Abstract
Growth hormone (GH) is a counter-regulatory hormone that plays an important role in preventing hypoglycemia during fasting. Because inhibition of the pyruvate dehydrogenase complex (PDC) by pyruvate dehydrogenase kinase 4 (PDK4) conserves substrates for gluconeogenesis, we tested whether GH increases PDK4 expression in liver by a signaling pathway sensitive to inhibition by metformin. The effects of GH and metformin were determined in the liver of wild-type, small heterodimer partner (SHP)-, PDK4-, and signal transducer and activator of transcription 5 (STAT5)-null mice. Administration of GH in vivo increased PDK4 expression via a pathway dependent on STAT5 phosphorylation. Metformin inhibited the induction of PDK4 expression by GH via a pathway dependent on AMP-activated protein kinase (AMPK) and SHP induction. The increase in PDK4 expression and PDC phosphorylation by GH was reduced in STAT5-null mice. Metformin decreased GH-mediated induction of PDK4 expression and metabolites in wild-type but not in SHP-null mice. In primary hepatocytes, dominant-negative mutant-AMPK and SHP knockdown prevented the inhibitory effect of metformin on GH-stimulated PDK4 expression. SHP directly inhibited STAT5 association on the PDK4 gene promoter. Metformin inhibits GH-induced PDK4 expression and metabolites via an AMPK-SHP–dependent pathway. The metformin-AMPK-SHP network may provide a novel therapeutic approach for the treatment of hepatic metabolic disorders induced by the GH-mediated pathway.
Metformin (1,1-dimetylbiguanide hydrochloride) is widely used for the treatment of type 2 diabetes (1). It lowers blood glucose levels, decreases levels of triglycerides and free fatty acid (FFA), improves glucose tolerance, and decreases insulin resistance by inhibition of hepatic glucose production (2,3). Metformin also increases glucose uptake and promotes fatty acid oxidation in peripheral tissues (4). AMP-activated protein kinase (AMPK) is stimulated by physiologic stimuli, such as exercise, hypoxia, and oxidative stress, and also by pharmacologic agents, metformin and thiazolidinediones (TZD), that lower blood glucose (5). AMPK is regulated by distinct upstream kinases, including Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β), LKB-1, transforming growth factor-β (TGF-β)–activated kinase-1 (Tak1), and ataxia telangiectasia mutated (ATM), a member of the phosphoinositide 3-kinase–related kinase family of protein kinases (5–7). AMPK functions as a master regulator of glucose and lipid homeostasis via its effects on target genes required for gluconeogenesis, lipogenesis, fatty acid oxidation, and lipolysis in diverse tissues (1,7).
The small heterodimer partner (SHP; NR0B2) is an atypical orphan nuclear receptor that lacks a classical DNA-binding domain but retains a putative ligand-binding domain (8). Widely expressed in tissues, SHP represses the transcriptional activity of several nuclear receptors and/or transcription factors, including hepatocyte nuclear factors-4α (HNF-4α), forkhead box class O1 (FoxO1), and HNF-3β/FoxA2, which play important roles in the regulation of glucose, lipid, and bile acid metabolism (8–10). Our previous studies have demonstrated that elevated gene expression of SHP is induced by pharmacologic agents, including metformin, hepatocyte growth factor (HGF), and sodium arsenite, all of which inhibit hepatic gluconeogenesis by repression of key transcription factors via an AMPK-SHP–dependent pathway (11–13). Moreover, loss of SHP exacerbates insulin resistance, hepatic fibrosis, inflammation, and bile acid homeostasis by increasing glucose intolerance and promoting the expression of profibrogenic or proinflammatory genes and the accumulation of bile acid (14–16).
Upon binding to its receptor, growth hormone (GH) activates the Janus kinase 2 (JAK2) and the downstream transcription factors signal transducer and activator of transcription 5 (STAT5) (17,18). Via its stimulation of IGF-I, GH stimulates anabolic processes that promote an increase in lean body mass. In conditions where food is not available and glucose levels are low, GH functions as a counter-regulatory hormone to insulin, stimulating the release of FFAs from the adipose tissue and the oxidation of FFA in the liver and peripheral tissues. In these conditions, GH antagonizes the action of insulin on glucose and lipid metabolism in most tissues (19), resulting in insulin resistance but preservation of lean muscle mass (20,21). Our previous findings have shown that loss of STAT5 causes liver fibrosis, hepatosteatosis, and insulin resistance by increasing TGF-β and STAT3 activation, fat mass, and intolerance of glucose and insulin (22,23).
Pyruvate dehydrogenase kinase (PDK) is a key regulator of pyruvate dehydrogenase complex (PDC) activity associated with the regulation of glucose oxidation (24). The PDC is activated by pyruvate dehydrogenase phosphatases through dephosphorylation in the well-nourished state but is inactivated by PDK via phosphorylation in response to fasting or the diabetic condition (25). Indeed, the expression of PDK4 is increased by starvation, diabetes, and insulin-resistance conditions in diverse tissues, whereas refeeding decreases PDK4 gene expression (26,27). Inactivation of PDC by upregulation of PDK4 conserves glucose and three carbon compounds that can be converted to glucose. Conservation of these three carbon compounds that can be recycled back to glucose conserves lean body mass by reducing the need for net glucose synthesis from amino acids (28), which is the same effect that GH exerts when food is sparse. Despite this, the potential importance of the regulation of PDC activity by GH had received little attention before a recent report that GH induces PDK4 expression in adipocytes (29). However, no study has monitored whether the effects of GH on liver metabolism can be explained in part by induction of PDK4. The current study shows this is the case. Likewise, whether the effects of GH on liver metabolism are sensitive to inhibition by metformin has not been investigated. Our findings indicate that the GH-activated STAT5-PDK4 signaling is sensitive to inhibition by a metformin-AMPK-SHP–dependent pathway and therefore may provide a new therapeutic approach for the treatment of hepatic metabolic disorders induced by the GH-dependent pathway.
RESEARCH DESIGN AND METHODS
Animal experiments.
Male and female wild-type C57BL/6 J mice and SHP-null mice were used in experiments. The study used null male mice for PDK4 (27,30), STAT5 (31), and SHP (16) (weight, 20–30 g; age, 12 weeks). Recombinant human GH (ProSpec, Rehovot, Israel) was administered intraperitoneally (i.p.) to fed mice at 2 μg/g body weight; metformin was given by oral gavage to fed mice at 200 mg/kg body weight. Protocols were approved by the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology, and experiments were performed in accordance with National Institutes of Health animal research standards.
