Abstract
We investigated the impact of heterogeneous nuclear ribonucleoprotein F (hnRNP F) overexpression on angiotensinogen (Agt) gene expression, hypertension, and renal proximal tubular cell (RPTC) injury in high-glucose milieu both in vivo and in vitro. Diabetic Akita transgenic (Tg) mice specifically overexpressing hnRNP F in their RPTCs were created, and the effects on systemic hypertension, Agt gene expression, renal hypertrophy, and interstitial fibrosis were studied. We also examined immortalized rat RPTCs stably transfected with control plasmid or plasmid containing hnRNP F cDNA in vitro. The results showed that hnRNP F overexpression attenuated systemic hypertension, suppressed Agt and transforming growth factor-β1 (TGF-β1) gene expression, and reduced urinary Agt and angiotensin II levels, renal hypertrophy, and glomerulotubular fibrosis in Akita hnRNP F-Tg mice. In vitro, hnRNP F overexpression prevented the high-glucose stimulation of Agt and TGF-β1 mRNA expression and cellular hypertrophy in RPTCs. These data suggest that hnRNP F plays a modulatory role and can ameliorate hypertension, renal hypertrophy, and interstitial fibrosis in diabetes. The underlying mechanism is mediated, at least in part, via the suppression of intrarenal Agt gene expression in vivo. hnRNP F may be a potential target in the treatment of hypertension and kidney injury in diabetes.
In addition to the systemic renin-angiotensin system (RAS), the existence of a local intrarenal RAS is well-established (1). We previously demonstrated that high glucose (25 mmol/L d-glucose) stimulates rat angiotensinogen (Agt; the sole precursor of all angiotensins) gene expression and induces renal proximal tubular cell (RPTC) hypertrophy and profibrotic gene expression in vitro (2–5). RAS blockers and transfer of antisense rat Agt cDNA prevent high-glucose stimulation of transforming growth factor-β1 (TGF-β1) and RPTC hypertrophy in RPTCs (6,7). RAS blockers also attenuate hypertension, proteinuria, and renal injury in diabetic transgenic (Tg) mice specifically overexpressing rat Agt in their RPTCs (8,9). Taken together, these data support a crucial role for intrarenal Agt gene expression in hypertension and kidney injury in diabetes.
We have established that insulin inhibits high-glucose stimulation of Agt gene expression and RPTC hypertrophy (10,11) via a putative insulin-responsive element (IRE) in the rat Agt gene promoter that binds two nuclear proteins, heterogeneous nuclear ribonucleoprotein F (hnRNP F) and hnRNP K (12–14). Overexpression of hnRNP F and/or hnRNP K inhibits Agt gene transcription in RPTCs in vitro (13,14). The physiological roles of hnRNP F and hnRNP K in RPTCs in vivo, however, remain undefined.
In the present studies, we investigated the effect of hnRNP F overexpression on Agt gene expression, hypertension, and RPTC injury in high-glucose milieu both in vivo and in vitro. Our results demonstrate that hnRNP F overexpression indeed attenuated hypertension, suppressed Agt and TGF-β1 gene expression, and ameliorated RPTC hypertrophy and glomerulotubular fibrosis in diabetes.
RESEARCH DESIGN AND METHODS
Chemicals and constructs.
d(+)-Glucose, d-mannitol, and monoclonal antibodies against β-actin were purchased from Sigma-Aldrich Canada (Oakville, Ontario, Canada). Rabbit polyclonal antibodies specific for hnRNP F (13) and rat Agt (15) were generated in our laboratory. Anti-p27Kip1 and anti-green fluorescence protein (GFP) antibodies were obtained from BD Biosciences (Mississauga, Ontario, Canada) and Zymed (South San Francisco, CA), respectively. Polyclonal anti–TGF-β1 antibody was procured from R&D Systems (Minneapolis, MN). Anti-angiotensin II (Ang II) antibody (immunohistochemistry grade) was bought from Phoenix Pharmaceuticals (Burlingame, CA). The plasmid pKAP2 containing the kidney-specific androgen-regulated protein (KAP) promoter that is responsive to androgen was a gift from Dr. Curt D. Sigmund (University of Iowa, Iowa City, IA) (16). Full-length rat hnRNP F cDNA [cloned in J.S.D.C.’s laboratory (13)] fused with HA-tag (which encodes amino acid residues 98–106 of human influenza virus hemagglutinin at the COOH-terminal with NotI site at both 5′ and 3′ termini) was inserted into pKAP2 plasmid at the NotI site or enhanced green fluorescent protein (pEGFP) C1 plasmid (Invitrogen, Burlington, Ontario, Canada). Oligonucleotides were synthesized by Invitrogen. Restriction and modifying enzymes were obtained from commercial sources. Mice heterozygous for the Akita spontaneous mutation of insulin 2 (Ins2) gene (C57BL/6-Ins2Akita/J) (viable and fertile) were purchased from The Jackson Laboratory (Bar Harbor, ME; http://jaxmice.jax.org).
Generation of Akita hnRNP F-Tg mice.
Tg mice specifically overexpressing hnRNP F in their RPTCs were generated as described previously for Tg mice specifically overexpressing rat Agt or catalase in their RPTCs (8,17).
