Modest regular exercise and reduction of dietary fat halves the risk of developing type 2 diabetes. In overt type 2 diabetes, higher levels of exercise training improve glycemic control, whereas the impact of diet and optimal dietary composition are presently unknown (1). Thus, one can assume that intensive exercise protects from diet-induced insulin resistance. Intravenous lipid infusion is an established model to increase plasma free fatty acids (FFAs) and induce insulin resistance (2,3). Although plasma FFAs and intramyocellular lipid (IMCL) inversely correlate with insulin sensitivity in sedentary humans, athletes store more IMCL despite greater insulin sensitivity. This has been termed the “athletes’ paradox” (4).
In this issue of Diabetes, Phielix et al. (5) hypothesize that, relative to their untrained counterparts, the high oxidative capacity of endurance-trained athletes attenuates lipid-induced insulin resistance during hyperinsulinemic-normoglycemic clamp tests. Results showed that the athletes’ higher Vo2max was associated with greater ex vivo muscle mitochondrial capacity, insulin sensitivity, and carbohydrate oxidation. Lipid infusion reduced glucose disposal by 63% in untrained individuals, thereby confirming previous reports (3), but only by 29% in the athletes. The authors explained the athletes’ reduction in glucose disposal exclusively by diminished carbohydrate oxidation. They interpret the concomitant dephosphorylation of muscle glycogen synthase as stimulation of glycogen synthesis reflecting shuttling of glucose into nonoxidative storage as glycogen, in line with the “substrate (glucose:FFA) competition” theory of Randle et al. (6) (Fig. 1). The strength of this article includes combining in vivo and in vitro methods to assess muscle metabolism and signaling without interference from acute exercise effects. Nonetheless, some limitations need to be considered: 1) the nominally higher body weight and plasma FFAs during lipid infusion could have contributed to greater insulin resistance in the untrained participants; 2) indirect calorimetry does not measure tissue-specific nonoxidative metabolism; and 3) assessment of protein expression after prolonged insulin stimulation, which cannot trace the sequence of signaling events.
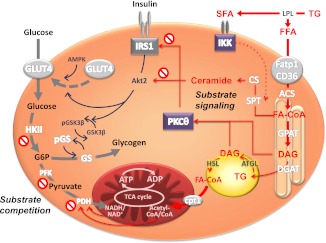
FIG. 1.
Mechanisms of lipid-induced insulin resistance in skeletal muscle. Lipolysis of plasma triglycerides (TG) by lipoprotein lipase (LPL) increases FFAs, including saturated fatty acids (SFA), which are taken up by fatty acid transporters (Fatp1/CD36) and activated to fatty acyl-CoA (FA-CoA) by acyl-CoA synthase. Substrate competition postulates that lipid oxidation in the tricarboxylic acid (TCA) cycle raises the NADH/NADH+ and acetyl-CoA/CoA ratios, which inhibits pyruvate dehydrogenase (PDH) and subsequently phosphofructokinase (PFK) and hexokinase II (HKII). Glucose-6-phosphate (G6P) would inhibit glucose uptake and feed glycogen synthesis via glycogen synthase (GSK). Substrate signaling involves the lipid intermediate DAG arising from triglyceride synthesis via glycerol phosphate synthase (GPAT) and DAG acyltransferase (DGAT) or from lipolysis via adipose tissue triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL). Certain DAG species activate novel PKC isoforms and inhibit insulin receptor substrate-1 (IRS1) signaling. Ceramides are synthesized via serine palmitoyl transferase (SPT), which is also transcriptionally activated by inhibitory-κB kinase (IKK) and ceramide synthase (CS) and in turn inhibit Akt signaling. Inhibition of insulin signaling will inhibit glucose transporter-4 (GLUT4) recruitment and thereby decrease glucose transport/phosphorylation. In this model, glycogen synthesis will decrease in turn and/or as a result of lower phosphorylation (p) of glycogen synthase kinase-3β (GSK3β), which would lead to lower glycogen synthase (GS) activity. AMPK, AMP-activated protein kinase.
Endurance training causes various adaptations such as increased muscle capillary density, glucose transporter-4 expression, and mitochondrial mass (7). Phielix et al. confirm this in that maximal oxidative phosphorylation expressed per muscle fiber was enhanced in athletes but not different from the untrained individuals when expressed per mitochondrial content. Nevertheless, baseline ATP synthase flux can be lower in relation to tricarboxylic acid cycle flux, thereby indicating less efficient mitochondrial coupling in athletes (8).
Without lipid infusion, the athletes’ higher insulin sensitivity resulted from increased oxidative, but not nonoxidative, carbohydrate metabolism. In contrast, a comparable group of athletes had augmented nonoxidative glucose disposal, muscle glycogen synthase activity, and glycogen accumulation (9). Also in sedentary individuals, muscle glycogen synthesis, resulting from increased glucose transport/phosphorylation, accounts for whole-body insulin sensitivity (10). Finally, endurance training improves insulin sensitivity in first-degree relatives of patients with type 2 diabetes by increasing myocellular glucose-6-phosphate and glycogen concentrations (11). These findings indicate that the current study’s observation requires confirmation by direct monitoring of muscle glycogen synthesis and glucose transport/phosphorylation.