Measurement of liver metabolites by liquid chromatography-mass spectrometry/mass spectrometry.
Liver tissues were isolated, freeze-clamped, and extracted with 80% methanol, as described previously (32).
Western blot analysis.
Western blot analysis from primary rat hepatocytes and mouse liver tissues were performed according to methods described previously (10). The membranes were probed with phospho-ATM, ATM, phospho-AMPK, AMPK phospho-STAT5 (Tyr694, Cell Signaling Technology), STAT5, SHP (H-160), GHR, β-actin (Santa Cruz Biotechnology), and phospho-PDH (Ser293, EMD Chemicals) and then developed using an enhanced chemiluminescence Western blot detection kit (Amersham Bioscience).
Plasmid constructions.
The small interfering (si)RNAs of human SHP (siSHP) and the plasmids encoding for the constitutively active form of AMPK (CA-AMPK) and the dominant-negative mutant AMPK (DN-AMPK) were described previously (10,33). The reporter plasmids STAT5-Luc and TEL-JAK2 were provided by Dr. Minho Shong (Chungnam National University School of Medicine), and the mouse PDK4-Luc was provided by Drs. Minho Shong and Robert A. Harris (Indiana University School of Medicine), respectively. STAT5 cDNA (34) was provided by Dr. Gregorio Gil, (Medical College of Virginia, USA). SHP cDNA, glutathione S-transferase (GST), and GST-fused SHP (GST-SHP) were described previously (10).
Preparation of recombinant adenovirus.
Adenoviruses (Ad) encoding full-length human SHP, siRNA for SHP (Ad-si SHP), c-myc–tagged DN-AMPK, and CA-AMPK have been previously described (11,33).
Culture of hepatocytes and transient transfection assay.
HepG2 and primary rat hepatocytes were cultured as previously described (11). Transient transfections were performed in accordance with the method described previously (11).
In vivo interaction and coimmunoprecipitation assay.
In vivo interaction assay between STAT5 and SHP was performed as described previously (10). For coimmunoprecipitation assay, total protein liver extract (700 μg) was immunoprecipitated with STAT5 antibody and then blotted with SHP.
Quantitative RT-PCR.
RT-PCR was used to analyze mRNAs for SHP, PDK2, and PDK4, as described previously (10,12). Individual PCRs were performed in triplicate on liver samples using mouse β-actin (Mm00607939_s1) as a housekeeping gene and experimental probes for PDK4 (Mm01166879_m1), PDK2 (Mm00446681_m1), and suppressor of cytokine signaling 2 (SOCS2; Mm00850544_g1) genes (Applied Biosystems, Foster City, CA).
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation (ChIP) assay was performed as described previously (10). The specific primers used for the analyses were proximal forward 5′-CTGTTCGCTAAGAAGAAAGT-3′ and proximal reverse 5′- CAATGTCACGCATTCCTAG-3′; and distal forward 5′-AGTGCATGTATTGTCCAAG-3′ and distal reverse 5′-TGCACACAGACACTGCCAGA-3′.
Statistical analysis.
Results are expressed as means ± SEM. Analysis of variance was used to determine significant differences as detected by Student t tests and/or one-way ANOVA methods using the Prism program. Statistical significance was considered at P < 0.05.
RESULTS
GH regulates PDK4 gene expression and metabolite parameters in liver.
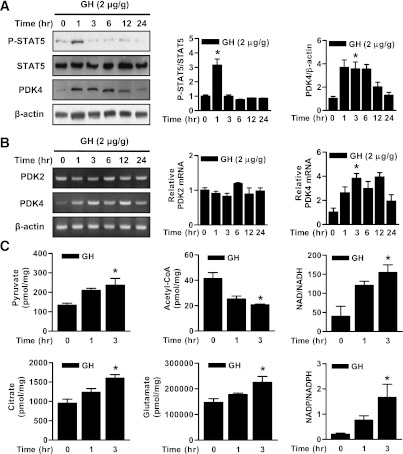
Previous studies have shown GH upregulates the expression of PDK4 via activation of STAT5 in 3T3 L1 adipocytes (29). On the basis of this finding, we hypothesized that activation of STAT5 by GH would upregulate PDK4 expression and thereby alter the levels of key metabolites in the liver. Indeed, administration of GH to intact mice significantly increased STAT5 phosphorylation and PDK4 expression in a time-dependent manner (Fig. 1A and B). In contrast, GH had no effect on PDK2 expression (Fig. 1B). Pyruvate concentration is markedly reduced in the liver of PDK4-null mice (27) because of higher PDC activity; therefore, we anticipated finding the pyruvate concentration increased in GH-treated mice due to reduced PDC activity. As expected, GH increased hepatic levels of pyruvate, citrate, and glutamate, as well as the NAD+-to-NADH and NADP+-to-NADPH ratios. In contrast, acetyl-CoA concentrations were decreased, consistent with less PDC activity (Fig. 1C). Overall, these results suggest that the induction of PDK4 by GH-mediated STAT5 activation plays an important role in regulating metabolite concentrations in the liver, including conservation of three carbon compounds that can be diverted into glucose rather than oxidized by way of PDC and the citric acid cycle.
FIG. 1.
GH regulates PDK4 gene expression and liver metabolites. A: Wild-type mice (12 weeks old) were injected i.p. with GH (2 μg/g body weight) at the observed time periods. Tissue extracts prepared from the tissues of the indicated groups were subjected to Western blot analysis with various antibodies. Protein levels were normalized to total form antibodies and/or β-actin levels. All mice were separated into experimental groups (n = 4–6 mice/group). *P < 0.05 compared with untreated controls. B: WT mice were determined at the indicated time periods after the administration of GH in the feeding condition. Total RNA extracts were isolated from liver samples at the indicated time periods after GH treatment. The levels of PDK2 and PDK4 mRNA were measured by RT-PCR analysis and then normalized to internal control (β-actin level). *P < 0.05 compared with untreated controls. C: Liver samples were measured at the observed time periods after GH treatment. Metabolites were analyzed with the liquid chromatography-mass spectrometry/mass spectrometry method as described in research design and methods. All mice were separated into experimental groups (n = 4 mice/group). *P < 0.05 compared with untreated controls.
