A small but significant amount of reactive oxygen metabolites (ROMs) is generated in the course of metabolism by mitochondria and numerous cellular oxygenases, oxidases, and peroxidases. Under normal conditions, ROMs produced in the course of metabolism and signal transduction processes are contained by the antioxidant system, which consists of numerous endogenous enzymes, substrates, and scavenger molecules as well dietary antioxidants. Individual components of the antioxidant system serve specific functions and work in concert to protect against tissue injury. Nuclear factor erythroid 2p45-related factor 2 (Nrf2) regulates constitutive expression and coordinated induction of numerous genes encoding antioxidant and phase-2 detoxifying enzymes and related proteins, such as superoxide dismutases (SODs), catalase, UDP-glucuronosyltransferase, NAD(P)H:quinone oxidoreductase-1 (NQO1), heme oxygenase-1, glutamate cysteine ligase, glutathione S-transferase, glutathione peroxidase, and thioredoxin (1). Nrf2 is kept as an inactive complex in the cytoplasm by a repressor molecule, Keap1 (Kelch-like ECH-associated protein 1). Oxidative or covalent modification of thiols in the cysteine residues of Keap1 by ROM or phosphorylation of threonine or serine residues of Nrf2 by upstream kinases results in the release and migration of Nrf2 to the nucleus where it binds to the antioxidant response elements in the promoter regions of the target genes (2,3) to promote transcription. Regulation of cellular antioxidant and anti-inflammatory machinery by Nrf2 is critical in defense against oxidative stress. In fact, Nrf2 disruption in mice attenuates or abrogates the induction of genes encoding antioxidant molecules in response to oxidative stress. In addition, ablation of the Nrf2 gene causes a lupus-like autoimmune nephritis, intensifies cyclosporine-induced tubulointerstial fibrosis, and exacerbates diabetes-induced oxidative stress, inflammation, and nephropathy in experimental animals (4–6).
The primary ROM generated in the cell is superoxide anion [O2·−], which is the byproduct of the single electron reduction of molecular oxygen [O2 + e− → O2·−]. Superoxide is normally converted to hydrogen peroxide [O2·− + 2H → H2O2] by the SOD family of enzymes located in the cytoplasm(CuZn SOD), mitochondria (MnSOD), and plasma membrane (extracellular SOD). Compared with superoxide, hydrogen peroxide is much more stable and less cytotoxic. It serves as the principal activator of redox-sensitive signal transduction pathways, transcription factors (including nuclear factor-κB [NF-κB] and activator protein-1), and growth factors. Hydrogen peroxide is normally converted to water by catalase [2H2O2 → 2 H2O + O2] and glutathione peroxidase [H2O2 + GSH → 2H2O + SG-GS], where GSH is glutathione and SG-GS is the oxidized GSH. However, it can serve as the substrate for nearly uncontainable and highly cytotoxic oxidants such as hydroxyl radical (·OH) in presence of catalytically active iron [H2O2 + Fe2+ → ·OH + OH− + Fe3+] or other transition metals and to hypochlorous acid (HOCl, commonly known as bleach) in presence of myeloperoxidase, which is abundantly expressed in granulocytes, monocytes, and macrophages [H2O2 + Cl− → HOCl]. Unlike superoxide and hydrogen peroxide, which are readily contained by the above enzymes, cells have no enzymes to neutralize hydroxyl radical or hypochlorous acid, which once formed freely damage tissues by attacking and denaturing nucleic acids, proteins, and lipids. Therefore, conditions that lead to impaired containment of hydrogen peroxide or facilitate its conversion to hydroxyl radical or hypochlorous acid can lead to cell damage and dysfunction. In this context, glycated proteins, which are produced in the hyperglycemic states, avidly bind iron and other transition metals, forming complexes in which the transition metals retain their catalytic activities (7). Conversely iron and other transition metals facilitate glycation of protein (8). In fact plasma nontransferrin-bound iron level is elevated in diabetic patients and has been implicated in the pathogenesis of the kidney and vascular complications (9).