Direct monitoring of cellular glucose fluxes would also be important for the article’s main conclusion that lipid-induced insulin resistance is prevented in athletes by shuttling glucose toward glycogen storage. This reasoning favors the substrate competition concept of Randle et al. above the alternative mechanism, which relies on “substrate signaling,” i.e., the interaction of lipids with insulin signaling. Randle et al. (6) inferred from rodent studies that FFAs increase the intramitochondrial acetyl-CoA/CoA and NADH/NAD+ ratios, leading to pyruvate dehydrogenase inhibition (Fig. 1). Subsequently, glycolytic intermediates and glucose-6-phosphate would accumulate and inhibit hexokinase II (HKII) activity and glucose uptake. The alternative substrate signaling mechanism postulates that myocellular lipid intermediates (diacylglycerol [DAG], ceramides) act as “lipotoxins” to inhibit insulin signaling directly or via activation of novel protein kinase C isoforms (PKC) with subsequent impairment of glucose transport/phosphorylation and reduction in glycogen synthesis (Fig. 1). Indeed, lower increases in glucose-6-phosphate precede lipid-induced reduction in insulin sensitivity and glycogen synthesis in sedentary humans (3). Phielix et al. confirm the reduced nonoxidative glucose disposal in untrained volunteers, whereas only glucose oxidation was lower in the athletes during lipid infusion. They speculate that the higher oxidative capacity of trained muscle allows for more efficient shifting from glucose to lipid oxidation. This would imply a rise in glucose-6-phosphate with decreased glucose uptake and continued glycogen synthesis. However, lipid oxidation was comparable between both groups in this study, and the lipid-induced decline of glucose oxidation was similar in another study (12). In the absence of data on glycolytic intermediates and glucose-6-phosphate, the operation of substrate competition remains to be proven for lipid-exposed athletes. Supporting substrate signaling, insulin failed to consistently stimulate Akt phosphorylation in both trained and untrained individuals. Nevertheless, glycogen synthase phosphorylation as surrogate of actual glycogen synthesis was decreased only in the athletes. It is noteworthy that increased AMP-activated protein kinase activity could stimulate glucose uptake via glucose transporter-4 translocation independently of insulin.
Endurance training also stimulates IMCL synthesis (13), which should diminish myocellular lipotoxins. Phielix et al. state that only untrained humans responded to lipid infusion with an increased total lipid fraction. However, total IMCL do not necessarily reflect true triglyceride turnover in that lipid infusion could require longer exposure times (14) or differently affect subsarcolemmal compartments, which are more closely associated with insulin resistance (15). Finally, endurance training can enhance lipoprotein lipase and FFA uptake (16), thereby raising myocellular lipotoxins. Indeed, a recent study reported accumulation of total muscle DAG as “another athletes’ paradox” (17). Detailed studies on subcellular DAG distribution, however, suggest that athletes have decreased membrane-associated saturated DAG species and membrane-to-cytosol PKCε/θ ratios (18). This could result from upregulation of DAG acyltransferase, which will take DAG to triacylglycerols and protects mice from lipid-induced insulin resistance (10). In sedentary humans, lipid infusion sequentially increases muscle DAG species, activates PKCθ, and induces insulin resistance (19). Thus, a diminished rise in DAG and novel PKC could account for the partial prevention of lipid-induced insulin resistance in athletes.
In conclusion, application of lipid infusion in athletes sheds new light on muscle metabolism, but future studies are needed to identify the sequence of events leading to lipotoxic effects not only on muscle but also on liver metabolism. This will contribute to improved characterization of subgroups at risk for type 2 diabetes as well as identification of innovative targets for the treatment of insulin resistance.
ACKNOWLEDGMENTS
The work of M.R. is supported by the German Federal Ministry of Health (BMG) and the State Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia and in part by the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). M.R. declares that there is an ongoing scientific cooperation on other topics with the group of Professor Patrick Schrauwen.
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2472.
REFERENCES
- 1.American Diabetes Association Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 1994;93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996;97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 5.Phielix E, Meex R, Ouwens DM, et al. High oxidative capacity due to chronic exercise training attenuates lipid-induced insulin resistance. Diabetes 2012;61:2472–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 7.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 1967;242:2278–2282 [PubMed] [Google Scholar]
- 8.Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 2008;105:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebeling P, Bourey R, Koranyi L, et al. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest 1993;92:1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996;335:1357–1362 [DOI] [PubMed] [Google Scholar]
- 12.Matzinger O, Schneiter P, Tappy L. Effects of fatty acids on exercise plus insulin-induced glucose utilization in trained and sedentary subjects. Am J Physiol Endocrinol Metab 2002;282:E125–E131 [DOI] [PubMed] [Google Scholar]
- 13.Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol 2010;108:1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhäusl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006;55:136–140 [PubMed] [Google Scholar]
- 15.Nielsen J, Mogensen M, Vind BF, et al. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E706–E713 [DOI] [PubMed] [Google Scholar]
- 16.Jeppesen J, Jordy AB, Sjøberg KA, et al. Enhanced fatty acid oxidation and FATP4 protein expression after endurance exercise training in human skeletal muscle. PLoS ONE 2012;7:e29391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amati F, Dubé JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 2011;60:2588–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 2012;55:1140–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szendrödi J, Yoshimura T, Phielix E, et al. The role of diacylglycerol concentrations in the development of lipid-mediated insulin resistance in human skeletal muscle. Diabetologia 2011;54(Suppl. 1):S30 [Google Scholar]