Induction of SHP by metformin inhibits GH-mediated STAT5 transactivation of PDK4 expression in primary hepatocytes.
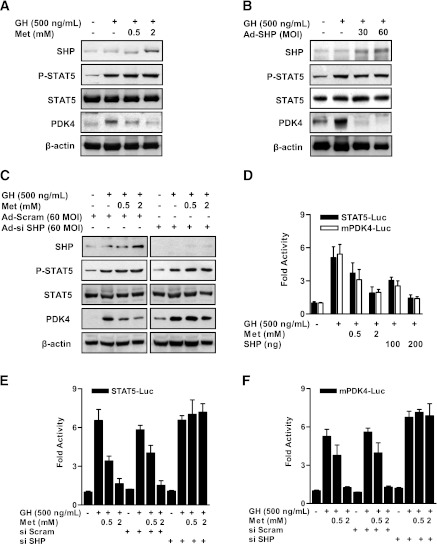
To determine the effect of metformin on the increase of GH-mediated STAT5 phosphorylation, we determined whether metformin treatment or overexpression of SHP with Ad-SHP transduction prevents activation of STAT5 or its transactivation of the PDK4 promoter in primary hepatocytes. As shown in Fig. 2A and B, GH-mediated STAT5 phosphorylation was not decreased by metformin or Ad-SHP, whereas GH-induced PDK4 expression was significantly reduced by metformin and Ad-SHP. Whether STAT5 transactivation is blocked by SHP was tested by knocking-down SHP with Ad-siSHP and oligonucleotide siSHP in primary hepatocytes. As expected, STAT5 activation by GH was not repressed under control and SHP-knockdown conditions, whereas SHP knockdown reversed metformin-mediated repression of GH-induced PDK4 expression compared with control siRNA (Ad-Scram; Fig. 2C). To test whether metformin or SHP regulate STAT5 and PDK4 promoter activities, transient transfection assays were performed with the STAT5 and PDK4 gene promoter. In HepG2 cells, GH-induced STAT5 and PDK4 promoter activities were significantly decreased by metformin or SHP in a dose-dependent manner (Fig. 2D). Metformin inhibited the increase of STAT5 and PDK4 promoter activities in the presence of GH, which is consistent with previous results, and this inhibition was significantly abolished by SHP knockdown (Fig. 2E and F). Overall, these results demonstrate that the metformin-SHP pathway represses hepatic PDK4 gene expression by inhibiting transactivation of the PDK4 promoter by STAT5.
FIG. 2.
Metformin inhibits GH-induced STAT5 transactivity and PDK4 expression in primary hepatocytes. Rat primary hepatocytes were cultured under serum-free conditions for 24 h. A: After pretreatment with metformin (Met) for 12 h, the cells were treated with GH for 1 h at the indicated dose (500 ng/mL). B: Rat primary hepatocytes were infected with Ad-SHP at a multiplicity of infection (MOI) of 30 or 60 for 36 h. After infection for 36 h, cells were treated with GH (500 ng/mL) for 1 h. Specific proteins were assayed in whole-cell extracts by Western blot analysis with the indicated antibodies and then normalized to an internal control (β-actin level and/or total forms). C: Rat primary hepatocytes were infected with 60 MOI of Ad-siSHP and Ad-Scram for 36 h. After infection with Ad-siSHP and Ad-Scram, cells were treated with GH (500 ng/mL) for 1 h, with or without metformin for 12 h at the indicated dose. Whole-cell extracts were isolated and analyzed by immunoblotting with the indicated antibodies, and then normalized to an internal control (β-actin level and/or total forms). The results shown are representative of at least three independent experiments. D: HepG2 cell lines were cotransfected with SHP in the indicated reporter genes and treated with GH for 1 h or metformin for 12 h after transfection. Luciferase (Luc) activity was measured after 36 h and normalized to β-galactosidase activity. All data are representative of three independently performed experiments and are shown as fold activations relative to the control (± SEM). E and F: HepG2 cell lines were transfected with the oligonucleotide siSHP and siScram. After transfection for 36 h, cells were transfected with the indicated reporter gene (STAT5-Luc [E], mPDK4-Luc [F]) and then treated with GH (500 ng/mL) for 1 h in the presence of metformin for 12 h. Luciferase (Luc) activity was normalized to β-galactosidase activity. The results shown are representative of at least three independent experiments. All data are indicated as fold activations relative to the control (± SEM).
AMPK specifically regulates hepatic PDK4 gene expression.
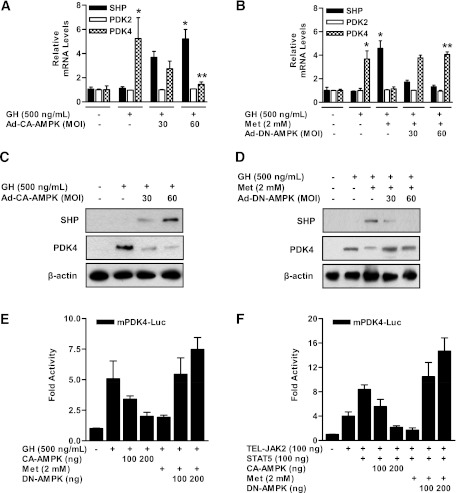
To verify that AMPK activation is involved in the regulation of PDK4 expression by metformin, we examined the effects of the metformin-AMPK pathway on the regulation of PDK4 gene expression in primary hepatocytes. GH-stimulated PDK4 expression was markedly reduced by overexpression of the CA form of AMPK (Ad-CA-AMPK) in a dose-dependent manner (Fig. 3A and C) but not by overexpression of the DN form of AMPK (Ad-DN-AMPK; Fig. 3B and D). As expected, the level of PDK2 mRNA was not changed by Ad-CA-AMPK and Ad-DN-AMPK in the presence of GH or metformin (Fig. 3A and B). We confirmed regulation of PDK4 promoter activity by AMPK with transient transfection assays. In HepG2 cells, the increase of PDK4 promoter activity by GH and/or TEL-JAK2-STAT5 was effectively reduced by metformin and CA-AMPK in a dose-dependent manner but not that of DN-AMPK (Fig. 3E and F).