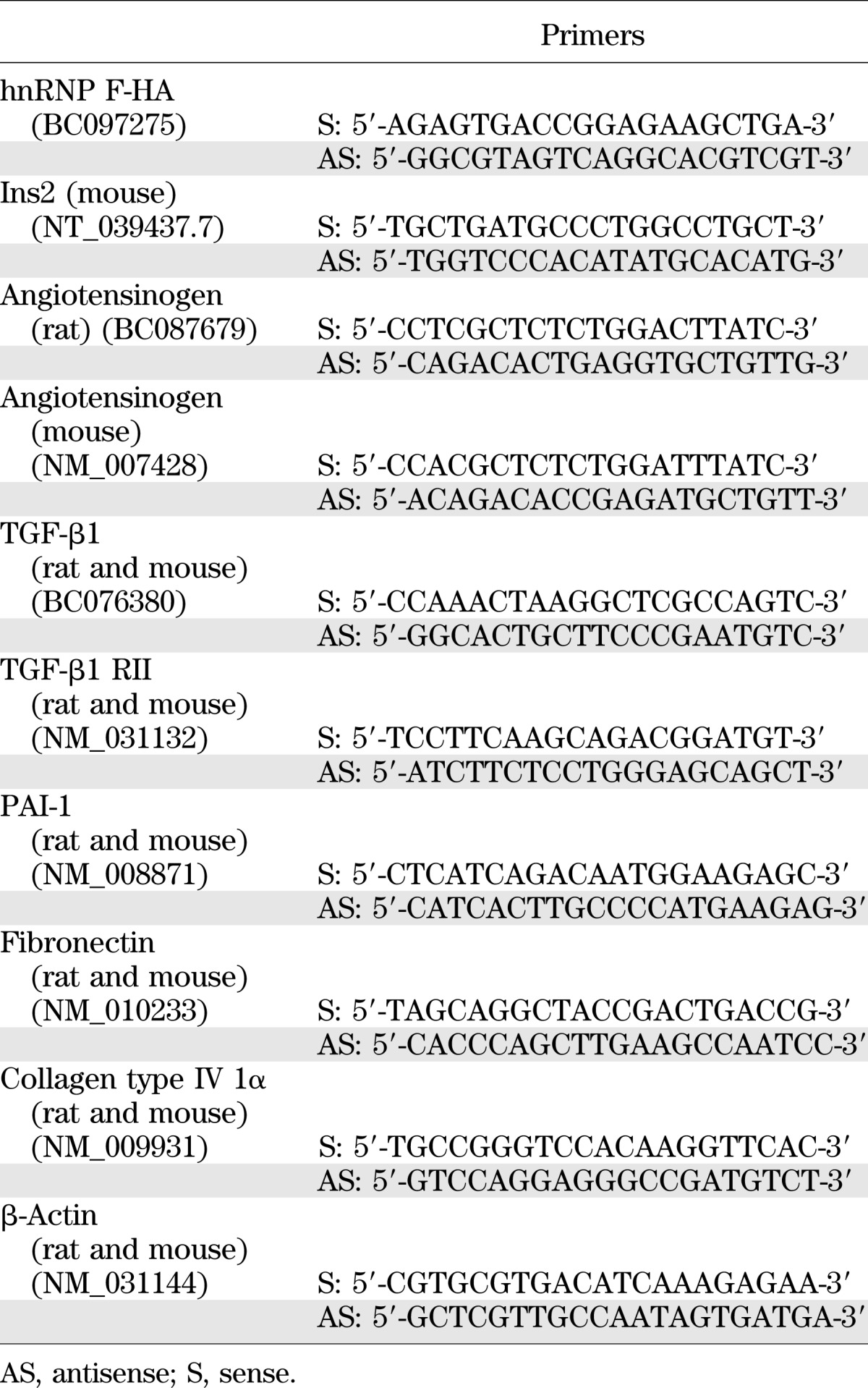
Akita hnRNP F-Tg mice were created by cross-breeding of homozygous hnRNP F-Tg mice with heterozygous Akita mice (homozygous Akita mice are infertile). Primers used for PCR genotyping of Akita hnRNP F-Tg mice are listed in Table 1.
TABLE 1.
Primers for genotyping and RT-qPCR
Physiological studies.
Male wild-type (WT) adult control littermates, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice (six mice per group) were used. All animals received standard mouse chow and water ad libitum. Animal care and procedures were approved by the Centre de Recherche du Centre Hospitalier de l’Université de Montréal’s Animal Committee.
Systolic blood pressure (SBP) was monitored with a BP-2000 tail-cuff pressure machine (Visitech Systems, Apex, NC) as described previously (8,9,18–20). Twenty-four hours prior to euthanasia (at 20 weeks of age), they were housed individually in metabolic cages. Body weight was recorded. Urine was collected and assayed for albumin and creatinine (ELISA; Albuwell and Creatinine Companion; Exocell, Philadelphia, PA) (8,9,18–20). Mouse serum and urinary Agt were assayed by ELISA (Immuno-Biological Laboratories, IBL America, Minneapolis, MN). The kidneys were removed, decapsulated, and weighed. The left kidneys were processed for histology and immunostaining study, and the right kidneys were used for isolation of renal proximal tubules (RPTs) by Percoll gradient (8,9,18–20). Aliquots of freshly isolated RPTs from individual animals were used immediately for total RNA isolation and Western blotting (WB).
Histology.
Kidneys were collected in Tissue-Tek cassettes (VWR Canlab, Montreal, Quebec, Canada), dipped immediately in ice-cold 4% paraformaldehyde, fixed for 24 h at 4°C, and then processed by the Centre Hospitalier de l’Université de Montréal Pathology Department. Tissue sections (four to five sections per kidney) were counterstained with periodic acid Schiff (PAS) or Masson’s Trichrome staining (8,9,18–20) and analyzed by light microscopy by two investigators blinded to treatment.
The mean glomerular and RPTC volumes and tubular luminal areas were determined as previously described (9,18–20).
Immunohistochemical staining was performed by the standard avidin-biotin-peroxidase complex method using four to five sections per kidney and three mouse kidneys per group (ABC Staining System; Santa Cruz Biotechnology) (18–20). Staining was analyzed by light microscopy by two independent investigators blinded to treatment. The collected images were analyzed and quantified using National Institutes of Health ImageJ software (17–19).
Real-time quantitative PCR.
Agt, TGF-β1, TGF-β1 receptor II (TGF-β1 RII), plasminogen activator inhibitor-1 (PAI-1), collagen type IV Iα, fibronectin, and β-actin mRNA expression in RPTs was quantified by real-time quantitative PCR (RT-qPCR) with forward and reverse primers (Table 1) (18–20).
Western blotting.
WB was performed as described previously (12–14,18–20). The relative densities of hnRNP F, Agt, p27Kip1, and β-actin bands were quantified by computerized laser densitometry (ImageQuant software, version 5.1; Molecular Dynamics, Sunnyvale, CA).
Serum and urinary Ang II measurement.
Mouse serum and urine samples were extracted with a kit and assayed for Ang II by specific ELISA (Bachem Americas, Torrance, CA) as described previously (20).
Cellular hypertrophy.
