and GFR (Fig. 4E), irrespective of whether the dams were nondiabetic or diabetic Hoxb7/CAT-GFP-Tg (ACR: Hoxb7/CAT-Con, 0.031 ± 0.004 [n = 9], vs. Hoxb7/CAT-DM, 0.087 ± 0.11 [n = 8]; GFR: Hoxb7/CAT-Con, 22.22 ± 2.39 [n = 7], vs. Hoxb7/CAT-DM, 22.35 ± 3.23 [n = 6]).
Renal morphology and TGF-β1 gene expression in adulthood.
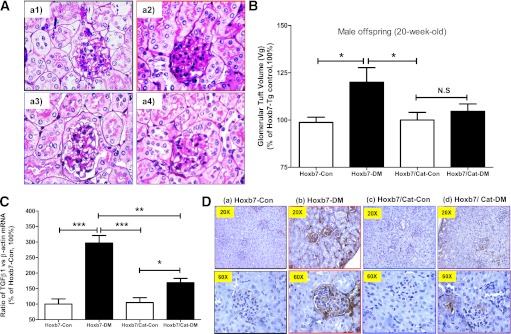
Enhanced extracellular matrix (ECM) protein expression and accumulation in glomeruli is a marker of glomerular injury. PAS staining of kidney sections revealed that ECM accumulation in the glomeruli (Figs. 5A) and higher mean Vg (Fig. 5B) were more pronounced in hypertensive offspring of diabetic Hoxb7-GFP-Tg dams; this finding was attenuated in offspring of both diabetic and nondiabetic Hoxb7/CAT-GFP-Tg dams (Fig. 5A and B).
FIG. 5.
Renal morphology and TGF-β1 gene expression in the male offspring at 20 weeks of age. A: PAS staining (original magnification ×60). Hoxb7-Con (a1); Hoxb7-DM (a2); Hoxb7/CAT-Con (a3); Hoxb7/CAT-DM (a4). B: Quantification of Vg value (Hoxb7-Con, n = 13; Hoxb7-DM, n = 9; Hoxb7/CAT-Con, n = 7; and Hoxb7/CAT-DM, n = 6). The y-axis shows the percentage of Vg compared with Hoxb7-Con (100%). C: RT-qPCR of renal TGF-β1 mRNA. The relative mRNA levels of TGF-β1 in the renal cortex were compared with their own β-actin mRNA. Hoxb7-Con values were considered as 100%. Each point represents the mean ± SD of three independent experiments. D: TGF-β1 IHC expression (original magnification ×20 and ×60). □, nondiabetic offspring (Hoxb7-Con; Hoxb7/CAT-Con); ■, diabetic offspring (Hoxb7-DM; Hoxb7/CAT-DM). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. NS, nonsignificant. (A high-quality digital representation of this figure is available in the online issue.)
TGF-β1 is a ROS-inducible gene that is overexpressed in diabetes; it is directly associated with increases in ECM accumulation and tubulointerstitial fibrosis (10,11,44). Increments of TGF-β1 gene expression (Fig. 5C), predominantly localized to glomeruli and the tubulointerstitium, were observed in kidneys of hypertensive Hoxb7-GFP-Tg offspring (Fig. 5D). Further, the heightened TGF-β1 expression was attenuated in kidneys of offspring of diabetic Hoxb7/CAT-GFP-Tg dams (Fig. 5C and D), indicating that suppressing ROS generation ameliorated glomerular and tubulointerstitial fibrosis.
Nrf2–HO-1 gene expression in adulthood.
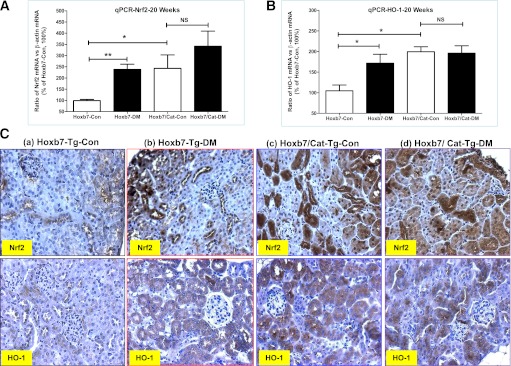
We assessed Nrf2 and HO-1 gene expression in the renal cortex using RT-qPCR (Fig. 6A and B). Compared with offspring of nondiabetic Hoxb7-GFP-Tg dams, both Nrf2 and HO-1 levels were significantly increased in hypertensive offspring of diabetic Hoxb7-GFP-Tg dams. Overexpression of CAT in RPTCs further enhanced Nrf2 and HO-1 gene expression in the affected male offspring of both nondiabetic and diabetic Hoxb7/CAT-GFP-Tg dams.
