Abstract
Adipose-derived mesenchymal stem cells (ADMSCs) display immunosuppressive properties, suggesting a promising therapeutic application in several autoimmune diseases, but their role in type 1 diabetes (T1D) remains largely unexplored. The aim of this study was to investigate the immune regulatory properties of allogeneic ADMSC therapy in T cell–mediated autoimmune diabetes in NOD mice. ADMSC treatment reversed the hyperglycemia of early-onset diabetes in 78% of diabetic NOD mice, and this effect was associated with higher serum insulin, amylin, and glucagon-like peptide 1 levels compared with untreated controls. This improved outcome was associated with downregulation of the CD4+ Th1-biased immune response and expansion of regulatory T cells (Tregs) in the pancreatic lymph nodes. Within the pancreas, inflammatory cell infiltration and interferon-γ levels were reduced, while insulin, pancreatic duodenal homeobox-1, and active transforming growth factor-β1 expression were increased. In vitro, ADMSCs induced the expansion/proliferation of Tregs in a cell contact–dependent manner mediated by programmed death ligand 1. In summary, ADMSC therapy efficiently ameliorates autoimmune diabetes pathogenesis in diabetic NOD mice by attenuating the Th1 immune response concomitant with the expansion/proliferation of Tregs, thereby contributing to the maintenance of functional β-cells. Thus, this study may provide a new perspective for the development of ADMSC-based cellular therapies for T1D.
Autoimmune type 1 diabetes (T1D) is an inflammatory T cell–mediated autoimmune destruction of insulin-producing β-cells at the pancreatic islets (1). This process is mainly mediated by Th1-effector CD4+ cells and by proinflammatory cytokines, such as interferon (IFN)-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α (2). Some studies show that the treatment of nonobese diabetic (NOD) mice with anti–IFN-γ antibody prevents the development of diabetes (3), and the transgenic expression of this cytokine in diabetes-resistant mice results in disease development (4). In addition, the in vitro combination of IL-1β, IFN-γ, and TNF-α has been shown to increase the β-cell vulnerability to autoimmune destruction (5). The autoimmune process in T1D is also composed of regulatory components, such as CD4+CD25+Foxp3+ regulatory T cells (Tregs), which are important for suppressing the activation of the immune system and thereby maintaining homeostasis and tolerance to self-antigens (6). The reduction of Treg frequency by disrupting the B7/CD28 pathway could accelerate the onset of autoimmune diabetes in NOD mice (7), while the expansion of these cells in pancreatic lymph nodes (PLNs) was correlated with disease resistance (8). Several successful experimental therapies for T1D show a correlation between a better outcome and an increased frequency of these cells (9–11).
As a result of their immune suppressive/regulatory and regenerative potential, mesenchymal stem cells (MSCs) have emerged as a potential new therapy for T1D. Several studies from the past few years show that MSCs are capable of suppressing the immune response by inhibiting the maturation of dendritic cells and suppressing the proliferation/function of T cells, B cells, and NK cells (12–15). Moreover, MSCs have been shown to induce the expansion of CD4+CD25+Foxp3+ Tregs (16–18), and some studies evaluate the therapeutic effect of allogeneic or syngeneic bone marrow–derived MSCs in the prevention or reversion of autoimmune diabetes in several experimental models (19–26). It is important that adipose-derived (AD)MSCs, which can be isolated from fat tissue after liposuction and easily expanded in culture, have become an attractive source of MSCs for cell therapy. In addition, it has been shown that ADMSCs can suppress in vivo T-cell autoimmune responses in graft-versus-host disease and some experimental models of autoimmune diseases, such as collagen-induced arthritis, experimental colitis, and autoimmune encephalomyelitis (27–29). However, the immunosuppressive effect of ADMSCs in the treatment of T1D remains largely unexplored. In this study, we evaluated the therapeutic potential of ADMSCs in ameliorating the recent onset of experimental autoimmune diabetes in a NOD mouse model with regard to their immune regulatory properties. Therefore, we investigated the potential of ADMSC therapy to simultaneously suppress the Th1 CD4 T cell–mediated immune response involved in this disease and promote the expansion of Tregs.
RESEARCH DESIGN AND METHODS
NOD (H2-Ag7) mice were purchased from Taconic (Germantown, NY), and Balb/c mice (H2-Ad) were purchased from The Jackson Laboratory (Bar Harbor, ME). Professor Alexandre Salgado Basso (Universidade Federal de São Paulo) provided the C57BL/6 Foxp3-GFP knock-in mice. All protocols were conducted in adherence to the Brazilian Committee for Experimental Animals and were approved by the institutional ethics committee on animal use of the University of São Paulo.
Isolation and characterization of ADMSCs.
ADMSCs were isolated from epididymal fat tissue from 8-week-old male Balb/c mice (n = 10) and characterized by immunophenotyping (Supplementary Fig. 1E), potential of multilineage differentiation (Supplementary Fig. 1B–D), and ability to inhibit the proliferation of CD4+ T cells (Supplementary Fig. 1F) in agreement with previous studies (30,31).
Experimental autoimmune diabetes in NOD mice and treatment protocol.
To study the immunomodulatory properties of ADMSCs, we used the model of experimental spontaneous autoimmune diabetes in NOD mice (32). Blood glucose levels were monitored once a week with an Accu-Chek Advantage glucometer (Roche, Mannheim, Germany) in female NOD mice from age 12 to 30 weeks under nonfasting conditions. The mice were considered diabetic when blood glucose levels were >240 mg/dL for at least two sequential determinations (day 0) (24). The incidence of diabetes at age 30 weeks reached 75%. For the treatment of animals, 1 × 106 ADMSCs were suspended in 0.2 mL PBS and administrated by intraperitoneal injection in female NOD diabetic mice (n = 9) on days 0, 7, and 14. At the same time, a control group of diabetic mice (n = 7) was injected with PBS. The mice from the same offspring that did not develop diabetes (hyperglycemia) were used as controls (normoglycemic control group) (n = 5). Blood glucose levels were determined once a week after treatment, and the animals were killed on day 35 (short-term experiment). In the long-term experiment (n = 9, ADMSC-treated group; n = 15, untreated diabetic group), the mice were killed 12 weeks after the first ADMSC administration.
Intraperitoneal glucose tolerance test.