FIG. 3.
Induction of SHP by AMPK inhibits GH-induced PDK4 expression in primary hepatocytes. A: Rat primary hepatocytes were infected with 30, 60 multiplicity of infection (MOI) of adenoviral vector expressing CA-AMPK for 36 h. After infection with Ad-CA-AMPK, cells were treated with GH (500 ng/mL) for 1 h. Total RNA extracts were isolated and analyzed by RT-PCR analysis with the indicated primers and then normalized to an internal control (β-actin level). *P < 0.05 and **P < 0.05 compared with untreated control, GH-treated cells. B: Rat primary hepatocytes were infected with Ad-DN-AMPK for 36 h and then treated with GH (500 ng/mL) for 1 h in the presence or absence of metformin (Met) for 12 h. Total RNA were isolated from hepatocytes and used by RT-PCR analysis. SHP, PDK2, and PDK4 mRNA levels were normalized to an internal control with β-actin level. *P < 0.05 and **P < 0.01 compared with untreated control, GH-treated cells. Rat primary hepatocytes were infected with Ad-CA-AMPK (C) and Ad-DN-AMPK (D) for 30 or 60 MOI for 36 h. After infection, cells were treated with GH (500 ng/mL) for 1 h with or without metformin for 12 h. Specific proteins were determined by Western blot analysis with the indicated antibodies, and then normalized to an internal control (β-actin level and/or total forms). E: HepG2 cells were cotransfected with CA-AMPK and DN-AMPK and then treated with GH (500 ng/mL) for 1 h in the presence of metformin for 12 h. F: Cells were cotransfected with TEL-JAK2, STAT5, CA-AMPK, and DN-AMPK and then treated with metformin for 12 h. Luciferase (Luc) activity was normalized to β-galactosidase activity to correct for variations in transfection efficiency. All data are representative of at least three independent experiments. All data are shown as fold activations relative to the control (± SEM).
To further confirm whether GH-mediated induction of PDK4 expression is altered by the metformin-AMPK-SHP pathway, we determined the effect of SHP on GH-induced PDK4 gene expression in primary hepatocytes. As expected, GH induced PDK4, and this effect was blocked by metformin (Supplementary Fig. 1). Also as anticipated, metformin induced SHP, suggesting the inhibition of the effect of GH may be mediated by SHP. That this is the case was proven by the finding that knockdown of SHP abolished the inhibitory effect of metformin on the induction of PDK4 expression by GH. Taken together with the data presented in Fig. 2, these results strongly suggest that GH-mediated induction of hepatic PDK4 expression is repressed by the metformin-AMPK-SHP pathway.
SHP physically interacts with STAT5 and inhibits the DNA binding of STAT5 on the PDK4 gene promoter.
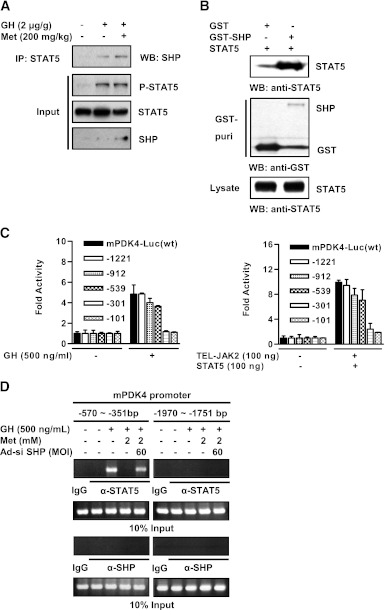
To investigate whether SHP and STAT5 physically interact, endogenous coimmunoprecipitation assays were performed with mouse liver samples prepared with control, GH alone, and GH plus metformin. Endogenous SHP strongly interacted with STAT5 in the presence of GH and metformin relative to control and GH alone (Fig. 4A). To determine the in vivo interaction between SHP and STAT5, we cotransfected mammalian expression vectors encoding GST alone or GST-SHP together with STAT5 into HepG2 cells. As shown in Fig. 4B, STAT5 strongly interacted with GST-SHP but not with GST. Collectively, these results show that SHP directly interacts with STAT5 in vivo. A proximal STAT5 consensus motif (TTCNNNGAA, -389/-378 bp) in the PDK4 promoter has been reported previously to be essential for GH- and prolactin-induced PDK4 expression in adipocytes (29). PDK4 promoter activity was continuously retained with deletions up to -539 bp. Promoter activity was largely lost with the -301 bp construct in response to GH treatment as well as TEL-JAK2/STAT5 cotransfection (Fig. 4C). These observations demonstrate that the GH-responsive region lies between -539 to -301 bp on the PDK4 gene promoter, consistent with the previously established STAT5 consensus sequence. To further confirm the inhibition of DNA binding of endogenous STAT5 proteins on the PDK4 gene promoter by SHP, we performed ChIP assays using anti-STAT5 (nonphosphorylated STAT5) or anti-phosphorylated STAT5 (p-STAT5) antibodies in primary hepatocytes. Endogenous STAT5 directly binds to the proximal (-570/-351 bp) site with GH, and this activity was clearly removed by metformin. However, the binding activity of STAT5 on the PDK4 gene promoter was restored by SHP knockdown compared with controls (Fig. 4D, left). No binding was observed in the nonspecific distal region (-1970/-1751 bp) on the PDK4 gene promoter (Fig. 4D, right). Endogenous SHP and control IgG was not bound to this promoter in either site (Fig. 4D). Furthermore, evidence of p-STAT5 occupancy on the PDK4 gene promoter was demonstrated by ChIP assay (Supplementary Fig. 2). Overall, these results demonstrate that induction of SHP by metformin decreased GH-stimulated PDK4 transcriptional activity via the blockage of STAT5 occupancy on the PDK4 gene promoter.
FIG. 4.