FIG. 6.
Nrf2 and HO-1 gene expression in the male offspring at 20 weeks of age. RT-qPCR of Nrf2 (A) and HO-1 mRNA (B). The relative mRNA levels of Nrf2 and HO-1 in the renal cortex were compared with their own β-actin mRNA. Hoxb7-Con values were considered as 100%. Each point represents the mean ± SD of three independent experiments. C: Nrf2 and HO-1 IHC expression (original magnification ×20). □, nondiabetic offspring (Hoxb7-Con; Hoxb7/CAT-Con); ■, diabetic offspring (Hoxb7-DM; Hoxb7/CAT-DM). *P ≤ 0.05 and **P ≤ 0.01. NS, nonsignificant. (A high-quality digital representation of this figure is available in the online issue.)
Consistent with the RT-qPCR data (Fig. 6A and B), our IHC studies (Fig. 6C) in paraffin-embedded renal sections showed that Nrf2 protein expression was detected not only in glomeruli, as reported by others (21,22), but also in RPTCs, whereas HO-1 appeared limited to the RPTCs. Most interestingly, the elevation of Nrf2 protein expression was strikingly increased in RPTCs, accompanied by nuclear translocation in offspring of Hoxb7/CAT-GFP-Tg dams, both diabetic and nondiabetic, indicating that overexpression of CAT in RPTCs activates the Nrf2–HO-1 defense system to ameliorate maternal diabetes–induced perinatal programming of kidney injury in offspring.
Nrf2–HO-1 gene expression in IRPTCs in vitro.
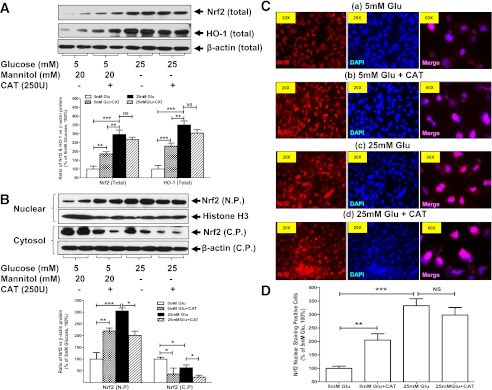
To confirm the effect of CAT on Nrf2 translocation, we performed additional in vitro studies with cultured IRPTCs (44). In this in vitro system, as in vivo, high glucose (Fig. 7A–D) significantly upregulates Nrf2 gene expression, as well as its translocation from the cytosol to the nucleus, which targets the downstream HO-1 gene, resulting in significant upregulation, implicating the Nrf2 antioxidative machinery in the operation. Meanwhile, CAT itself could trigger Nrf2 translocation and further upregulate HO-1 expression in IRPTCs, indicating that the Nrf2–HO-1 antioxidative action could be mediated in a CAT-dependent manner.
FIG. 7.
High glucose (Glu) effects on Nrf2 and/or HO-1 protein expression as well as Nrf2 nuclear translocation analyzed by WB (A and B) and IF staining (C and D) in IRPTCs in vitro. A: WB performed on the total cell lysis. B: WB performed on isolated nuclear protein (N.P.) and cytosolic protein (C.P.) extracts. C: IF images (original magnification ×20 and ×60). D: Semiquantification of Nrf2 IF nuclear-positive cells. The relative blot densities of Nrf2 and HO-1 protein expression in IRPTCs were compared with their own β-actin or histone H3. The values in 5 mmol/L glucose medium were considered as 100%. Each point represents the mean ± SD of three independent experiments. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. NS, nonsignificant. (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
The present work demonstrates that intrarenal ROS generation induced by maternal diabetes can exert a direct effect on nephrogenesis in utero, and consequently trigger the perinatal programming of hypertension and renal injury in the offspring of diabetic dams when they reach adulthood. CAT overexpression in RPTCs appears to prevent this phenomenon, mediated, at least in part, by the Nrf2–HO-1 defense system.
In women with gestational diabetes, there is evidence of increased oxidative stress and impairment of antioxidant defense mechanisms, as seen in maternal plasma and cord blood as well as in placental tissue (46). It appears that ROS can directly impair nephrogenesis and elicit growth retardation and congenital kidney anomalies (5–7), and may also lead to adverse perinatal programming (26,29). In the current study, we created a unique murine model, hybrid Hoxb7/CAT-GFP-Tg mice, allowing us to directly study the functional role of CAT in the impact of maternal diabetes on nephrogenesis during the prenatal period and on the development of perinatal programming of hypertension and kidney injury in adulthood in the exposed male offspring.