The peripheral response to glucose was evaluated by the intraperitoneal glucose tolerance test (IGTT) 32 days after the first ADMSC injection. The glucose (1.5 mg/g body wt) was administrated intraperitoneally in 12-h fasting mice, and blood glucose levels were determined before and 15, 30, 60, 90, and 120 min after glucose administration.
Histology and immunohistochemistry.
For pancreas histology, paraffin-embedded sections of 5 µm in size were stained by hematoxylin-eosin (H-E), and the extent of inflammatory cell infiltration (or insulitis score) into the pancreatic islets was evaluated. Furthermore, immunohistochemical staining was performed using anti-mouse insulin (1:400, C27C9; Cell Signaling Technology, Danvers, MA) and anti-mouse pancreatic duodenal homeobox-1 (PDX-1) (1:500, ab47267; Abcam, Cambridge, MA) antibodies. The reaction was developed using the Envision Dual Link System-HRP kit (Dako, Glostrup, Denmark) according to the manufacturer’s recommendations.
Intracellular cytokine analysis and Foxp3 staining.
Cells were obtained from pancreatic PLNs and analyzed by flow cytometry for surface markers CD4 (PerCP) and CD25 (fluorescein isothiocyanate) and afterward for intracellular transcription factor Foxp3 using allophycocyanin anti-mouse/rat Foxp3 staining (eBioscience, San Diego, CA). For intracellular cytokine staining with allophycocyanin-conjugated anti–TNF-α and fluorescein isothiocyanate-conjugated anti–IFN-γ antibodies, the BD Cytofix/Cytoperm Fixation/Permeabilization solution kit (BD Biosciences) was used according to the manufacturer’s recommendations.
Cytokine levels in serum and pancreatic tissue.
The levels of IL-2, IFN-γ, and TNF-α cytokines in the serum and pancreatic tissue homogenate were quantified using a Bio-Plex cytokine assay kit according to the manufacturer’s recommendations (Bio-Rad Laboratories, Hercules, CA).
Hormone levels.
The blood was obtained by intracardiac puncture of anesthetized nonfasting mice before they were killed on day 35. The levels of glucagon-like peptide 1 (GLP-1) (active), glucose-dependent insulinotropic polypeptide (GIP) (total), peptide YY (PYY) (total), amylin (active), leptin, and insulin were quantified in serum samples using the Mouse Gut Multiplex Assay Kit (Millipore, Billerica, MA).
Homing of ADMSCs.
To study the in vivo homing of ADMSCs, cells were labeled for 10 min at 37°C with 5 μmol/L 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR). A total of 2 × 106 cells were intraperitoneally injected into diabetic NOD mice. The CFSE+ cells in the spleen, pancreas, peritoneal cavity, and PLNs were analyzed by flow cytometry or immunofluorescence microscopy 18 and 72 h after cell injection. Age-matched untreated female NOD mice were used to verify and establish the basal tissue autofluorescence.
Enzyme-linked immunosorbent assay for TGF-β1.
An ELISA (TGFβ1 Emax; Promega, Madison, WI) was used to measure the amount of TGF-β1 protein in pancreatic tissue lysates and conditioned media according to the manufacturer’s recommendations.
In vitro expansion/proliferation of Foxp3+ cells by ADMSCs.
To evaluate the in vitro expansion of CD4+CD25+Foxp3+ cells induced by ADMSCs, CD4+ T cells were isolated from the spleen of Foxp3-GFP C57BL/6 knock-in mice using CD4 microbeads (L3T4; Miltenyi Biotec, Auburn, CA). CD4+ T cells (1 × 106) were cocultured with ADMSCs (1 × 105 cells) in a six-well plate (cell-to-cell contact) or in a transwell system (Millipore) (ADMSCs in the upper chamber and CD4+ cells in the bottom chamber) in the presence of IL-2 (50 units/mL). Where indicated, an anti–programmed death ligand 1 (PD-L1) neutralizing antibody (clone 10F.9G2; Biolegend) or isotype control was used at 5 µg/mL. Cell proliferation was verified using the CellTrace Violet Cell Proliferation Kit (Molecular Probes). The frequency and proliferation of CD25+Foxp3+ cells in the CD4+ T-cell gate were analyzed by flow cytometry after 4 days.
Statistical analysis.
Differences among groups were compared using ANOVA (Tukey posttest) or Student t test. All statistical analyses were performed using GraphPad PRISM 4 software, and the differences were considered significant at P < 0.05.
RESULTS
ADMSC treatment attenuates hyperglycemia of early-onset autoimmune diabetes.
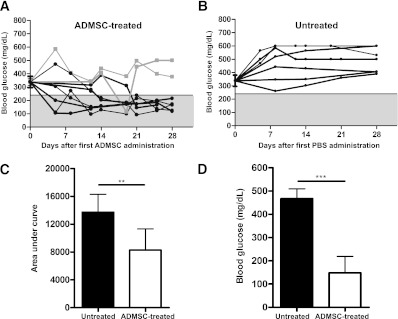
Blood glucose levels were monitored once a week in female NOD mice from age 10 to 30 weeks under nonfasting conditions. When their blood glucose levels were >240 mg/dL after two consecutive determinations, 1 × 106 ADMSCs (ADMSC-treated group) or PBS (untreated group) were injected intraperitoneally on days 0, 7, and 14 into the NOD mice. It is important that the blood glucose measurements upon initiation of treatment did not differ between groups (P = NS). The ADMSC treatment reversed the hyperglycemia of early-onset diabetes in NOD mice in 78% (seven of nine) of the animals by 28 days after the treatment. The blood glucose levels in nonfasting mice were lower in the ADMSC-treated group compared with those of the untreated diabetic mice, which remained hyperglycemic throughout the entire period studied (Fig. 1A–C). To better evaluate the hyperglycemia attenuation promoted by ADMSC treatment, the 12-h fasting blood glucose levels were determined 32 days after treatment. It is interesting that in the ADMSC-treated mice, the fasting blood glucose levels were lower compared with those of the nontreated mice (147.8 ± 70.9 vs. 467.0 ± 42.4 mg/dL, P < 0.001) (Fig. 1D). Moreover, ADMSC-treated mice did not lose weight, indicating lower morbidity caused by the disease (data not shown).
FIG. 1.