Immortalized rat RPTCs (21) were stably transfected with the plasmid pEGFP C1 or pEGFP C1/hnRNP F and cultured in normal glucose (5 mmol/L d-glucose plus 20 mmol/L d-mannitol) or high glucose (25 mmol/L d-glucose) as described previously (18–20) and observed under an ECLIPSE TE2000 fluorescence microscope (Nikon, Melville, NY). Cellular hypertrophy was assessed by several indices of hypertrophy: total cellular protein content, [3H]leucine incorporation, and cellular p27kip1 expression, as described previously (5–7,11).
Statistical analysis.
Statistical significance between the experimental groups was analyzed initially by Student t test or one-way ANOVA and the Bonferroni test as appropriate. The data are expressed as means ± SEM. The P values <0.05 were considered to be statistically significant.
RESULTS
RPTC-specific expression of the hnRNP F transgene in Akita and Tg mouse kidneys.
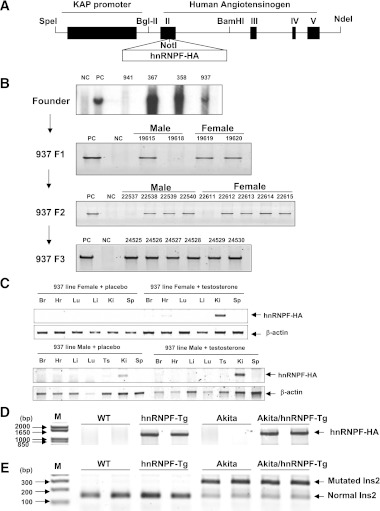
KAP2-hnRNP F transgenic mice were generated to produce specific and inducible expression of hnRNP F in RPTs. This was accomplished by inserting hnRNP F cDNA including the stop codon into a construct containing the KAP promoter and exons 2–5 of the human Agt gene, including noncoding DNA at the 3′ terminal (Fig. 1A). (Note: human Agt could not be expressed because exon I of the human Agt gene containing the starting site of transcription is deleted in the plasmid.) Southern blot analysis revealed the presence of the transgene in heterozygote and homozygote animals (Fig. 1B). Testosterone implant induced hnRNP F-HA transgene expression in the kidney of female and male Tg mice but not in other tissues, and placebo pellet had no effect (Fig. 1C). Similarly, hnRNP F-HA transgene was detected in RPTs of hnRNP F-Tg and Akita hnRNP F-Tg mice but not in WT or Akita mice (Fig. 1D). Mutated Ins2 gene was detected in Akita and Akita hnRNP F-Tg mice but not in WT or hnRNP F-Tg mice (Fig. 1E). These results confirm that the KAP gene promoter directs hnRNP F-HA transgene expression in RPTCs of hnRNP F-Tg and Akita hnRNP F-Tg mice.
FIG. 1.
Generation of hnRNP F-Tg and Akita hnRNP F-Tg mice. A: Schematic map of the KAP2-rat hnRNP F construct. The isolated 17-kb KAP2-hnRNP F-HA transgene (digested with SpeI and NdeI) was microinjected into one-cell fertilized mouse embryos obtained from superovulated C57Bl6 × C3H mice (performed at the Clinical Research Institutes of Montreal, Montreal, Quebec, Canada). B: Southern blotting of genomic DNA for founders with radioactive hnRNP F probe. Heterozygous and homozygous F1, F2, and F3 were screened by PCR with specific primers (Table 1). C: RT-PCR product showing tissue expression of hnRNP F-HA mRNA in female and male Tg mice uninduced or induced with testosterone. β-Actin and hnRNP F-HA fragments are indicated. Transgenic mice (line #937) were induced with placebo pellets or pellets containing 5 mg testosterone with a 21-day release schedule (catalog number A-121; Innovative Research of America, Sarasota, FL) for 2 weeks prior to RNA isolation. D: Specific PCR analysis of hnRNP F-HA transgene in offspring of hnRNP F-Tg line 937 crossbred with heterozygous Akita mice. Akita hnRNP F-Tg mice displaying hnRNP F-HA transgene were used in subsequent experiments. E: Genotyping of Ins2 gene mutation in WT control littermates, hnRNP F-Tg mice, Akita, and Akita hnRNP F-Tg mice with primers of the mouse Ins2 gene (Table 1). The PCR-DNA fragment (280 base pairs) of mouse Ins2 gene was then digested with the restriction enzyme Fnu4H1 for 1 h at 37°C. If an allele was mutated, two DNA fragments with 280 and 140 base pairs were observed on 3% agarose gel electrophoresis. Br, brain; Hr, heart; Ki, kidney; Li, liver; Lu, lung; Sp, spleen; Ts, testis.
HnRNP F overexpression attenuates Agt expression in Akita hnRNP F-Tg mice.
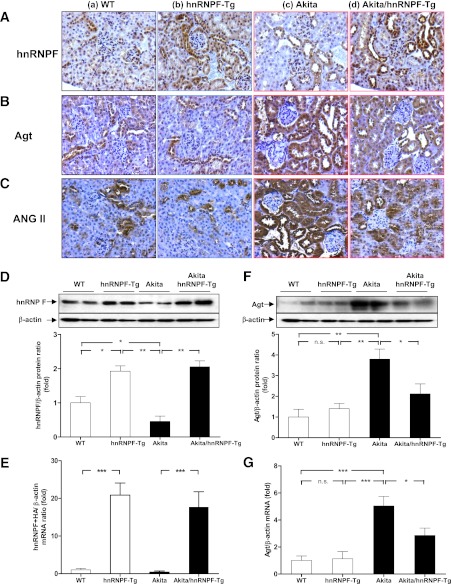
Immunostaining of renal sections (Fig. 2A), WB of isolated RPTs (Fig. 2D), and RT-qPCR of hnRNP F-HA transgene (Fig. 2E) revealed that hnRNP F expression was significantly higher in RPTCs from hnRNP F-Tg and Akita hnRNP F-Tg mice compared with WT or Akita mice, respectively.
FIG. 2.