First, we characterized our hybrid Hoxb7/CAT-GFP-Tg mice and documented that the offspring of those Hoxb7/CAT-GFP-Tg mice rendered diabetic during pregnancy overexpress CAT in their RPTCs in isolated embryonic or newborn kidneys, which could prevent maternal diabetes–induced renal dysmorphogenesis (small kidneys with decreased nascent nephron number as well as less UB branching morphogenesis). One possible explanation is that since common progenitors in the S-shaped body migrate spontaneously and differentiate to form tubules (both proximal and distal) and glomeruli (glomerular tufts), tubulogenesis and glomerulogenesis directly influence each other (47). Thus, if CAT overexpression in RPTCs eliminates the impairment of maternal diabetes–induced ROS in tubulogenesis, glomerulogenesis is improved as well. Nevertheless, our data suggest that maternal diabetes–induced renal ROS may exert a direct effect on nephrogenesis in utero, and that the impaired nephrogenesis induced by maternal diabetes could be ameliorated by CAT overexpression in RPTCs.
We previously reported that CAT overexpression in RPTCs of db/db mice, which spontaneously develop diabetes, effectively attenuates hypertension, albuminuria, interstitial fibrosis, tubular apoptosis, and proapoptotic gene expression (32,33), suggesting that CAT overexpression in the RPTCs might provide a novel approach to obviating or reversing the pathophysiological manifestations of maternal diabetes–induced perinatal programming of hypertension and kidney injury. Hence, we hypothesized that the protective role of CAT in RPTCs programmed for hypertension and kidney injury by maternal diabetes might be mediated through the Nrf2–HO-1 defense system.
To test this hypothesis, we followed male offspring of nondiabetic and diabetic dams until adulthood (20 weeks of age). We observed that the adult male offspring of diabetic Hoxb7-GFP-Tg dams displayed higher renal ROS generation and developed hypertension and renal injury features such as microalbuminuria and renal hyperfiltration (increased GFR and mean Vg) and apparent glomerular injury (ECM accumulation and tubulointerstitial fibrosis) with heightened TGF-β1 expression, as compared with male offspring of diabetic Hoxb7-CAT-GFP-Tg dams. Thus, CAT overexpression in RPTCs of the male offspring of diabetic Hoxb7/CAT-GFP-Tg dams appears to normalize these abnormalities with the upregulation of Nrf2 and HO-1 gene expression in the kidney.
It has recently been reported that the Nrf2–HO-1 defense system is renoprotective (20–24); further, a causal link between Nrf2 antioxidative pathways and oxidative stressors (e.g., ROS, angiotensin II, TGF-β1, and NF-κB, etc.) has been established (20–24,48). Thus, it seems likely that Nrf2-mediated antioxidative capacity could act to counterbalance the stress induced by increased ROS production. When Nrf2 signals are impaired, either by reduction of Nrf2 pathway activation (20–24,48) or by disruption of Nrf2 gene expression (Nrf2 knockout mice) (21,49,50), renal damage may worsen, suggesting Nrf2-dependent regulation. Our present data indicate that overexpression of CAT in RPTCs promotes Nrf2 gene expression, and then decreases TGF-β1–related glomerular ECM accumulation, which confirms the findings of Jiang et al. (21), who reported that knockdown of Nrf2 by small interfering RNA enhanced TGF-β1 transcription and fibronectin production in cultured human mesangial cells. Moreover, previously, we established that there is a functional relationship between intrarenal ROS generation, the activation of the intrarenal renin-angiotensin system, and the NF-κB signaling pathway in our maternal diabetes murine model of perinatal programming (26,27,29). Taken together, our data suggest that CAT is capable of triggering Nrf2 translocation and then targeting downstream genes, such as the HO-1 gene, which then interacts with the intrarenal renin-angiotensin system and NF-κB signaling, improving renal outcome (27,29,34).
Finally, we observed that the augmented upregulation of Nrf2 with nuclear translocation was most evident in the RPTCs, rather than in glomeruli, as reported by others (21,22), whereas heightened HO-1 IHC expression seems to only be localized in the RPTCs, in agreement with other reports (20,22). Given this specific localization of Nrf2 expression in RPTCs, we further validated our in vivo data by using IRPTCs in vitro (44). In addition to showing that CAT eliminates ROS generation produced by either high glucose milieu, we confirmed that CAT itself could trigger Nrf2 translocation and further upregulate HO-1 expression in IRPTCs, indicating that the antioxidative action of Nrf2–HO-1 occurs in a CAT-dependent manner.