ADMSC treatment attenuates hyperglycemia of early-onset autoimmune diabetes in NOD mice. ADMSCs (1 × 106) were administrated by intraperitoneal injection in female NOD diabetic mice (blood glucose >240 mg/dL by two consecutive measurements) on days 0, 7, and 14. A: Blood glucose (mg/dL) was measured in nonfasting mice once a week after treatment for 28 days, and the mice were killed on day 35. Black lines indicate mice that showed a reduction in blood glucose levels after treatment (n = 7). A reduction in blood glucose levels was observed mainly on day 21 after the first ADMSC injection. Gray lines indicate mice that did not show a reduction in blood glucose levels after treatment (n = 2). B: Blood glucose levels in the untreated group: a group of diabetic mice was treated with PBS (n = 7) on the same days of ADMSC administration as in the treated group. The gray region denotes blood glucose values <240 mg/dL. Average ± SEM of the blood glucose levels in the initiation of treatment is shown and did not differ between groups (P = NS). C: Area under the curve of blood glucose levels from day 1 to 28. The area under the curve was determined for each animal, and the average ± SD of each group is shown. D: Blood glucose levels (mg/dL) at 12 h of fasting were determined 32 days after treatment, and lower levels were detected in ADMSC-treated mice as compared with the untreated group. Data are average ± SD. **P < 0.01, ***P < 0.0001.
Improvement of the peripheral response to glucose and glucosuria after treatment with ADMSCs.
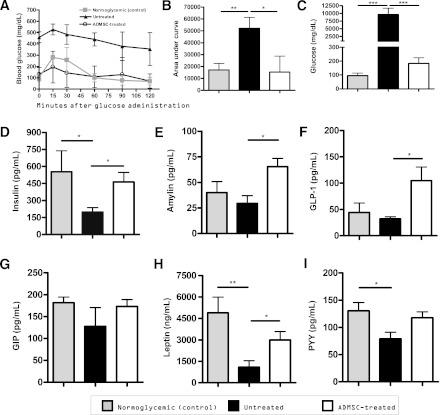
To evaluate the peripheral response to glucose, an IGTT was performed after ADMSC treatment in ADMSC-treated, untreated, and normoglycemic NOD mice. The ADMSC-treated mice showed a significant improvement in response to intraperitoneal glucose administration (Fig. 2A), and a statistically significant difference was observed when comparing the area under the curve of the IGTT among the treated and untreated mouse groups (Fig. 2B). In addition, the glucose levels in the urine samples were lower in the treated mice compared with the nontreated mice (Fig. 2C).
FIG. 2.
Improvement of the peripheral response to glucose and glucosuria after treatment with ADMSCs. A: An IGTT was performed in 12-h fasted mice 32 days after the first ADMSC administration. Glucose was administrated intraperitoneally in mice, and blood glucose levels (mg/dL) were determined 0, 15, 30, 60, 90, and 120 min after administration. B: The area under the curve corresponding from time 0 to 120 was determined for each animal, and the average ± SD of each group is shown. C: Glucosuria (mg/dL) levels were determined 28 days after treatment in urine samples using a glucometer (averages ± SD are shown). Urine was diluted in 0.9% NaCl to fit samples into the linear range of the glucometer system. Administration of ADMSCs promotes an increase in hormone levels, which contributes to the amelioration of diabetes. Blood was obtained by intracardiac puncture of anesthetized mice before they were killed on day 35 (n = 5 mice/group). The levels of insulin (D), amylin (pg/mL) (E), GLP-1 (pg/mL) (F), GIP (pg/mL) (G), leptin (ng/mL) (H), and PYY (pg/mL) (I) are shown, and all data are expressed as average ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
As shown in Fig. 2D, the serum insulin levels of ADMSC-treated mice were significantly higher than those of the untreated mice (552.2 ± 186.0 vs. 198.5 ± 38.14 pg/mL, respectively, P < 0.05) and similar to the normoglycemic control group (462.6 ± 85.85 pg/mL).
Administration of ADMSCs promotes an increase in some hormone levels reflective of autoimmune diabetes improvement.
The levels of amylin, a peptide hormone secreted with insulin by pancreatic β-cells, were higher in the treated mice than in the untreated mice (65.33 ± 8.27 vs. 29.43 ± 7.54 pg/mL, P < 0.05) (Fig. 2E). In addition, the ADMSC treatment significantly increased serum GLP-1 levels (104.5 ± 25.98 in treated mice vs. 38.82 ± 7.55 pg/mL in untreated mice) (Fig. 2F).
Treatment with ADMSCs reduces the amount of inflammatory cell infiltration and maintains insulin expression in pancreatic islets.
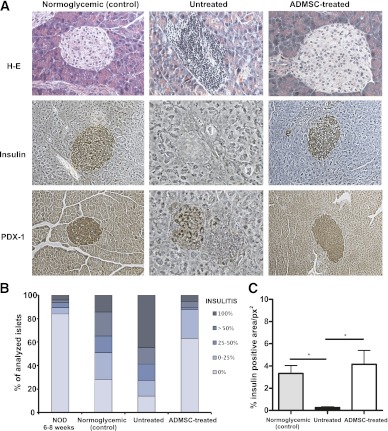
To evaluate the ability of ADMSC treatment to reduce the inflammatory cell infiltration in pancreatic islets (insulitis), the following groups were analyzed: 1) female NOD mice at age 6 weeks, 2) normoglycemic female NOD mice at age 12 weeks, 3) untreated diabetic mice, and 4) ADMSC-treated mice. The analysis of groups 1, 2, and 3 showed a spontaneous increase in insulitis with the age of NOD mice (Fig. 3B).
FIG. 3.