Interaction of between SHP and STAT5 in vivo. A: Wild-type mice were injected with GH (2 μg/g body weight) for 1 h or metformin (Met; 200 mg/kg body weight) for 6 h at the indicated concentrations. Coimmunoprecipitation assays with liver extracts demonstrate the functional association between SHP and STAT5. Tissue extracts (700 μg/lane) were immunoprecipitated with STAT5 antibody and blotted with SHP antibody. Expression of p-STAT5, STAT5, and SHP from 10% input were analyzed by immunoblotting. All mice were separated into experimental groups (n = 4–6 mice/group). B: In vivo interaction between SHP and STAT5. HepG2 cells were cotransfected with expression vectors for STAT5 together with p-EBG-SHP (GST-SHP) and p-EBG (GST alone) as a control. The complex formation (top) and the amount of STAT5 used for the in vivo binding assay (bottom, lysate) were analyzed by Western blot (WB) using anti-STAT5 antibody. The same blot was stripped and reprobed with an anti-GST antibody (middle) to confirm the expression levels of the GST-SHP and the GST control. C: Schematic diagrams show the wild-type (wt) and the deletion forms of the mPDK4 promoter constructs. HepG2 cells were cotransfected with wild-type, deletion form mPDK4 reporter, and TEL-JAK2, STAT5, respectively. After transfection for 36 h, cells were treated with GH (500 ng/mL) for 1 h. Luciferase (Luc) activity was normalized to β-galactosidase activity to correct for transfection efficiency. All data are shown as fold activations relative the control (± SEM). D: ChIP assay. Rat primary hepatocytes were infected with Ad-siSHP for 36 h and then treated with GH and metformin at the indicated concentrations. Before immunoprecipitation, an aliquot of the sample was stored and then purified and represents input for each sample. Cell extracts were immunoprecipitated with STAT5 and SHP antibodies, and purified DNA samples were used to perform PCR using primers binding the specific proximal (left) and nonspecific distal (right) regions on the mPDK4 gene promoter. All data are representative of at least three independent experiments.
GH-mediated STAT5 expression increases PDC phosphorylation by induction of PDK4.
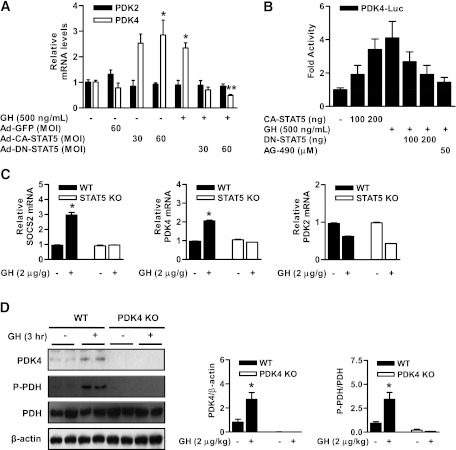
To determine whether the expression of PDK4 by GH is mediated by STAT5, we evaluated the role of STAT5 on the regulation of PDK4 gene expression using Ad-CA-STAT5 or Ad-DN-STAT5. As shown in Fig. 5A, STAT5 overexpression with Ad-CA-STAT5 increased PDK4 gene expression in primary hepatocytes. Furthermore, the stimulatory effect of GH on PDK4 expression was decreased markedly by Ad-DN-STAT5. We next confirmed CA-STAT5 and GH regulate the PDK4 transcriptional activity with a PDK4 gene promoter-luciferase reporter construct in hepatocytes. The activity of PDK4 gene promoter was significantly increased by GH or CA-STAT5 but not in DN-STAT5 and AG490, a JAK2 inhibitor (Fig. 5B). Overall, these results indicate that GH-mediated induction of hepatic PDK4 expression and activation of the PDK4 gene promoter were mediated by STAT5 in primary hepatocytes. Next, we evaluated the physiologic effect of regulation of PDK4 expression by GH in wild-type and STAT5-null mice. GH increased PDK4 expression in the liver of wild-type but not in STAT5-null mice (Fig. 5C). Under the same conditions, the mRNA of the positive control SOCS2 increased in parallel with PDK4, while PDK2 mRNA levels were not increased (Fig. 5C). To elucidate whether PDK4 induction by GH is required for phosphorylation of PDC under these conditions, we determined whether upregulation of PDK4 expression correlated with phosphorylation of PDC in the livers of GH-treated wild-type and PDK4-null mice. As shown Fig. 5D, GH significantly increased PDC phosphorylation in the liver of wild-type but not in PDK4-null mice. Taken together, these findings suggest that the GH-STAT5-PDK4 network plays a key role in the regulation of the phosphorylation state and therefore the activity of PDC.
FIG. 5.
GH-mediated induction of PDC phosphorylation is mediated by the STAT5-PDK4 pathway in the liver. A: Rat primary hepatocytes were infected with Ad-GFP, Ad-CA-STAT5, and Ad-DN-STAT5 for 36 h and then treated with GH for 3 h. Total RNA extracts were isolated and analyzed by RT-PCR analysis with the indicated primers and then normalized to an internal control (β-actin level). *P < 0.05 and **P < 0.05 compared with untreated control, GH-treated cells. B: HepG2 cells were cotransfected with CA-STAT5 and DN-STAT5 at the indicated reporter gene and then treated with GH for 3 h or the JAK2 inhibitor AG490 for 6 h after transfection for 36 h. Luciferase (Luc) activity was normalized to β-galactosidase activity to correct for variations in transfection efficiency. All data are shown as fold activations relative to the control (± SEM). C: For GH stimulation, mice were injected with GH (2 μg/g body weight i.p.), killed 4 h after injection, and livers were harvested for analyses. Total RNA was isolated from liver tissues of Stat5f/f (wild-type [WT]) and Stat5f/f;Alb-Cre (liver-specific STAT5 KO) mice. The levels of PDK2, PDK4, and SOCS2 mRNA were measured by RT-PCR analysis and then normalized to internal control (β-actin level). Three mice from each experimental group were evaluated. *P < 0.05 compared with untreated wild-type mice. D: WT and PDK4-null mice were injected with GH (2 μg/g body weight i.p.) for 3 h. Tissue extracts were isolated from livers of the indicated groups and assessed by Western blot analysis with various antibodies. The protein levels were normalized to total form antibody and/or β-actin level. All mice were separated into experimental groups (n = 5 mice/group). *P < 0.05 compared with untreated wild-type mice.
Induction of SHP by metformin regulates liver metabolites through the inhibition of PDK4 expression in vivo.