In conclusion, we demonstrated that CAT overexpression in RPTCs could exert a direct effect on nephrogenesis in utero and ameliorate maternal diabetes–induced dysnephrogenesis, consequently preventing maternal diabetes–induced perinatal programming, mediated at least in part, by the Nrf2–HO-1 defense system.
ACKNOWLEDGMENTS
This project was supported by grants to S.-L.Z. from the Canadian Institutes of Health Research (MOP115025), the American Society of Nephrology (Carl W. Gottschalk Research Scholar), and the Bourse de Chercheure-Boursier Juniors 2-Fonds de Recherche en Santé du Québec.
No potential conflicts of interest relevant to this article were reported.
S.-Y.C., Y.-W.C., X.-P.Z., and I.C., researched data and contributed to discussion. S.T. and A.S. researched data. J.R.I. contributed to discussion and reviewed and edited the manuscript. S.-L.Z. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. S.-L.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank former mentors Dr. Julie R. Ingelfinger (Massachusetts General Hospital) and Dr. John S.D. Chan (CRCHUM) for their unconditional support (and research reagents and transgenic mice) and valuable comments on the manuscript. Editorial assistance was provided by the CRCHUM’s Research Support Office.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0248/-/DC1.
See accompanying commentary, p. 2400.
REFERENCES
- 1.Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med 2009;14:119–124 [DOI] [PubMed] [Google Scholar]
- 2.Simeoni U, Ligi I, Buffat C, Boubred F. Adverse consequences of accelerated neonatal growth: cardiovascular and renal issues. Pediatr Nephrol 2011;26:493–508 [DOI] [PubMed] [Google Scholar]
- 3.Ingelfinger JR. Pathogenesis of perinatal programming. Curr Opin Nephrol Hypertens 2004;13:459–464 [DOI] [PubMed] [Google Scholar]
- 4.Plagemann A. Maternal diabetes and perinatal programming. Early Hum Dev 2011;87:743–747 [DOI] [PubMed] [Google Scholar]
- 5.Kanwar YS, Nayak B, Lin S, et al. Hyperglycemia: its imminent effects on mammalian nephrogenesis. Pediatr Nephrol 2005;20:858–866 [DOI] [PubMed] [Google Scholar]
- 6.Kanwar YS, Akagi S, Nayak B, et al. Renal-specific oxidoreductase biphasic expression under high glucose ambience during fetal versus neonatal development. Kidney Int 2005;68:1670–1683 [DOI] [PubMed] [Google Scholar]
- 7.Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol 2007;24:31–41 [DOI] [PubMed] [Google Scholar]
- 8.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res 2009;66:121–127 [DOI] [PubMed] [Google Scholar]
- 9.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 2010;15:191–195 [DOI] [PubMed] [Google Scholar]
- 10.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 12.Grabenbauer M, Fahimi HD, Baumgart E. Detection of peroxisomal proteins and their mRNAs in serial sections of fetal and newborn mouse organs. J Histochem Cytochem 2001;49:155–164 [DOI] [PubMed] [Google Scholar]
- 13.Johkura K, Usuda N, Liang Y, Nakazawa A. Immunohistochemical localization of peroxisomal enzymes in developing rat kidney tissues. J Histochem Cytochem 1998;46:1161–1173 [DOI] [PubMed] [Google Scholar]
- 14.Oberley TD, Oberley LW, Slattery AF, Lauchner LJ, Elwell JH. Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol 1990;137:199–214 [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MR, Miller FJ, Jr, Li WG, et al. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 1999;85:524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo JH, Erzurum SC, Hay JG, Lemarchand P, Crystal RG. Vulnerability of the human airway epithelium to hyperoxia. Constitutive expression of the catalase gene in human bronchial epithelial cells despite oxidant stress. J Clin Invest 1994;93:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Haan JB, Stefanovic N, Nikolic-Paterson D, et al. Kidney expression of glutathione peroxidase-1 is not protective against streptozotocin-induced diabetic nephropathy. Am J Physiol Renal Physiol 2005;289:F544–F551 [DOI] [PubMed] [Google Scholar]
- 18.Lee JM, Li J, Johnson DA, et al. Nrf2, a multi-organ protector? FASEB J 2005;19:1061–1066 [DOI] [PubMed] [Google Scholar]
- 19.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 1994;91:9926–9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin DH, Park HM, Jung KA, et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic Biol Med 2010;48:1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010;59:850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu QQ, Wang Y, Senitko M, et al. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol Renal Physiol 2011;300:F1180–F1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 2010;298:F662–F671 [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Sato T, Rodríguez-Iturbe B, Vaziri ND. Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. J Pharmacol Exp Ther 2011;337:583–590 [DOI] [PubMed] [Google Scholar]