ADMSC therapy reduces inflammatory cellular infiltrate and improves insulin and PDX-1 expression in pancreatic islets. A: Histological analysis of the pancreas by H-E and immunohistochemistry for insulin and PDX-1 expression, respectively. Original magnification ×400. B: Quantification of mononuclear cellular infiltrate (or insulitis score) in the pancreatic islets was determined by counting approximately 60 islets/experimental group from at least five mice/group using the following classification: 0 (no cellular infiltration), 0–25, 25–50, >50, and 100% of cell infiltration in each islet. C: The positive area for insulin in the pancreatic islets was quantified using the imaging software NIS-Elements AR 3.2 (Nikon). Data are average of % insulin positive area/squared pixels (px2) ± SEM. *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
In the pancreatic islets of the untreated diabetic mice, a severe insulitis score was found. In most of the islets analyzed (44.6%), the cell infiltration covered the pancreatic islets entirely (score of 100% for insulitis) (Supplementary Fig. 2 and Fig. 3A and B). It is noteworthy that the ADMSC-treated mice showed a significant reduction in insulitis compared with the untreated group (P < 0.0001) because a large number of islets had no cellular infiltration or peri-insulitis (63.0% of analyzed islets for ADMSC-treated mice vs. 14.0% of analyzed islets for untreated mice) (Supplementary Fig. 2 and Fig. 3A and B).
Because ADMSC therapy could reduce the inflammatory cellular infiltrate, it was interesting to analyze the expression of insulin and PDX-1 in pancreatic islets, which reflect the maintenance/survival of functional β-cells, in treated mice. The islets from ADMSC-treated mice showed a higher expression of insulin compared with the untreated diabetic mice and were similar to the normoglycemic control group (P < 0.05) (Fig. 3A and C). Moreover, the PDX-1 staining of the islets from the treated mice was more similar to that observed in the normoglycemic control animals, while in the untreated mice, PDX-1 expression was barely detected (Fig. 3A).
Modulation of the CD4+ Th1 immune response in PLNs.
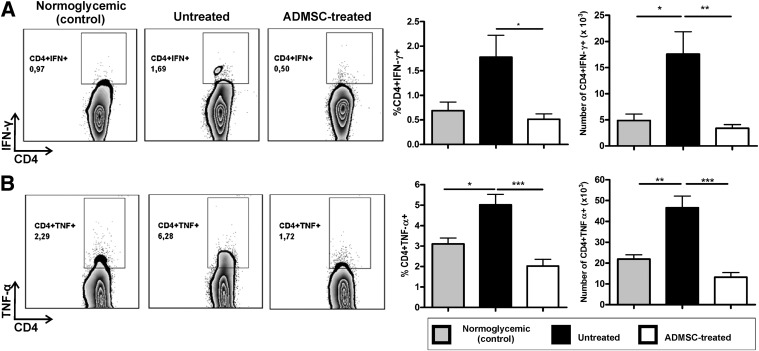
Considering that autoimmune diabetes is characterized by a Th1 immune response, we evaluated whether ADMSC treatment could attenuate the high levels of IFN-γ and TNF-α produced by CD4+ T cells in the PLNs of the diabetic NOD mice. The frequency of both CD4+IFN-γ+ and CD4+TNF-α+ T cells was lower in ADMSC-treated mice compared with the untreated group (Fig. 4A and B). To study the in vivo homing capacity of ADMSCs, the cells were CFSE-stained and then administered to diabetic NOD mice by intraperitoneal injection. It is noteworthy that ADMSCs expressed chemokine receptors (Supplementary Fig. 3A) and that CFSE+ cells were detected in the peritoneal cavity, pancreas, and PLNs 72 h after administration (Supplementary Fig. 3).
FIG. 4.
ADMSC treatment modulates the Th1 immune response by decreasing CD4+IFN-γ+ and CD4+TNF-α+ T cells in the PLNs of diabetic mice. The cells obtained from the PLNs were stimulated in vitro with phorbol-12-myristate-13-acetate (100 ng/mL) + ionomycin (1 µg/mL) and brefeldin A (1 µg/mL) for 5 h and after analysis by flow cytometry for intracellular cytokine staining. Dot plots represent CD4+IFN-γ+ (A) and CD4+TNF-α+ (B) T-cell frequencies in the CD4+ cell gate. Graphs show frequency of CD4+IFN-γ+ and CD4+TNF-α+ T cells in PLNs in the CD4+ cell gate and absolute number of CD4+IFN-γ+ and CD4+TNF-α+ T cells in the PLNs. Normoglycemic (control), female NOD from the same offspring that did not develop diabetes (hyperglycemia), used as controls (n = 5); Untreated, diabetic untreated mice (n = 5); ADMSC-treated, group of diabetic mice treated with ADMSCs (n = 5). Data are average ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
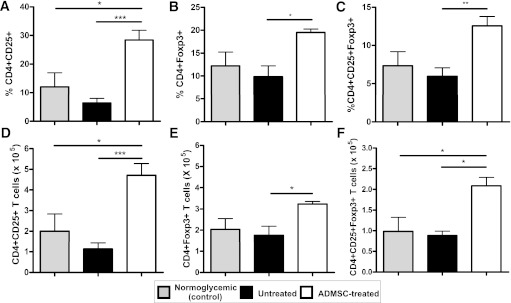
Therapy with ADMSCs increases the frequency of CD4+CD25+Foxp3+ T cells in the PLNs.
To evaluate the hypothesis that the therapeutic effect of ADMSC treatment could be associated with the expansion of Tregs, we analyzed the frequency of CD4+CD25+Foxp3+ T cells in the PLNs of treated mice compared with that of both the untreated and normoglycemic mice. The frequency and absolute numbers of CD4+CD25+Foxp3+ T cells were higher in the PLNs of treated mice than those of the untreated mice (12.55 ± 1.22 vs. 5.96 ± 1.12%, P < 0.01) (Fig. 5).
FIG. 5.