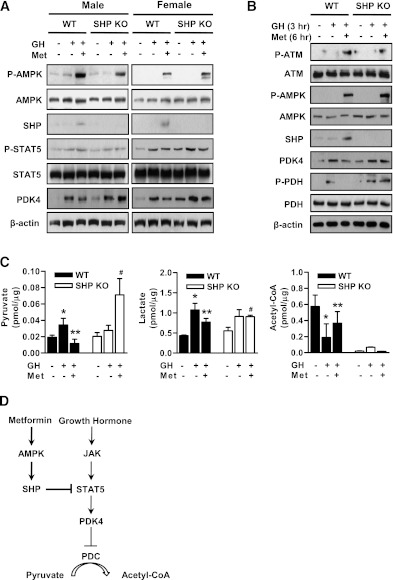
As reported previously, the pattern of GH secretion differs markedly between males and females, the basal GH level being higher in females than males (35,36). Initially, we examined basal expression of hepatic PDK2 and PDK4 in wild-type and SHP-null mice. Whereas PDK2 mRNA was similar in both genotypes, PDK4 mRNA level was significantly higher in the liver of male and female SHP-null versus the wild-type mice (data not shown). Also, endogenous PDK4 expression was higher in the liver of female relative to male mice (data not shown). Moreover, we evaluated the endogenous expression of GH receptor in wild-type, PDK4-null, and SHP-null mice. Our results show that endogenous growth hormone receptor expression was not impaired in the liver of wild-type, PDK4-null, and SHP-null mice (Supplementary Fig. 3). Metformin increased SHP expression via the activation of AMPK in both male and female wild-type mice (Fig. 6A). Induction of SHP by metformin significantly decreased GH-induced PDK4 expression in wild-type male and female mice but not in SHP-null mice (Fig. 6A). GH-induced STAT5 activation was not decreased by metformin in both mice, consistent with data presented in Fig. 2. To elucidate the correlation between PDC activation by GH-mediated PDK4 expression and metformin-induced SHP expression, GH and metformin were administered to wild-type and SHP-null mice. As expected, the increase of SHP by metformin-mediated activation of ATM and AMPK significantly decreased PDC phosphorylation via the reduction of PDK4 expression in the liver of wild-type but not in SHP-null mice (Fig. 6B). Collectively, these results demonstrate that GH induces hepatic PDK4 expression in a sex-dependent manner and metformin inhibits GH-stimulated hepatic PDK4 expression and PDC activation in wild-type but not in SHP-null mice, thereby indicating a requirement for SHP in metformin-mediated inhibition of PDK4 expression.
FIG. 6.
Metformin (Met) alters GH-dependent pathway and liver metabolites through SHP induction in vivo. A: Male and female wild-type (WT) and SHP-null mice were orally injected with metformin (200 mg/kg body weight) for 6 h and then intraperitoneally treated with GH (2 μg/g body weight) for 1 h in the feeding condition. Tissue extracts were isolated from liver tissue harvested from the mice of the indicated groups and assessed by Western blot analysis with appropriate antibodies. Protein levels were normalized to total form antibodies and/or β-actin levels. All mice were separated into experimental groups (n = 4–6 mice/group). B: WT and SHP-null mice were orally administered with metformin (200 mg/kg body weight) for 6 h and then intraperitoneally treated with GH (2 μg/g body weight) for 3 h in the fed condition. Tissue extracts were isolated from liver tissue harvested from the mice of the indicated groups and assessed by Western blot analysis with appropriate antibodies. Protein levels were normalized to total form and/or β-actin levels. All mice were separated into experimental groups (n = 4–6 mice/group). C: WT and SHP-null mice were measured at the observed time periods after metformin and GH treatment. Metabolites were analyzed with the liquid chromatography-mass spectrometry/mass spectrometry method as described in research design and methods. *P < 0.05, **P < 0.005, and #P < 0.005 compared with untreated control wild-type mice, GH-treated wild-type mice, and GH- and Met-treated wild-type mice. D: Growth hormone upregulates PDK4 gene expression by STAT5 transactivation, whereas metformin, a known activator of SHP via AMPK pathway, represses the GH-STAT5 pathway via downregulation of DNA-binding activity of STAT5 on the PDK4 gene promoter.
Finally, we measured the effect of metformin on liver metabolites in GH-treated wild-type and SHP-null mice. Metformin prevented the increase in pyruvate and lactate induced by GH in the livers of wild-type but not SHP-null mice (Fig. 6C). Metformin also prevented the decrease in acetyl-CoA concentration caused by GH in wild-type mice. This effect of metformin was not rescued in SHP-null mice because of low acetyl-CoA levels in all conditions (Fig. 6C). Overall, these results suggest that the induction of PDK4 by GH-mediated STAT5 activation plays an important role in the regulation of liver metabolite levels.
DISCUSSION
In the current study, we have demonstrated that changes induced in the phosphorylation state of PDC by the GH-STAT5-PDK4 pathway are prevented by the metformin-AMPK-SHP pathway under both in vitro and in vivo conditions. However, the inhibitory effects of metformin on this GH-dependent pathway were reversed by Ad-DN-AMPK and by Ad-si SHP in vitro conditions. The fundamental molecular mechanism of our current study demonstrated that metformin-induced SHP expression represses STAT5 occupancy on the PDK4 gene promoter through a protein–protein interaction. Moreover, the increase of PDK4 expression and PDC phosphorylation by GH was abolished in both STAT5-null and PDK4-null mice. Finally, these effects of metformin were lost in SHP-null mice.
As previously mentioned, the expression of the PDK4 gene is mediated by GH- and prolactin-stimulated STAT5 activation in adipocytes (29). However, the correlation between GH-induced PDK4 expression and the opposing effects of metformin in the liver had not been studied previously. We observed that GH significantly induced hepatic PDK4 expression through the JAK2-STAT5 signal pathway. Interestingly, we found that metformin-mediated inhibition of PDK4 expression was not related to STAT5 phosphorylation, whereas the inhibitory effects of metformin were altered by STAT5 transcriptional activity. Our results in the in vitro and in vivo experiments strongly suggest that GH acts as a positive regulator of PDK4 expression through the JAK2-STAT5 pathway and that metformin downregulated the stimulatory effects of GH by inhibition of STAT5 transactivation of the PDK4 promoter. Recent work has demonstrated that PDK4 expression is synergistically induced by AMPK and fatty acids in hepatoma cells and cardiomyocytes, whereas AMPK activation by hypoxia or AICAR (AMPK activator) had no significant effect on PDK4 expression by itself (37). However, our present findings demonstrate that metformin significantly repressed GH-induced PDK4 expression through AMPK activation in wild-type but not in SHP-null mice (Fig. 6). Furthermore, Ad-CA-AMPK inhibited GH-stimulated PDK4 expression but not that of Ad-DN-AMPK (Fig. 3), indicating that the metformin-AMPK-SHP cascade acts as a negative regulator of GH-induced PDK4 expression in both in vitro and in vivo conditions. Overall, these findings suggest that the metformin-AMPK-SHP pathway may provide with a key checkpoint for regulating PDK4 expression on hepatic metabolic disorders, such as diabetes, obesity, and insulin resistance, through the GH-dependent pathway.