HnRNP F, Agt, and Ang II expression in Tg mouse kidneys at week 20. Immunohistochemical staining for hnRNP F (A), Agt (B), and Ang II (C) in mouse kidneys. a: WT control mouse. b: hnRNP F-Tg mouse. c: Akita mouse. d: Akita hnRNP F-Tg mouse. Original magnification ×200. D: WB analysis of hnRNP F expression in RPTs from kidneys of WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. E: RT-qPCR of hnRNP F mRNA expression in RPTs of WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. F: WB analysis of Agt expression in RPTs from kidneys of WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. G: RT-qPCR of Agt mRNA expression in RPTs of WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. Values are expressed as means ± SEM; N = 6. WT and hnRNP F-Tg mice, empty bars; Akita and Akita hnRNP F-Tg mice, filled bars. *P < 0.05; **P < 0.01; ***P < 0.005. (A high-quality digital representation of this figure is available in the online issue.)
In contrast, immunostaining (Fig. 2B), WB (Fig. 2F), and RT-qPCR (Fig. 2G) revealed significantly increased Agt expression in RPTs of Akita mice as compared with WT controls and hnRNP F-Tg mice but was reduced in Akita hnRNP F-Tg mice. The findings of Agt immunostaining in mouse kidneys (Fig. 2B) were further confirmed by immunostaining for Ang II in mouse kidneys (Fig. 2C and Supplementary Fig. 1A and B). These data demonstrated that hnRNP F overexpression suppresses Agt expression in RPTs of Akita mice.
Overexpression of hnRNP F attenuates hypertension and kidney hypertrophy in Akita hnRNP F-Tg mice.
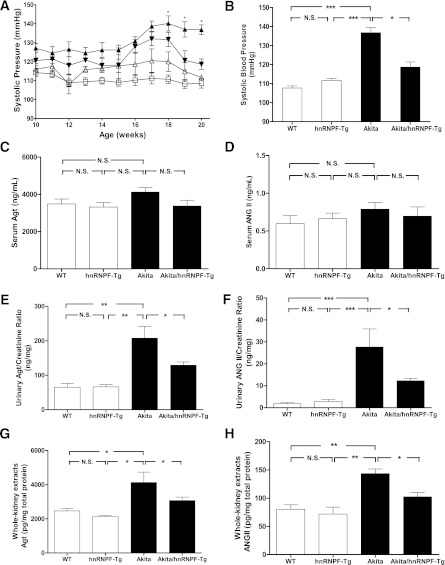
Longitudinal SBP measurement showed that SBP was higher in Akita mice than WT or hnRNP F-Tg mice from week 10 to 20; however, there was no significant difference in SBP between Akita mice and Akita hnRNP F-Tg mice detected until week 18 (Fig. 3A). Cross-sectional SBP measurement at week 20 revealed that SBP was significantly elevated in Akita mice compared with WT and hnRNP F-Tg mice but significantly attenuated in Akita hnRNP F-Tg mice (Fig. 3B), demonstrating that hnRNP F overexpression attenuates systemic hypertension.
FIG. 3.
Overexpression of hnRNP F attenuates systemic hypertension in Akita Tg mice. A: Longitudinal changes in mean SBP in male WT control (□), hnRNP F-Tg (△), Akita (▲), and Akita hnRNP F-Tg mice (▼). The mice were trained in the procedure for at least 15–20 min per day for 5 days before the first SBP measurements. B: Cross-sectional analysis of SBP (measured two to three times per animal per week in the morning without fasting, week 20). Serum Agt levels (C), serum Ang II levels (D), urinary Agt levels (E), urinary Ang II levels (F), Agt levels in whole-kidney extracts (G), and Ang II levels in whole-kidney extracts (H) in WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. Serum and urinary Agt were assayed by specific enzyme-linked immunosorbent assay (ELISA), and urinary Agt levels were normalized with urinary creatinine levels. Serum and urinary Ang II were extracted, assayed by specific ELISA, and normalized with urinary creatinine levels. Agt and Ang II levels in whole-kidney extracts were assayed by respective ELISA for Agt and Ang II. All data are expressed as means ± SEM; N = 6. WT and hnRNP F-Tg mice, empty bars; Akita and Akita hnRNP F-Tg mice, filled bars. *P < 0.05; **P < 0.01; ***P < 0.005.
Serum Agt and Ang II levels did not differ significantly in the four different groups of mice studied (Fig. 3C and D, respectively). In contrast, urinary Agt and Ang II levels were significantly higher in Akita mice as compared with WT control littermates or hnRNP F-Tg mice but attenuated in Akita hnRNP F-Tg mice (Fig. 3E and F, respectively). Furthermore, Agt (Fig. 3G) and Ang II (Fig. 3H) levels in whole-kidney extracts were significantly higher in Akita mice than in WT or hnRNP F-Tg mice and were attenuated in Akita hnRNP F-Tg mice, supporting the notion that augmented renal Ang II might be responsible, at least in part, for the development of hypertension in Akita mice.
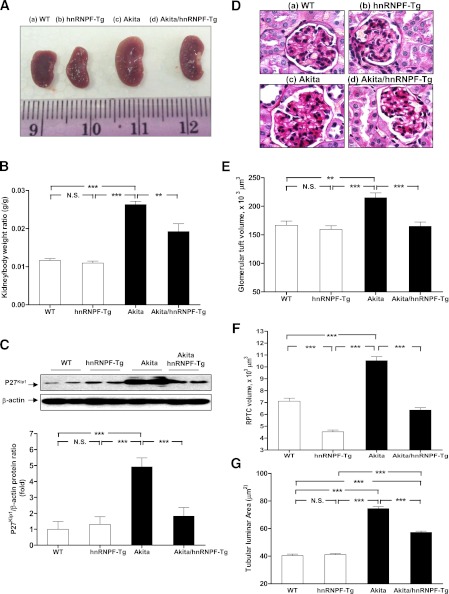
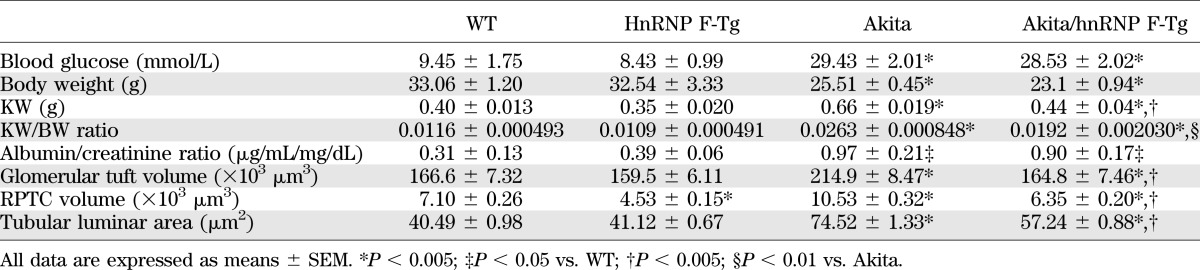
Kidney size and kidney weight/body weight (KW/BW) ratio were significantly higher in Akita mice as compared with WT controls and hnRNP F-Tg mice at 20 weeks of age, and these parameters were significantly attenuated in Akita hnRNP F-Tg mice (Fig. 4A and B and Table 2). WB of p27Kip1 expression (a marker of cell hypertrophy) showed that p27Kip1 expression was significantly elevated in RPTs of Akita mice compared with WT controls and hnRNP F-Tg mice (Fig. 4C), and it was significantly attenuated in Akita hnRNP F-Tg mice. These data further confirmed the kidney hypertrophy in Akita mice and that hnRNP F overexpression could partially attenuate the kidney hypertrophy. In contrast, blood glucose levels or urinary albumin/creatinine ratio in Akita mice were significantly higher than in WT controls and hnRNP F-Tg mice and did not normalize in Akita hnRNP F-Tg mice (Table 2).