- 25.Hosick PA, Stec DE. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am J Physiol Regul Integr Comp Physiol 2012;302:R207–R214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YW, Chenier I, Chang SY, Tran S, Ingelfinger JR, Zhang SL. High glucose promotes nascent nephron apoptosis via NF-kappaB and p53 pathways. Am J Physiol Renal Physiol 2011;300:F147–F156 [DOI] [PubMed] [Google Scholar]
- 27.Tran S, Chen YW, Chenier I, et al. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol 2008;19:943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SL, Chen YW, Tran S, Chenier I, Hébert MJ, Ingelfinger JR. Reactive oxygen species in the presence of high glucose alter ureteric bud morphogenesis. J Am Soc Nephrol 2007;18:2105–2115 [DOI] [PubMed] [Google Scholar]
- 29.Chen YW, Chenier I, Tran S, Scotcher M, Chang SY, Zhang SL. Maternal diabetes programs hypertension and kidney injury in offspring. Pediatr Nephrol 2010;25:1319–1329 [DOI] [PubMed] [Google Scholar]
- 30.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet 1999;24:241–251 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol 2004;271:98–108 [DOI] [PubMed] [Google Scholar]
- 32.Brezniceanu ML, Liu F, Wei CC, et al. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 2007;71:912–923 [DOI] [PubMed] [Google Scholar]
- 33.Brezniceanu ML, Liu F, Wei CC, et al. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 2008;57:451–459 [DOI] [PubMed] [Google Scholar]
- 34.Godin N, Liu F, Lau GJ, et al. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int 2010;77:1086–1097 [DOI] [PubMed] [Google Scholar]
- 35.Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol 1980;137:293–298 [DOI] [PubMed] [Google Scholar]
- 36.Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol 2006;155:365–370 [DOI] [PubMed] [Google Scholar]
- 37.Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum Reprod 1998;13:460–464 [DOI] [PubMed] [Google Scholar]
- 38.Jeremy SD. Winter: sexual differentiation. In Endocrinology and Metabolism 2nd ed. Felig P, Baxter JD, Broadus AE, Frohman LA, Eds. New York, McGraw-Hill Book Company, 1987, p. 996 [Google Scholar]
- 39.Barkley MS, Michael SD, Geschwind II, Bradford GE. Plasma testosterone during pregnancy in the mouse. Endocrinology 1977;100:1472–1475 [DOI] [PubMed] [Google Scholar]
- 40.Chang SY, Chen YW, Chenier I, Tran SleM, Zhang SL. Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp Diabetes Res 2011;2011:521076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi Z, Fujita H, Jin J, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 2005;54:2628–2637 [DOI] [PubMed] [Google Scholar]
- 42.Weibel ER. Numerical density: shape and size of particles. In Stereological Methods Volume 2 Theoretical Foundations Weibel ER, Ed. London, Academic Press, 1980, p. 149–152 [Google Scholar]
- 43.Bertram JF. Counting in the kidney. Kidney Int 2001;59:792–796 [DOI] [PubMed] [Google Scholar]
- 44.Brezniceanu ML, Wei CC, Zhang SL, et al. Transforming growth factor-beta 1 stimulates angiotensinogen gene expression in kidney proximal tubular cells. Kidney Int 2006;69:1977–1985 [DOI] [PubMed] [Google Scholar]
- 45.Zhang SL, Moini B, Ingelfinger JR. Angiotensin II increases Pax-2 expression in fetal kidney cells via the AT2 receptor. J Am Soc Nephrol 2004;15:1452–1465 [DOI] [PubMed] [Google Scholar]
- 46.Karacay O, Sepici-Dincel A, Karcaaltincaba D, et al. A quantitative evaluation of total antioxidant status and oxidative stress markers in preeclampsia and gestational diabetic patients in 24-36 weeks of gestation. Diabetes Res Clin Pract 2010;89:231–238 [DOI] [PubMed] [Google Scholar]
- 47.Vaughan MR, Quaggin SE. How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 2008;19:24–33 [DOI] [PubMed] [Google Scholar]
- 48.Kang SJ, You A, Kwak MK. Suppression of Nrf2 signaling by angiotensin II in murine renal epithelial cells. Arch Pharm Res 2011;34:829–836 [DOI] [PubMed] [Google Scholar]
- 49.Yoh K, Itoh K, Enomoto A, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int 2001;60:1343–1353 [DOI] [PubMed] [Google Scholar]
- 50.Yoh K, Hirayama A, Ishizaki K, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells 2008;13:1159–1170 [DOI] [PubMed] [Google Scholar]