Therapy with ADMSCs increases the frequency of Tregs in PLNs of diabetic ADMSC-treated mice. Frequency of CD4+CD25+Foxp3+ T cells was analyzed by flow cytometry in cells obtained from the PLNs. The cells were stained for surface markers CD4 and CD25 and afterward for transcription factor Foxp3. A–C: The frequency of CD4+CD25+, CD4+Foxp3+, and CD4+CD25+Foxp3+ T cells in the CD4+ cell gate, respectively, for each group (n = 5/group). D–F: Absolute numbers of CD4+CD25+, CD4+Foxp3+, and CD4+CD25+Foxp3+ T cells regarding total number of cells in the PLNs were determined for each experimental group. Data are average ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
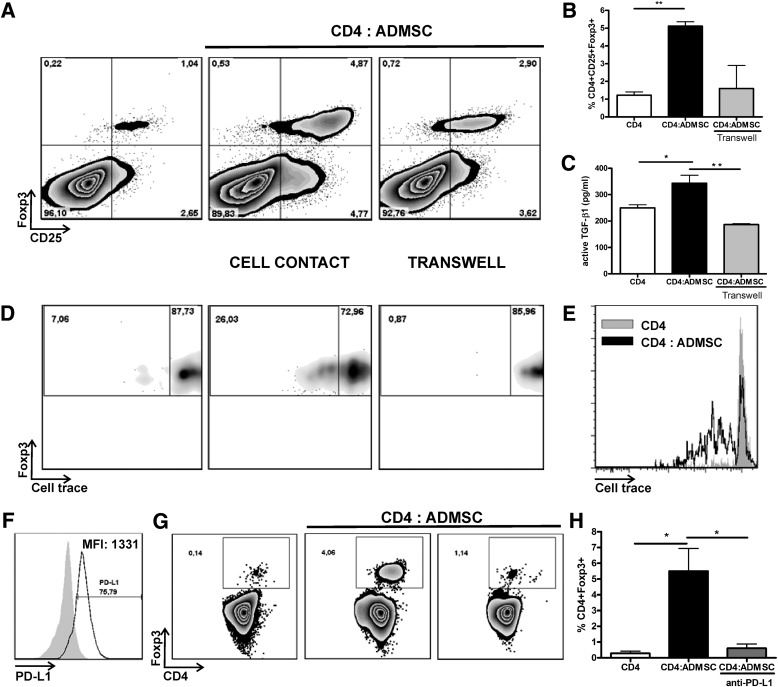
In vitro expansion of CD4+CD25+Foxp3+ induced by ADMSCs is partially dependent on cell-to-cell contact and mediated by PD-L1 costimulatory molecule.
Because ADMSC treatment promoted an increase in the frequency of CD4+CD25+Foxp3+ T cells in PLNs, we evaluated the in vitro expansion of these cells in the presence of ADMSCs. As shown in Fig. 6, both a higher frequency (Fig. 6A and B and Supplementary Fig. 4) and proliferation (Fig. 6D and E) of CD4+CD25+Foxp3+ T cells were observed after coculture of CD4+ T cells with ADMSCs (cell-to-cell contact) compared with CD4+ T cells cultured alone. This effect was accompanied by higher active TGF-β1 levels (P < 0.01) (Fig. 6C) and could be partially reversed by culturing cells in a transwell system, indicating that cell-to-cell contact mediated this process (Fig. 6).
FIG. 6.
In vitro, ADMSCs promote the expansion/proliferation of CD4+CD25+Foxp3+ T cells in a cell-to-cell contact-dependent manner in a mechanism mediated by PD-L1. A and B: In vitro expansion/proliferation of CD4+CD25+Foxp3+ cells by ADMSCs. CD4+ T cells were isolated from the spleen of Foxp3-GFP C57BL/6 knock-in mice and cocultured together (cell-to-cell contact) or in a transwell system with ADMSCs (10 CD4+:1 ADMSC). The frequency of putative Tregs is shown as CD4+CD25+Foxp3+ cells in the CD4+ T-cell gate. C: Active TGF-β levels were quantified by enzyme-linked immunosorbent assay in the conditioned mediums and are shown as average ± SEM. D: CD4+CD25+Foxp3+ T-cell proliferation was verified using Cell Trace Violet staining. The percentage of CD4+CD25+Foxp3+ divided cells after 4 days is shown by density plot representation. E: Histogram analysis showing the proliferation of CD4+CD25+Foxp3+ T cells in the presence or absence of ADMSCs. F: PD-L1 expression by ADMSCs. Expression of PD-L1 was verified in ADMSCs by flow cytometry, and the frequency and median intensity of fluorescence (MFI) are shown. G: CD4+ T cells were cocultured with ADMSCs in the presence of anti-PD-L1 neutralizing antibody (clone 10F.9G2) or isotypic control. H: Frequency of CD4+Foxp3+ cells of three independent experiments is shown (average ± SEM). *P < 0.05, **P < 0.01.
Recent studies identify a role for PD-L1 in the development, maintenance, and function of Tregs (33,34). It is interesting that PD-L1 is expressed at high levels by ADMSCs (Fig. 6F). Therefore, we hypothesized that PD-L1 could mediate the expansion of CD4+Foxp3+ T cells induced by ADMSCs. Blocking PD-L1 impaired the expansion of CD4+Foxp3+ T cells exposed to ADMSCs (Fig. 6G and H) in a dose-dependent manner (Supplementary Fig. 5), thereby indicating a role for this negative costimulatory molecule in the expansion/maintenance of Tregs induced by ADMSCs.
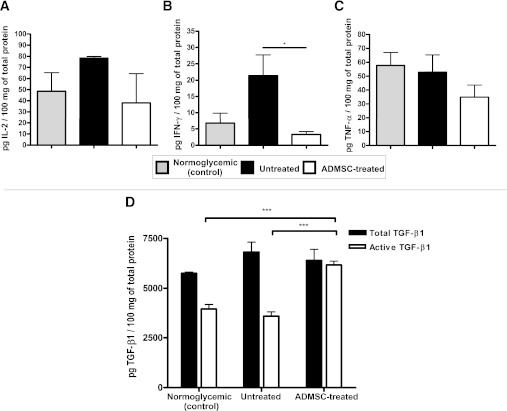
ADMSC treatment suppresses the Th1 immune response in the pancreas and promotes the high expression of active TGF-β1.
The levels of Th1 proinflammatory cytokines IL-2, IFN-γ, and TNF-α in the pancreatic tissue lysate were determined. A major reduction was observed in IFN-γ cytokine levels (3.2 ± 0.89 pg/100 mg of total protein for the ADMSC-treated group vs. 21.33 ± 6.38 pg/100 mg of total protein for the untreated group, P < 0.05) (Fig. 7B).
FIG. 7.
ADMSCs attenuate the Th1 immune response in the pancreas and promote high expression of active TGF-β1. The levels of IL-2 (A), IFN-γ (B), and TNF-α (C) were quantified in pancreatic tissue homogenate. The normalization between samples was performed using the total protein content, and the results were expressed by cytokine levels (pg)/100 mg of total protein. D: Total and biologically active TGF-β1 levels in pancreatic tissue homogenates were detected by enzyme-linked immunosorbent assay (n = 5). Results show TGF-β1 (pg)/100 mg of total protein (average ± SEM). Normoglycemic (control), female NOD from the same offspring that did not develop diabetes (hyperglycemia), used as controls; Untreated, diabetic untreated mice; ADMSC-treated, group of diabetic mice treated with ADMSCs. Data are average ± SEM. *P < 0.05, ***P < 0.001.