FIG. 4.
Overexpression of hnRNP F attenuates renal hypertrophy in Akita Tg mice. A: Kidney size. B: KW/BW ratio. C: WB of p27kip1 protein expression in RPTs of WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. The relative densities of the p27Kip1 band were normalized with the β-actin band. p27Kip1 level in WT control mouse RPTs was considered as the control (100%). D: Periodic acid Schiff staining of mouse kidneys: WT control mouse (a), hnRNP F-Tg mouse (b), Akita mouse (c), and Akita hnRNP F-Tg mouse (d) at week 20. Original magnification ×600. Mean glomerular volume (E), mean RPTC volume (F), and mean tubular luminal area (G) of WT control mice, hnRNP F-Tg mice, Akita mice, and Akita hnRNP F-Tg mice at week 20. **P < 0.01; ***P < 0.005. (A high-quality digital representation of this figure is available in the online issue.)
TABLE 2.
Physiological measurements
Histologic studies.
Unlike WT mice (Fig. 4Da) and hnRNP F-Tg mice (Fig. 4Db), renal structural damage was evident in Akita mice (Fig. 4Dc). Histological findings included glomerular expansion, tubular luminal dilation, vacuolar degeneration in RPTCs, accumulation of cell debris in the tubular lumen, and loss of RPTC brush borders. The kidneys of Akita hnRNP F-Tg mice (Fig. 4Dd) ameliorated these morphological changes but did not completely reverse these abnormalities.
Morphological analysis revealed significantly augmented glomerular volume (Fig. 4E), RPTC volume (Fig. 4F), and tubular luminal areas (Fig. 4G) in Akita mice compared with non-Akita WT and hnRNP F-Tg mice. Overexpression of hnRNP F prevents the increases in glomerular and RPTC volumes and tubular luminal area in Akita hnRNP F-Tg mice (Fig. 4E–G and Table 2). Interestingly, RPTC volume was also significantly attenuated in hnRNP F-Tg compared with non-Akita WT controls.
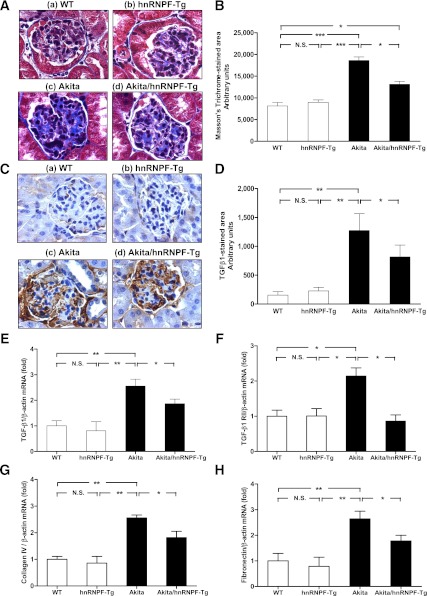
HnRNP F overexpression suppresses TGF-β1 and profibrotic gene expression in Akita hnRNP F-Tg mice.
Significant increase of Masson’s Trichrome staining and TGF-β1 immunostaining was observed in glomerulotubular areas of Akita mice (Fig. 5Ac and C) as compared with WT controls (Fig. 5Aa and C) and hnRNP F-Tg mice (Fig. 5Ab and C) but was reduced in Akita hnRNP F-Tg mice (Figs. 5Ad and C). Quantification of Masson’s Trichrome-stained (Fig. 5B) and TGF-β1–stained areas (Fig. 5D) confirmed these findings. Furthermore, TGF-β1 mRNA (Fig. 5E) by RT-qPCR confirmed TGF-β1 expression. These data demonstrated that hnRNP F overexpression suppresses tubulointerstitial fibrosis and TGF-β1 expression in kidneys of Akita mice.
FIG. 5.
Overexpression of hnRNP F ameliorates glomerulotubular fibrosis and suppresses profibrotic gene expression in Akita Tg mice. A: Masson’s Trichrome staining of collagenous components expression in mouse kidneys: WT control mouse (a), hnRNP F-Tg mouse (b), Akita mouse (c), and Akita hnRNP F-Tg mouse (d). Original magnification ×600. B: Semiquantitative analysis of Masson’s Trichrome staining in glomerulotubular areas of kidney sections from WT control, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice at week 20. C: Immunohistochemistry of TGF-β1 protein expression in mouse kidneys: WT control mouse (a), hnRNP F-Tg mouse (b), Akita mouse (c), and Akita hnRNP F-Tg mouse (d). Original magnification ×600. D: Semiquantitative analysis of TGF-β1 staining in kidney sections from WT control mice, hnRNP F-Tg mice, Akita mice, and Akita hnRNP F-Tg mice at week 20. RT-qPCR of TGF-β1 (E), TGF-β1 RII (F), collagen type IV 1α (G), and fibronectin (H) mRNA expression in RPTs of WT controls, hnRNP F-Tg, Akita, and Akita hnRNP F-Tg mice. Values are expressed as means ± SEM; N = 6. WT and hnRNP F-Tg mice, empty bars; Akita and Akita hnRNP F-Tg mice, filled bars. *P < 0.05; **P < 0.01; ***P < 0.005. (A high-quality digital representation of this figure is available in the online issue.)