The imbalance between the rate of ROM production and the antioxidant capacity leads to oxidative stress in which the uncontained ROMs cause tissue injury and cytotoxicity by attacking, denaturing, and modifying structural and functional molecules and activating redox-sensitive transcription factors and signal transduction pathways. These events lead to necrosis, apoptosis, inflammation, fibrosis, and other disorders that participate in the pathogenesis and progression of many acute, chronic, and degenerative disorders. Oxidative stress is caused by either increased ROM production, impaired antioxidant defense capacity, or both.
Exposure of the embryo to sustained elevation of glucose during the early stages of gestation in humans and experimental animals can result in cardiovascular, neurological, skeletal, and urogenital birth defects and partial or total renal agenesis (10). In fact elevated maternal glucose concentration has been shown to result in impaired renal morphogenesis, disruption of the ureteric bud iterations, reduction of the nascent nephron population, and increased apoptosis in the mice embryos. These defects are mediated by increased generation of ROM to which embryos are particularly vulnerable because of their low antioxidant capacity occasioned by the hypoxic intrauterine environment (11–15). By activating the redox-sensitive transcription factor, NF-κB, which is the master regulator of proinflammatory cytokines and chemokines, oxidative stress promotes inflammation. In fact, maternal diabetes-induced changes in the renal morphogenesis are accompanied by oxidative stress and inflammation (16). It is of interest that conditions associated with chronic inflammation and oxidative stress such as chronic granulomatous disease (17), asthma (18), and chronic kidney disease (19,20) are paradoxically associated with impaired Nrf2 activity, which renders the host vulnerable to the ravages of ROMs. This is due to the interference of the NF-κB P65 and P53 subunits with the dissociation of Nrf2 from Keap 1 and binding of Nrf2 to the antioxidant response elements of the target genes (21,22)
ROM production in mitochondria rises when oxygen supplies are low and/or substrate delivery is high. Increased supply of glucose in uncontrolled diabetes in the face of the hypoxic intrauterine milieu can raise mitochondrial production of ROS in the embryo. The proximal tubular epithelial cells in the embryo are particularly susceptible to hyperglycemia-induced oxidative stress. First, the impact of hyperglycemia on glucose load is far greater in proximal tubular epithelial cells than in any other cell type in the body because they serve as the vehicle for uptake of massive quantities of filtered glucose via their apical sodium/glucose cotransporter. Second, the heavy demand for generation of ATP to accommodate reabsorption of filtered sodium by the Na/K ATPase pump in the face of intrauterine hypoxic milieu can increase ROM production. Together these factors render the proximal tubular epithelial cells highly susceptible to oxidative injury (Fig. 1). In fact, in this issue of the journal, Chang et al. (23) have shown significant reduction in the size of the kidney and the number of glomeruli in the neonatal period and marked elevation of blood pressure, renal tissue ROM production, transforming growth factor-β expression, and matrix protein accumulation in adulthood in the offspring of female mice with severe diabetes. They have further shown prevention or significant attenuation of these abnormalities in the offspring of diabetic mice with targeted overexpression of catalase in the renal proximal tubular epithelial cells. The protective effect of the overexpression of catalase was accompanied by increased abundance and activity (nuclear translocation) of Nrf2 and expression of heme oxygenase-1 which is one of the many Nrf2 target gene products. These findings have provided irrefutable evidence for the role of ROMs, particularly H2O2, in the pathogenesis of and the protective effects of Nrf2 and its target gene products, especially catalase, against the maternal diabetes–induced perinatal programming of renal disease and hypertension.
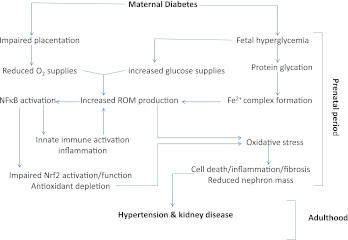
FIG. 1.
Uncontrolled maternal diabetes results in impaired placentation and fetal hyperglycemia, which respectively lead to diminished oxygen delivery and increased filtered glucose load to the developing fetal kidney. Together, the reduction in oxygen supplies and increased substrate delivery heighten mitochondrial production of ROMS. This is compounded by production of glycated proteins, which bind iron to form complexes in which iron can readily catalyze conversion of hydrogen peroxide to hydroxyl radical, the most cytotoxic ROM known. ROMs, in turn, activate NF-κB, which simultaneously results in immune cell activation and ROM generation as well as inhibition of Nrf2-mediated production of antioxidant and cytoprotective molecules. The combination of excess ROM generation and antioxidant depletion leads to oxidative stress in the developing embryonic kidney. The associated oxidative stress and inflammation results in impaired renal morphogenesis, diminished nascent nephron population, and other abnormalities that can predispose the offspring to development of hypertension and chronic kidney disease later in life. (A high-quality color representation of this figure is available in the online issue.)