TGF-β1 is a suppressor cytokine associated with an improvement of the autoimmune response in T1D. Increased levels of biologically active TGF-β1 were observed in the pancreas of ADMSC-treated mice compared with those of both untreated mice (6,183 ± 184 vs. 3,590 ± 217 pg/100 mg of total protein, P < 0.001) and normoglycemic control mice (3,956 ± 232 pg/100 mg of total protein, P < 0.001) (Fig. 7D).
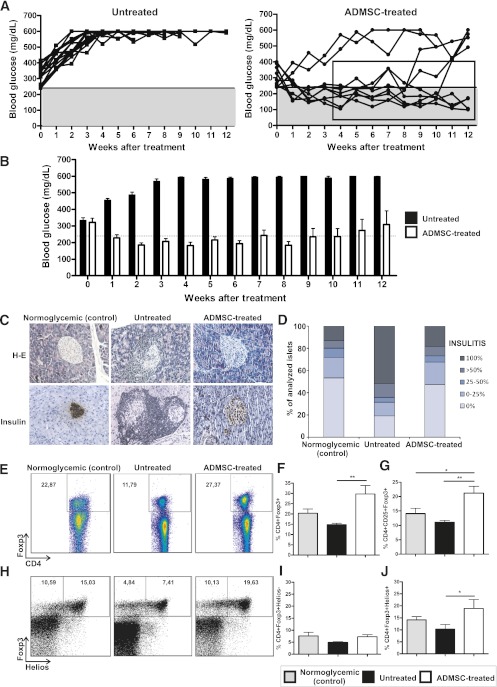
ADMSC therapy maintains long-term reversion of hyperglycemia in diabetic NOD mice.
Initially, our aim was to evaluate the short-term immune regulatory effect of ADMSCs in the treatment of experimental autoimmune diabetes. However, it was also important to evaluate the maintenance of this protective effect over long periods. Thus, ADMSC-treated mice were followed for 12 weeks after treatment. All untreated control mice had hyperglycemia during the entire monitored period (Fig. 8A). It is noteworthy that the reversion of hyperglycemia was maintained for 8 weeks in 78% (seven of nine) of the ADMSC-treated mice (Fig. 8B). The weekly mean blood glucose levels of the responder mice were significantly reduced compared with the untreated group from 2 to 12 weeks after the initial treatment (Fig. 8B and C). ADMSC-treated mice also showed a significant reduction in insulitis and a higher expression of insulin in their pancreatic islets compared with the untreated group at 12 weeks after treatment (Fig. 8C and D, respectively). In addition, a higher frequency of CD4+CD25+Foxp3+ cells was found in the PLNs of ADMSC-treated mice compared with those of the untreated controls (Fig. 8E–G).
FIG. 8.
ADMSC therapy promotes long-term reversion of hyperglycemia in diabetic NOD mice. ADMSCs (1 × 106) were administrated by intraperitoneal injection in female NOD diabetic mice (blood glucose >240 mg/dL by two consecutive measurements) on days 0, 7, and 14. A: Blood glucose (mg/dL) was measured once a week after treatment under nonfasting conditions for 12 weeks. The gray region denotes blood glucose values <240 mg/dL. Average ± SEM of blood glucose levels in the initiation of treatment is shown and did not differ between groups (P = NS). The reversion of hyperglycemia could be maintained for 8 weeks in seven of nine of the ADMSC-treated mice. B: The weekly mean blood glucose levels of responder mice were significantly reduced compared with the untreated group from 2 to 12 weeks. Normoglycemic levels were detected in 57% (four of seven) of responder mice with no insulin administration. C: Histological analysis of the pancreas by H-E and immunohistochemistry for insulin expression, respectively. Original magnification ×200. D: The quantification of mononuclear cellular infiltrate (or insulitis score) in the pancreatic islets was determined by counting at least 80 islets/experimental group from at least five mice/group using the following classification: 0 (no cellular infiltration), 0–25, 25–50, >50, and 100% of cell infiltration in each islet. The frequency of Tregs was analyzed by flow cytometry in cells obtained from the PLNs of responder mice. The cells were stained for surface markers CD4 and CD25 and afterward for transcription factor Foxp3. E: Dot plots represent the frequency of CD4+Foxp3+ cells at the CD4+ cell gate. F and G: Frequency of CD4+Foxp3+ and CD4+CD25+Foxp3+ T cells in the CD4+ cell gate, respectively, for each group. H: Dot plots represent the frequency of CD4+Foxp3+Helios+ cells at the CD4+ cell gate. I and J: Frequency of CD4+Foxp3+Helios− and CD4+Foxp3+Helios+ in the CD4+ cell gate, respectively. Data are average ± SEM. *P < 0.05; **P < 0.01. (A high-quality color representation of this figure is available in the online issue.)
Helios has recently been suggested as a potential marker for thymic-derived natural Tregs, regarding its absence of expression in induced Foxp3+ Tregs (35). It is important that ADMSC-treated mice showed a higher frequency of CD4+Foxp3+Helios+ cells in the PLNs (Fig. 8H–J and Supplementary Fig. 6), indicating that the Foxp3+ Treg subpopulation that was increased after ADMSC treatment could be derived from natural thymic-derived Tregs. Taken together, these results demonstrate an efficient long-term immune regulatory effect of ADMSCs in the treatment of recent-onset autoimmune diabetes in NOD mice.
DISCUSSION
Over the past few years, the incidence of T1D has increased, making this disease an important public health challenge worldwide (36,37). This fact has stimulated the research of new, safe, and effective therapies that could control/reverse or even prevent the disease onset. In this regard, cell-based therapies have become promising alternatives for the prevention/treatment of T1D. Human pancreatic islet transplantation provides a promising treatment for T1D; however, the lack of sufficient donors, high costs, and long-term immunosuppression limit the widespread use of this approach (38,39). Previous clinical studies suggest that moderate immunosuppression in newly diagnosed T1D can both prevent further loss of insulin production and reduce insulin needs later. In this regard, hematopoietic stem cells have been shown to have immunomodulatory potential, and the transplantation of these cells after immunosuppression in newly diagnosed T1D patients has been shown to improve β-cell function (40).