Previous studies from our group have demonstrated that loss of PDK4 improves glucose homeostasis by lowering blood glucose and improving insulin sensitivity and glucose tolerance in diet-induced obese mice (27,30). As mentioned above, the concentration of three carbon substrates (pyruvate, lactate, and alanine) for gluconeogenesis is decreased in the liver of PDK4-null mice (27). Our results demonstrated that GH-mediated induction of PDK4 expression induced PDC phosphorylation in the liver of wild-type but not in PDK4- and STAT5-null mice. GH induced PDK4 expression and PDC activation elevated liver metabolites (pyruvate and lactate) associated with gluconeogenesis and reduced acetyl-CoA concentrations associated with pyruvate metabolism (Fig. 5 and Fig. 6). Furthermore, the change of metabolites caused by activation of the GH-PDK4–dependent pathway was significantly reduced by metformin in wild-type but not in SHP-null mice (Fig. 6). These results suggest that GH-mediated PDK4 expression and the resulting decrease in PDC activity may play an important role in glucose homeostasis during fasting.
Because upregulation of PDK4 and inactivation of the PDC promote gluconeogenesis in the diabetic state, our present findings further suggest that AMPK activators that mediate an increase of SHP expression may have a beneficial effect on hepatic metabolites and blood glucose in diabetes via inhibition of the GH-PDK4–signaling pathway. On the basis of the findings of this study, we suggest that metformin suppresses hepatic gluconeogenesis via inhibition of the expression of gluconeogenic genes and the reduction of gluconeogenic substrates. At this point, however, we are unable to evaluate the relative importance of these two pathways, which will surely vary in the short- and long-term.
We previously demonstrated that various pharmacologic agents, including metformin, HGF, sodium arsenite, and fenofibrate, induce SHP gene expression and repress hepatic gluconeogenesis and fibrosis through the AMPK-SHP cascade (11–13,16). We confirmed that metformin-induced SHP expression regulates GH-mediated target genes in both in vitro and in vivo conditions. Indeed, our result demonstrated that the increase of SHP by metformin markedly repressed the GH-activated PDK4 expression in both male and female wild-type mice, whereas this inhibitory effect of metformin was blunted effectively with the Ad-DN-AMPK and SHP-null mice (Fig. 3 and Fig. 6). Therefore, it is suggested that AMPK activators that increase SHP, including fenofibrate, HGF, and other natural products, may induce significant effects on hepatic PDK4 expression by the GH-STAT5 signaling pathway, whereas induction of SHP by different pathways, including bile acids, may provide a weak correlation between the AMPK-dependent pathway and GH-induced hepatic PDK4 expression.
As previously mentioned, GH is well-known to be associated with its target genes, such as IGF-1, SOCS2, and HNF-6, in hepatocytes via the activation and/or DNA-binding activity of STAT5 to these gene promoters (38-40). Thus, we determined whether the fundamental molecular mechanism of GH-induced PDK4 expression is mediated by the metformin-AMPK-SHP cascade in the liver. Our results demonstrate that endogenous STAT5 binds directly to a proximal site on the PDK4 gene promoter, that metformin prevented the association of STAT5 with the PDK4 gene promoter, and that this effect of metformin was reversed by SHP knockdown (Fig. 4D). We further observed endogenous interaction between SHP and STAT5 by coimmunoprecipitation and in vivo interaction assays (Fig. 4A and B). Therefore, our results strongly suggest that inhibition of the binding of STAT5 to DNA is the molecular mechanism responsible for SHP-mediated inhibition of GH-stimulated PDK4 expression.
In conclusion, we have shown that GH induces PDK4 gene expression via the JAK2-STAT5–dependent pathway and that the metformin-AMPK-SHP cascade represses GH-induced hepatic PDK4 expression and PDC phosphorylation, as described in Fig. 6D. Our findings provide evidence for a novel pathway for the regulation of hepatic PDK4 expression and metabolites by GH and a potential therapeutic approach for the treatment of hepatic metabolic disorders.
ACKNOWLEDGMENTS
This work was supported by a National Creative Research Initiatives Center for Nuclear Receptor Signals Grant funded from the Korean Ministry of Education, Science and Technology (20110018305), Future-based Technology Development Program (BIO Fields) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (20100019512 to H.-S.C.), and by the KRIBB Research Initiative Program of Korea (to C.-H.L.), and the World Class University program through the NRF funded by the Ministry of Education, Science and Technology (R32-10064), a Grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare Grant A111345 (to I.-K.L.).
No potential conflicts of interest relevant to this article were reported.
Y.D.K. performed the experiments, researched data, and wrote and reviewed the manuscript. Y.-H.K. performed the animal experiments and reviewed the manuscript. S.T., J.H.Y., and Y.-H.Y. researched data and contributed to discussion. N.H.J., M.S., and L.H. contributed to discussion and critical review of the manuscript. R.A.H. and I.-K.L. contributed to the analysis and interpretation of data and to the critical review and revision of the manuscript. C.-H.L. and H.-S.C. contributed to experimental design and critical review and revision of the manuscript. H.-S.C. is the guarantor of this work, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. David D. Moore (Baylor College of Medicine, Houston, TX) for permitting them to use SHP-null mice and Dr. Seok-Yong Choi (Chonnam National University Medical School, Gwangju, Republic of Korea) for his critical suggestions and helpful discussions. Specific antibodies for PDK4 and PDC were kindly provided by Drs. In-Kyu Lee (Kyungpook National University School of Medicine) and Robert A. Harris (Indiana University School of Medicine).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1665/-/DC1.