Enhanced expression of TGF-β1 RII (Fig. 5F), collagen type IV (Fig. 5G), fibronectin (Fig. 5H), and PAI-1 (not shown) mRNA expression by RT-qPCR was also observed in RPTs of Akita mice as compared with WT controls and hnRNP F-Tg mice. Once again, hnRNP F overexpression attenuated the expression of these genes in RPTs of Akita hnRNP F-Tg mice. The data indicate that hnRNP F overexpression effectively prevents tubulointerstitial fibrosis in Akita hnRNP F-Tg mice.
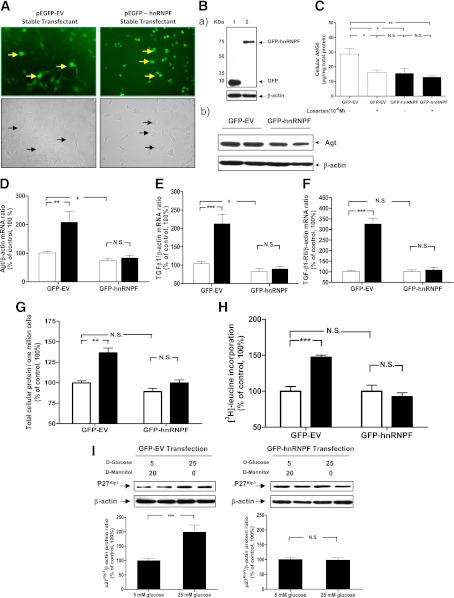
HnRNP F overexpression prevents high glucose–induced RPTC hypertrophy.
In vitro studies showed that fluorescence was predominantly cytoplasmic in pEGFP C1 empty vector (GFP-EV) stable transfectants, but was exclusively nuclear in GFP-hnRNP F transfectants (Fig. 6A). Thus, hnRNP F is a nuclear protein. Fig. 6B displays the WB of GFP-EV and GFP-hnRNP F stable transfectants. GFP-hnRNP F fusion protein was expressed as predicted at 72 kDa (the apparent molecular weights of GFP and hnRNP F were 26 and 46 kDa, respectively) (Fig. 6Ba). Furthermore, cellular Agt (Fig. 6Bb) and Ang II (Fig. 6C) expression is suppressed in GFP-hnRNP F stable transfectants as compared with that in GFP-EV stable transfectants. Losartan (10−6 M) significantly inhibited Ang II expression in GFP-EV stable transfectants but had no additional inhibitory effect on Ang II expression in GFP-hnRNP F stable transformants. These findings are consistent with our in vivo data that hnRNP F overexpression suppressed Agt and Ang II expression in RPTs of Akita hnRNP F-Tg mice.
FIG. 6.
hnRNP F overexpression prevents high-glucose stimulation of Agt, TGF-β1, and TGF-β1 RII mRNA expression and cellular hypertrophy in RPTCs. A: RPTCs were stably transfected with empty vector (GFP-EV) or pEGFP C1 containing the hnRNP F cDNA (GFP-hnRNP F) and observed under an ECLIPSE TE2000 fluorescence microscope (Nikon). B: WB of GFP fusion protein (a) and Agt (b) expressions in stable transfectants. C: Cellular Ang II levels in stable transfectants cultured with or without losartan (10−6 M) was assayed by ELISA and normalized by cellular proteins. RT-qPCR analysis of Agt mRNA (D), TGF-β1 (E), and TGF-β1 RII (F) mRNA expression in stable transfectants. Total cellular protein content per million cells (G), [3H]leucine incorporation (H), and WB of p27Kip1 protein expression (I) in RPTCs stably transfected with GFP-EV or GFP-hnRNP F. The cells were incubated in medium containing 5 mmol/L d-glucose plus 20 mmol/L d-mannitol or 25 mmol/L d-glucose for 24 h. Values are expressed as means ± SEM; N = 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005. (A high-quality color representation of this figure is available in the online issue.)
High glucose induced a significant increase in Agt mRNA (Fig. 6D), TGF-β1 mRNA (Fig. 6E), TGF-β1 RII mRNA (Fig. 6F), total cellular protein content (Fig. 6G), [3H]leucine incorporation (Fig. 6H), and p27Kip1 expression (Fig. 6I) in GFP-EV stable transfectants. hnRNP F overexpression prevented the stimulatory effect of high glucose on these parameters in GFP-hnRNP F stable transfectants.
DISCUSSION
This report demonstrates that selective overexpression of hnRNP F in RPTCs effectively suppresses Agt and TGF-β1 gene expression and attenuates systemic hypertension, kidney hypertrophy, and glomerulotubular fibrosis in Akita hnRNP F-Tg mice, suggesting a protective role for hnRNP F in preventing Ang II-induced hypertension and kidney injury in diabetes.
hnRNPs are pre-mRNA–binding proteins involved in mRNA processing, and ∼30 of them have been identified (22,23). Accumulating evidence indicates that hnRNP F may regulate gene expression at both the transcriptional and posttranscriptional levels. Indeed, hnRNP F was reported to be engaged in alternative splicing of c-src (24), β-tropomyosin gene (25), thyroid hormone receptor gene (26), Bcl-x gene (27,28), and p53 gene (29) as well as in the 3′-end processing of pre-mRNA in B-cell differentiation (30). hnRNP F is also associated with TATA-binding protein, RNA polymerase II, and even with the nuclear cap-binding protein complex (31,32). The molecular mechanism of hnRNP F action on gene transcription, however, is not well-defined.
We previously reported that hnRNP F binds to the IRE of rat Agt gene promoter and inhibits Agt promoter transcriptional activity (13). hnRNP F interacts with hnRNP K (another nuclear protein that binds to the same IRE in rat Agt gene promoter) and further inhibits Agt gene expression in RPTCs in vitro (14). Chen et al. (33) observed that hnRNP F interacts with the osmoregulatory transcription factor TonEBP/OREBP and modulates the expression of Hsp90 and PARP-1 gene. These findings indicate that hnRNP F may act alone or interact with other transcriptional factors to modulate specific gene transcription.