During T1D onset, residual β-cells still produce insulin, thus providing an opportunity for therapeutic intervention to stop/attenuate the autoimmune destruction and rescue the β-cell function. The MSCs regulate the immune and inflammatory responses, mainly through suppressive effects, and thereby provide therapeutic potential for immune intervention in several autoimmune diseases, including T1D (41). Recently, clinical trials evaluating the effects of MSCs have been initiated for several diseases, including T1D; however, more preclinical studies evaluating the safety, feasibility, and efficacy of their use are necessary. In addition, the mechanisms of immune regulation by MSCs in autoimmune diseases have only begun to be elucidated. The NOD mouse is an experimental model quite similar to human T1D, in which a combination of immune cell dysfunction and the presence of the Th1 proinflammatory autoimmune response leads to a failure of immune tolerance to β-cells (42). Previous studies show the immunosuppressive effect of ADMSCs on Th1 immune responses combined with an expansion of Tregs in other models of autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and colitis (27–29). Herein, we hypothesized that ADMSCs would ameliorate the recent onset of autoimmune diabetes in NOD mice by downregulating the CD4+ Th1 proinflammatory immune response concomitant with the expansion of Tregs. Although previous studies using allogeneic or syngeneic/congeneic bone marrow–derived MSCs successfully treated autoimmune diabetes in the NOD mouse model (21–24), the therapeutic potential of ADMSC treatment for autoimmune diabetes, with regard to their immune regulatory effects, was previously unexplored.
Fiorina et al. (21) showed that allogeneic bone marrow–derived MSCs homed to the PLNs and reversed hyperglycemia when administered to diabetic NOD mice. It is interesting that autologous NOD bone marrow–derived MSCs did not result in a therapeutic benefit and even caused the formation of soft tissue and visceral tumors. Therefore, we decided to use allogeneic ADMSCs, although the benefits of treatment with syngeneic/congeneic ADMSCs cannot be ignored. However, although allogeneic MSCs are considered immune-privileged cells, thereby avoiding or suppressing the immunological responses, it is important to note that the long-term recognition and rejection of these cells by the immune system cannot be disregarded.
In our study, a reduction in fasting blood glucose levels, improvement in the IGTT, and higher levels of insulin were observed in the serum of ADMSC-treated mice. Moreover, a decrease in the insulitis score accompanied by higher expression of insulin and PDX-1 in pancreatic islets was also observed, thereby indicating the maintenance of functional β-cells. It is noteworthy that higher levels of hormones that contribute to the amelioration of T1D, such as amylin, leptin, and GLP-1, were observed in ADMSC-treated mice. Amylin is a peptide hormone secreted by β-cells simultaneously with insulin, and this hormone has been shown to improve long-term glycemic control in patients with T1D (43). The combination of GLP-1 and gastrin can restore normoglycemia in diabetic NOD mice by concomitantly increasing the pancreatic β-cell mass and downregulating the autoimmune response (44). It recently was observed that GLP-1 receptor signaling can regulate lymphocyte proliferation and maintain peripheral Tregs in NOD mice (45). In our study, we observed higher levels of both GLP-1 expression and Treg frequency in ADMSC-treated mice, suggesting that these factors have a role in the improvement of the recent-onset autoimmune diabetes observed after treatment.
In other experimental models of autoimmune diseases, such as arthritis and colitis, treatment with ADMSCs has been shown to modulate the CD4+ immune response (27,46). In a model of inflammatory bowel disease, the treatment of colitic and septic mice with human or murine ADMSCs, intraperitoneally injected, significantly reduced the severity of the disease by abrogating body weight loss, diarrhea, and inflammation. This therapeutic effect was associated with the downregulation of the Th1 inflammatory response in the mucosa and draining lymph nodes and the induction of Tregs, which reduced the infiltration of inflammatory cells in various organs targeted by sepsis (28,46). Tregs are essential in the protection/attenuation of organ-specific autoimmune T1D. Weber et al. (47) showed that the transfer of Tregs resulted in the prevention of T1D in NOD mice by inhibiting the inflammatory cell infiltrate in the pancreas and blocking the onset of disease by Th1 effector cells in the PLNs. Madec et al. (22) studied the prevention of spontaneous insulitis after the injection of allogeneic bone marrow–derived MSCs in 4-week-old female NOD mice. They observed that the MSCs migrated to the PLNs and prevented autoimmune β-cell destruction and, therefore, T1D by inducing IL-10–secreting Foxp3+ T cells (22). In our study, the ADMSCs were detected in the pancreas and PLNs 72 h after administration, and a higher frequency of Tregs as well as a diminished frequency of both CD4+IFN-γ+ and CD4+TNF-α+ cells were observed in the PLNs of ADMSC-treated mice. It is important that in the long-term experiment, a higher frequency of CD4+Foxp3+Helios+ was observed in PLNs of ADMSC-treated mice, suggesting that this increase could be derived from natural thymic-derived but not peripherally induced Tregs. In addition, the levels of Th1 cytokines, such as IFN-γ, were lower and inversely correlated with a high expression of active TGF-β in the pancreas of the ADMSC-treated mice 35 days after treatment. It has been widely demonstrated that TGF-β is an important suppressor cytokine in the islet compartment for protection against CD4+ and CD8+ effector lymphocytes (48). In addition, Tonkin and Haskins (49) showed that Tregs enter the pancreas during T1D suppression and can inhibit effector T cells and macrophages in a TGF-β–dependent manner.
Herein, ADMSCs were shown to promote the in vitro expansion of Tregs in a cell-to-cell contact-dependent manner. Previous studies highlight the role of the PD-L1 negative costimulatory molecule in the development, expansion, and maintenance of Tregs (33,34). In addition, it has been shown that bone marrow–derived MSC inhibition of lymphocyte proliferation is mediated by PD-L1 (50). We showed that ADMSCs express PD-L1 and promote the in vitro expansion of CD4+Foxp3+ cells through a mechanism that is partially mediated by this molecule. In summary, the current study shows that ADMSC treatment efficiently modulated the CD4+ T cell–mediated immune response and ameliorated the recent onset of T1D pathogenesis in diabetic NOD mice by attenuating the Th1 immune response and favoring the expansion of Tregs in both short- and long-term periods. ADMSCs have many advantages over other sources of regenerative cells, such as high yield, and the procedure for obtaining the cells is minimally invasive. This work encourages further studies to confirm the safety and therapeutic potential of ADMSCs as attractive candidates for T1D treatment by exploring their immune regulatory effects.