REFERENCES
- 1.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White MF. Metformin and insulin meet in a most atypical way. Cell Metab 2009;9:485–487 [DOI] [PubMed] [Google Scholar]
- 4.Edgerton DS, Johnson KM, Cherrington AD. Current strategies for the inhibition of hepatic glucose production in type 2 diabetes. Front Biosci 2009;14:1169–1181 [DOI] [PubMed] [Google Scholar]
- 5.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 2009;9:407–416 [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Connors KE, Yang DQ. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol Cell Biochem 2007;306:239–245 [DOI] [PubMed] [Google Scholar]
- 7.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 2007;403:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YS, Chanda D, Sim J, Park YY, Choi HS. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol 2007;261:117–158 [DOI] [PubMed] [Google Scholar]
- 9.Chanda D, Park JH, Choi HS. Molecular basis of endocrine regulation by orphan nuclear receptor Small Heterodimer Partner. Endocr J 2008;55:253–268 [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Kim HJ, Kim KT, et al. Orphan nuclear receptor small heterodimer partner represses hepatocyte nuclear factor 3/Foxa transactivation via inhibition of its DNA binding. Mol Endocrinol 2004;18:2880–2894 [DOI] [PubMed] [Google Scholar]
- 11.Kim YD, Park KG, Lee YS, et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008;57:306–314 [DOI] [PubMed] [Google Scholar]
- 12.Chanda D, Li T, Song KH, et al. Hepatocyte growth factor family negatively regulates hepatic gluconeogenesis via induction of orphan nuclear receptor small heterodimer partner in primary hepatocytes. J Biol Chem 2009;284:28510–28521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanda D, Kim SJ, Lee IK, Shong M, Choi HS. Sodium arsenite induces orphan nuclear receptor SHP gene expression via AMP-activated protein kinase to inhibit gluconeogenic enzyme gene expression. Am J Physiol Endocrinol Metab 2008;295:E368–E379 [DOI] [PubMed] [Google Scholar]
- 14.Park YJ, Kim SC, Kim J, et al. Dissociation of diabetes and obesity in mice lacking orphan nuclear receptor small heterodimer partner. J Lipid Res 2011;52:2234–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YJ, Qatanani M, Chua SS, et al. Loss of orphan receptor small heterodimer partner sensitizes mice to liver injury from obstructive cholestasis. Hepatology 2008;47:1578–1586 [DOI] [PubMed] [Google Scholar]
- 16.Chanda D, Lee CH, Kim YH, et al. Fenofibrate differentially regulates plasminogen activator inhibitor-1 gene expression via adenosine monophosphate-activated protein kinase-dependent induction of orphan nuclear receptor small heterodimer partner. Hepatology 2009;50:880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter-Su C, Schwartz J, Smit LS. Molecular mechanism of growth hormone action. Annu Rev Physiol 1996;58:187–207 [DOI] [PubMed] [Google Scholar]
- 18.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev 2008;22:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res 2010;20:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda A, Chang KT, Matsumoto Y, et al. Obesity and insulin resistance in human growth hormone transgenic rats. Endocrinology 1998;139:3057–3063 [DOI] [PubMed] [Google Scholar]
- 21.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009;30:152–177 [DOI] [PubMed] [Google Scholar]
- 22.Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-β and STAT3 activation. J Exp Med 2009;206:819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Hosui A, Sun R, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 2007;46:504–513 [DOI] [PubMed] [Google Scholar]
- 24.Kwon HS, Harris RA. Mechanisms responsible for regulation of pyruvate dehydrogenase kinase 4 gene expression. Adv Enzyme Regul 2004;44:109–121 [DOI] [PubMed] [Google Scholar]
- 25.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans 2003;31:1143–1151 [DOI] [PubMed] [Google Scholar]
- 26.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 2003;284:E855–E862 [DOI] [PubMed] [Google Scholar]
- 27.Jeoung NH, Wu P, Joshi MA, et al. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J 2006;397:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields AL, Falk N, Cheema-Dhadli S, Halperin ML. Accelerated loss of lean body mass in fasting rats due to activation of pyruvate dehydrogenase by dichloroacetate. Metabolism 1987;36:621–624 [DOI] [PubMed] [Google Scholar]
- 29.White UA, Coulter AA, Miles TK, Stephens JM. The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes 2007;56:1623–1629 [DOI] [PubMed] [Google Scholar]
- 30.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab 2008;295:E46–E54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu JH, Zhu BM, Wickre M, et al. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology 2010;52:1808–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni Q, Reid KR, Burant CF, Kennedy RT. Capillary LC-MS for high sensitivity metabolomic analysis of single islets of Langerhans. Anal Chem 2008;80:3539–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M, Hwang JT, Lee HJ, et al. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem 2003;278:39653–39661 [DOI] [PubMed] [Google Scholar]
- 34.Subramanian A, Wang J, Gil G. STAT 5 and NF-Y are involved in expression and growth hormone-mediated sexually dimorphic regulation of cytochrome P450 3A10/lithocholic acid 6beta-hydroxylase. Nucleic Acids Res 1998;26:2173–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pincus SM, Gevers EF, Robinson IC, et al. Females secrete growth hormone with more process irregularity than males in both humans and rats. Am J Physiol 1996;270:E107–E115 [DOI] [PubMed] [Google Scholar]
- 36.MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 1991;131:395–399 [DOI] [PubMed] [Google Scholar]
- 37.Houten SM, Chegary M, Te Brinke H, et al. Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell Mol Life Sci 2009;66:1283–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chia DJ, Ono M, Woelfle J, Schlesinger-Massart M, Jiang H, Rotwein P. Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem 2006;281:3190–3197 [DOI] [PubMed] [Google Scholar]
- 39.Vidal OM, Merino R, Rico-Bautista E, et al. In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol Endocrinol 2007;21:293–311 [DOI] [PubMed] [Google Scholar]
- 40.Lahuna O, Rastegar M, Maiter D, Thissen JP, Lemaigre FP, Rousseau GG. Involvement of STAT5 (signal transducer and activator of transcription 5) and HNF-4 (hepatocyte nuclear factor 4) in the transcriptional control of the hnf6 gene by growth hormone. Mol Endocrinol 2000;14:285–294 [DOI] [PubMed] [Google Scholar]