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2565.
REFERENCES
- 1.Li W, Khor TO, Xu C, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 2008;76:1485–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 2008;74:1526–1539 [DOI] [PubMed] [Google Scholar]
- 3.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 2004;279:20108–20117 [DOI] [PubMed] [Google Scholar]
- 4.Yoh K, Itoh K, Enomoto A, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int 2001;60:1343–1353 [DOI] [PubMed] [Google Scholar]
- 5.Yoh K, Hirayama A, Ishizaki K, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells 2008;13:1159–1170 [DOI] [PubMed] [Google Scholar]
- 6.Shin DH, Park HM, Jung KA, et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic Biol Med 2010;48:1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming redox active “glycochelates”: implications for the pathogenesis of certain diabetic complications. Biochem Biophys Res Commun 1998;250:385–389 [DOI] [PubMed] [Google Scholar]
- 8.Young IS, Tate S, Lightbody JH, McMaster D, Trimble ER. The effects of desferrioxamine and ascorbate treatment on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med 1995;18:833–840 [DOI] [PubMed] [Google Scholar]
- 9.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol 2007;18:16–28 [DOI] [PubMed] [Google Scholar]
- 10.Kanwar YS, Nayak B, Lin S, et al. Hyperglycemia: its imminent effects on mammalian nephrogenesis. Pediatr Nephrol 2005;20:858–866 [DOI] [PubMed] [Google Scholar]
- 11.Kanwar YS, Akagi S, Nayak B, et al. Renal-specific oxidoreductase biphasic expression under high glucose ambience during fetal versus neonatal development. Kidney Int 2005;68:1670–1683 [DOI] [PubMed] [Google Scholar]
- 12.Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol 2007;24:31–41 [DOI] [PubMed] [Google Scholar]
- 13.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 2010;15:191–195 [DOI] [PubMed] [Google Scholar]
- 14.Tran S, Chen YW, Chenier I, et al. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol 2008;19:943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang SL, Chen YW, Tran S, Chenier I, Hébert MJ, Ingelfinger JR. Reactive oxygen species in the presence of high glucose alter ureteric bud morphogenesis. J Am Soc Nephrol 2007;18:2105–2115 [DOI] [PubMed] [Google Scholar]
- 16.Chen YW, Chenier I, Chang SY, Tran S, Ingelfinger JR, Zhang SL. High glucose promotes nascent nephron apoptosis via NF-kappaB and p53 pathways. Am J Physiol Renal Physiol 2011;300:F147–F156 [DOI] [PubMed] [Google Scholar]
- 17.Rieber N, Hector A, Kuijpers T, Roos D, Hartl D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin and Dev Immunol 2012;2012:252460 [DOI] [PMC free article] [PubMed]
- 18.Dworski R, Han W, Blackwell TS, Hoskins A, Freeman ML. Vitamin E prevents NRF2 suppression by allergens in asthmatic alveolar macrophages in vivo. Free Radic Biol Med 2011;51:516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 2010;298:F662–F671 [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Sato T, Rodríguez-Iturbe B, Vaziri ND. Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired Nrf2 activity in the progression of focal glomerulosclerosis. J Pharmacol Exp Ther 2011;337:583–590 [DOI] [PubMed] [Google Scholar]
- 21.Yu M, Li H, Liu Q, et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal 2011;23:883–892 [DOI] [PubMed] [Google Scholar]
- 22.Faraonio R, Vergara P, Di Marzo D, et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 2006;281:39776–39784 [DOI] [PubMed] [Google Scholar]
- 23.Chang S-Y, Chen Y-W, Zhao X-P, et al. Catalase prevents maternal diabetes–induced perinatal programming via the Nrf2–HO-1 defense system. Diabetes 2012;61:2565–2574 [DOI] [PMC free article] [PubMed]