ACKNOWLEDGMENTS
This study was supported by grants from the State of São Paulo Foundation for Research Support (07/07139-3, 09/51649-1, 2010/52180-4, 2010/12295-7, and 2010/16213-5), the Brazilian Council of Scientific and Technologic Development (501278/2010-9, 500842/2010-8, and 470456/2010-8), CNPq/DECIT/MS (573815/2008-9), and the National Institute of Science and Technology on Complex Fluids.
No potential conflicts of interest relevant to this article were reported.
Ê.J.B. researched data, performed the experiments, and wrote the manuscript. P.M.M.M.-V. researched data, performed the experiments, and reviewed the manuscript. C.S.R.M.S., D.C.A., and L.M.V. performed the experiments and reviewed the manuscript. C.S.C. and M.I.H. contributed technical assistance. A.S.B. and A.P.-S. contributed to discussion. N.O.S.C. contributed to discussion and reviewed the manuscript. N.O.S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Paulo Albe (Universidade de São Paulo) for histological technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0844/-/DC1.
REFERENCES
- 1.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell 1996;85:291–297 [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev 1998;14:129–151 [DOI] [PubMed] [Google Scholar]
- 3.Debray-Sachs M, Carnaud C, Boitard C, et al. Prevention of diabetes in NOD mice treated with antibody to murine IFN gamma. J Autoimmun 1991;4:237–248 [DOI] [PubMed] [Google Scholar]
- 4.Sarvetnick N, Liggitt D, Pitts SL, Hansen SE, Stewart TA. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell 1988;52:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachlin G, Augstein P, Schröder D, et al. IL-1beta, IFN-gamma and TNF-alpha increase vulnerability of pancreatic beta cells to autoimmune destruction. J Autoimmun 2003;20:303–312 [DOI] [PubMed] [Google Scholar]
- 6.Juedes AE, von Herrath MG. Regulatory T-cells in type 1 diabetes. Diabetes Metab Res Rev 2004;20:446–451 [DOI] [PubMed] [Google Scholar]
- 7.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000;12:431–440 [DOI] [PubMed] [Google Scholar]
- 8.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A 2003;100:10878–10883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002;51:1367–1374 [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee R, Chaturvedi P, Qin HY, Singh B. CD4+CD25+ regulatory T cells generated in response to insulin B:9-23 peptide prevent adoptive transfer of diabetes by diabetogenic T cells. J Autoimmun 2003;21:221–237 [DOI] [PubMed] [Google Scholar]
- 11.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010;207:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367–372 [DOI] [PubMed] [Google Scholar]
- 13.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008;26:212–222 [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 2004;13:263–271 [DOI] [PubMed] [Google Scholar]
- 15.Bassi EJ, de Almeida DC, Moraes-Vieira PM, Câmara NO. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev 2012;8:329–342 [DOI] [PubMed] [Google Scholar]
- 16.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010;90:1312–1320 [DOI] [PubMed] [Google Scholar]
- 17.Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 2010;185:302–312 [DOI] [PubMed] [Google Scholar]
- 18.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 2009;156:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boumaza I, Srinivasan S, Witt WT, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 2009;32:33–42 [DOI] [PubMed] [Google Scholar]
- 20.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 2006;103:17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009;183:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madec AM, Mallone R, Afonso G, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 2009;52:1391–1399 [DOI] [PubMed] [Google Scholar]
- 23.Jurewicz M, Yang S, Augello A, et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 2010;59:3139–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Wang Y, Wang D, et al. TGF-beta expression by allogeneic bone marrow stromal cells ameliorates diabetes in NOD mice through modulating the distribution of CD4+ T cell subsets. Cell Immunol 2008;253:23–30 [DOI] [PubMed] [Google Scholar]
- 25.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 2008;14:631–640 [DOI] [PubMed] [Google Scholar]
- 26.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 2003;21:763–770 [DOI] [PubMed] [Google Scholar]
- 27.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009;60:1006–1019 [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009;58:929–939 [DOI] [PubMed] [Google Scholar]
- 29.Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009;27:2624–2635 [DOI] [PubMed] [Google Scholar]
- 30.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 2008;45:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho KS, Park HK, Park HY, et al. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells 2009;27:259–265 [DOI] [PubMed] [Google Scholar]
- 32.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005;23:447–485 [DOI] [PubMed] [Google Scholar]
- 33.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010;184:3433–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 37.The global challenge of diabetes. Lancet 2008;371:1723. [DOI] [PubMed] [Google Scholar]
- 38.Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 2004;114:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 2004;4:259–268 [DOI] [PubMed] [Google Scholar]
- 40.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568–1576 [DOI] [PubMed] [Google Scholar]
- 41.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 2008;57:1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity 1997;7:727–738 [DOI] [PubMed] [Google Scholar]
- 43.Ceriello A, Piconi L, Quagliaro L, et al. Effects of pramlintide on postprandial glucose excursions and measures of oxidative stress in patients with type 1 diabetes. Diabetes Care 2005;28:632–637 [DOI] [PubMed] [Google Scholar]
- 44.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 2008;57:3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 2010;53:730–740 [DOI] [PubMed] [Google Scholar]
- 46.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009;136:978–989 [DOI] [PubMed] [Google Scholar]
- 47.Weber SE, Harbertson J, Godebu E, et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol 2006;176:4730–4739 [DOI] [PubMed] [Google Scholar]
- 48.Moritani M, Yoshimoto K, Wong SF, et al. Abrogation of autoimmune diabetes in nonobese diabetic mice and protection against effector lymphocytes by transgenic paracrine TGF-beta1. J Clin Invest 1998;102:499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tonkin DR, Haskins K. Regulatory T cells enter the pancreas during suppression of type 1 diabetes and inhibit effector T cells and macrophages in a TGF-beta-dependent manner. Eur J Immunol 2009;39:1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 2005;35:1482–1490 [DOI] [PubMed] [Google Scholar]