A novel observation in our study is that hnRNP F overexpression suppresses Agt gene expression and urinary Agt and Ang II levels and attenuates systemic hypertension and renal hypertrophy in Akita hnRNP F-Tg mice as compared with Akita mice. These observations are consistent with our previous finding that Agt-Tg mice overexpressing specifically Agt in their RPTCs develop hypertension and renal hypertrophy (8,9,19,20), demonstrating an important role of the intrarenal Agt gene expression and RAS activation in hypertension and renal hypertrophy development.
The Akita mouse is an autosomal-dominant model of spontaneous type 1 diabetes in which the Ins2 gene is mutated. These mice exhibit decreased numbers of β-cells of the pancreatic islets and develop hyperglycemia at age 3 to 4 weeks (34). By age 30 weeks, male Akita mice manifest impaired renal function with elevated serum IgA, glomerulosclerosis, and diffuse granular mesangial deposits of IgA as well as increases in oxidative stress markers in RPTs (35) closely resembling those in type 1 diabetic patients.
Consistent with studies by others (36), we also detected significantly larger kidney size, KW/BW ratio, and higher glomerular and RPTC volumes in Akita mice than WT. Expanding these studies, we also observed renal structural damage in Akita mice as compared with control littermates. Histological findings included glomerular hypertrophy, tubular luminal dilation, vacuolar degeneration in RPTCs, accumulation of cell debris in the tubular lumen, and some RPTCs were even flattened. Selective overexpression of hnRNP F in RPTCs strikingly suppressed, but did not completely reverse, these changes in Akita hnRNP F-Tg mice. Thus, intrarenal Agt overexpression and RAS activation would affect kidney size, glomerular tuft, RPTC volume, and tubular injury in diabetes, which could be attenuated by hnRNP F overexpression-mediated inhibition of Agt expression. However, whether intrarenal hnRNP F overexpression modulates glomerular function remains to be determined.
There were no significant differences in plasma Agt and Ang II levels among the four different groups of mice. Further, RPT Agt mRNA and protein levels and urinary Agt and Ang II levels did not differ significantly in the WT and hnRNP F-Tg groups. In contrast, they were significantly higher in Akita mice than WT and were markedly decreased in Akita hnRNP F-Tg mice. These observations raise the possibility that urinary Agt and Ang II in nondiabetic mice could be derived from a nonkidney source, whereas they could be predominantly derived from RPTCs in diabetic mice. Indeed, recent studies by Pohl et al. (37) demonstrated that Agt could be filtered through the glomerulus and that proximal tubules were capable of uptake of filtered Agt in nondiabetic rats.
Suppression of Agt expression by hnRNP F did not ameliorate microalbuminuria (assessed by the albumin/creatinine ratio) in Akita hnRNP F-Tg mice. One possible explanation might be that local suppression of Agt expression in RPTCs, resulting in diminished RAS activation in RPTCs, is insufficient to repair structural changes, such as loss of podocytes, in diabetic Akita glomeruli (38). It is also possible that the elevated SBP in Akita animals may induce albuminuria independently of intrarenal RAS activation. We speculate that the failure of hnRNP F to ameliorate proteinuria occurs because of the marked podocytopathy associated with this model (38) and lack of hnRNP F effect on podocyte function. Systemic RAS blockade would ameliorate the function of podocytes, glomeruli, and renal efferent vessels and therefore prevent albuminuria in diabetic mice as previously reported (9).
TGF-β1 is a risk factor for the initiation and progression of renal disease, and its expression is markedly elevated in diabetic kidneys (18,39,40). We observed higher TGF-β1 expression in the kidneys of Akita mice as compared with WT, confirming these observations. Our present studies indicate that hnRNP F overexpression attenuates Agt and TGF-β1 expression in RPTs of Akita hnRNP F-Tg mice and in RPTCs cultured in high-glucose medium. These changes attenuate cellular hypertrophy via suppression of TGF-β1 and TGF-β1 RII as well as profibrotic gene (PAI-1, collagen type IV, and fibronectin mRNA) expression in RPTCs.
The molecular mechanism(s) by which hnRNP F suppresses Agt gene expression remain undefined. One possibility is that hnRNP F acts as a negative transacting factor and competes with other positive transacting factor(s), such as cAMP-response element-binding protein (CREB) and activating transcription factor-1 for binding to TATA-binding protein and RNA polymerase II, subsequently attenuating Agt gene expression. This possibility is supported by our previous findings that CREB and activating transcription factor-1 expression is enhanced in RPTCs cultured in high-glucose medium (5) and that CREB overexpression augments Agt gene transcription (41). Another possibility is that hnRNP F overexpression may exhaust the availability of nuclear CAP-binding proteins for capping pre-mRNAs, which might then attenuate the formation of mature Agt mRNA in the cytoplasm. Finally, it is also possible that hnRNP F would form a heterodimer with hnRNP K that effectively binds other positive transcriptional factors to prevent their interaction with the initiation complex.
The physiological importance of attenuation of RPTC hypertrophy in diabetes by hnRNP F overexpression remains to be elucidated. Because targeted disruption of the p27Kip1 gene was found to attenuate kidney hypertrophy and progression of nephropathy in streptozotocin-induced diabetic mice (42,43), we propose that RPTC hypertrophy may be an initial mechanism leading to nephropathy in diabetes.
In summary, we have demonstrated that hnRNP F overexpression in RPTCs suppresses Agt and TGF-β1 gene expression and subsequently attenuates systemic hypertension and RPTC hypertrophy in vivo and in vitro, implying that dysregulation of hnRNP F expression might contribute to hypertension development and renal injury in diabetes via altering local intrarenal RAS activation.
ACKNOWLEDGMENTS
This work was supported by grants from the Canadian Institutes of Health Research (MOP-84363, MOP-93650, and MOP-16088 to J.S.D.C., MOP-86450 to S.L.Z., and MOP-64283 to J.G.F.), the Heart and Stroke Foundation of Canada (to J.S.D.C.), and the National Institutes of Health (HL-48455 to J.R.I.).
No potential conflicts of interest relevant to this article were reported.
C.-S.L. and S.L.Z. researched data and contributed to discussion. S.-Y.C. and I.C. researched data. J.G.F. and J.R.I. contributed to discussion and reviewed and edited the manuscript. J.S.D.C. contributed to discussion and wrote, reviewed, and edited the manuscript. J.S.D.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as a poster communication at the Annual Meeting of the American Society of Nephrology, Philadelphia, Pennsylvania, 8–13 November 2011.
The creation of hnRNP F-Tg mice and stable transfectants by Dr. Chih-Chang Wei (former PhD student of J.S.D.C. at the Université de Montréal, Centre Hospitalier de l’Université de Montréal-Hôtel Dieu Hospital) are greatly appreciated and acknowledged. Editorial assistance was provided by the Centre de Recherche du Centre Hospitalier de l’Université de Montréal’s Research Support office.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1349/-/DC1.
REFERENCES
- 1.Dzau VJ, Ingelfinger JR. Molecular biology and pathophysiology of the intrarenal renin-angiotensin system. J Hypertens Suppl 1989;7:S3–S8 [DOI] [PubMed] [Google Scholar]
- 2.Zhang SL, Filep JG, Hohman TC, Tang SS, Ingelfinger JR, Chan JS. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney Int 1999;55:454–464 [DOI] [PubMed] [Google Scholar]
- 3.Zhang SL, Tang SS, Chen X, Filep JG, Ingelfinger JR, Chan JS. High levels of glucose stimulate angiotensinogen gene expression via the P38 mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology 2000;141:4637–4646 [DOI] [PubMed] [Google Scholar]
- 4.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 2002;143:2975–2985 [DOI] [PubMed] [Google Scholar]
- 5.Hsieh TJ, Fustier P, Zhang SL, et al. High glucose stimulates angiotensinogen gene expression and cell hypertrophy via activation of the hexosamine biosynthesis pathway in rat kidney proximal tubular cells. Endocrinology 2003;144:4338–4349 [DOI] [PubMed] [Google Scholar]
- 6.Zhang SL, To C, Chen X, et al. Essential role(s) of the intrarenal renin-angiotensin system in transforming growth factor-beta1 gene expression and induction of hypertrophy of rat kidney proximal tubular cells in high glucose. J Am Soc Nephrol 2002;13:302–312 [DOI] [PubMed] [Google Scholar]
- 7.Zhang SL, To C, Chen X, et al. Effect of renin-angiotensin system blockade on the expression of the angiotensinogen gene and induction of hypertrophy in rat kidney proximal tubular cells. Exp Nephrol 2001;9:109–117 [DOI] [PubMed] [Google Scholar]
- 8.Sachetelli S, Liu Q, Zhang SL, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int 2006;69:1016–1023 [DOI] [PubMed] [Google Scholar]
- 9.Liu F, Brezniceanu ML, Wei CC, et al. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol 2008;19:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SL, Chen X, Filep JG, Tang SS, Ingelfinger JR, Chan JS. Insulin inhibits angiotensinogen gene expression via the mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology 1999;140:5285–5292 [DOI] [PubMed] [Google Scholar]
- 11.Zhang SL, Chen X, Wei CC, et al. Insulin inhibits dexamethasone effect on angiotensinogen gene expression and induction of hypertrophy in rat kidney proximal tubular cells in high glucose. Endocrinology 2002;143:4627–4635 [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Zhang SL, Pang L, et al. Characterization of a putative insulin-responsive element and its binding protein(s) in rat angiotensinogen gene promoter: regulation by glucose and insulin. Endocrinology 2001;142:2577–2585 [DOI] [PubMed] [Google Scholar]
- 13.Wei CC, Guo DF, Zhang SL, Ingelfinger JR, Chan JS. Heterogenous nuclear ribonucleoprotein F modulates angiotensinogen gene expression in rat kidney proximal tubular cells. J Am Soc Nephrol 2005;16:616–628 [DOI] [PubMed] [Google Scholar]
- 14.Wei CC, Zhang SL, Chen YW, et al. Heterogeneous nuclear ribonucleoprotein K modulates angiotensinogen gene expression in kidney cells. J Biol Chem 2006;281:25344–25355 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Lei C, Zhang SL, et al. Synergistic effect of dexamethasone and isoproterenol on the expression of angiotensinogen in immortalized rat proximal tubular cells. Kidney Int 1998;53:287–295 [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Davisson RL, Hardy DO, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem 1997;272:28142–28148 [DOI] [PubMed] [Google Scholar]
- 17.Brezniceanu ML, Liu F, Wei CC, et al. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 2007;71:912–923 [DOI] [PubMed] [Google Scholar]
- 18.Brezniceanu ML, Liu F, Wei CC, et al. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 2008;57:451–459 [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Wei CC, Wu SJ, et al. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 2009;75:156–166 [DOI] [PubMed] [Google Scholar]
- 20.Godin N, Liu F, Lau GJ, et al. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int 2010;77:1086–1097 [DOI] [PubMed] [Google Scholar]
- 21.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol 1999;276:F218–F227 [DOI] [PubMed] [Google Scholar]
- 22.Dreyfuss G, Matunis MJ, Piñol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem 1993;62:289–321 [DOI] [PubMed] [Google Scholar]
- 23.Han SP, Tang YH, Smith R. Functional diversity of the hnRNPs: past, present and perspectives. Biochem J 2010;430:379–392 [DOI] [PubMed] [Google Scholar]
- 24.Min H, Chan RC, Black DL. The generally expressed hnRNP F is involved in a neural-specific pre-mRNA splicing event. Genes Dev 1995;9:2659–2671 [DOI] [PubMed] [Google Scholar]
- 25.Chen CD, Kobayashi R, Helfman DM. Binding of hnRNP H to an exonic splicing silencer is involved in the regulation of alternative splicing of the rat beta-tropomyosin gene. Genes Dev 1999;13:593–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hastings ML, Wilson CM, Munroe SH. A purine-rich intronic element enhances alternative splicing of thyroid hormone receptor mRNA. RNA 2001;7:859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garneau D, Revil T, Fisette JF, Chabot B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J Biol Chem 2005;280:22641–22650 [DOI] [PubMed] [Google Scholar]
- 28.Dominguez C, Fisette JF, Chabot B, Allain FH. Structural basis of G-tract recognition and encaging by hnRNP F quasi-RRMs. Nat Struct Mol Biol 2010;17:853–861 [DOI] [PubMed] [Google Scholar]
- 29.Decorsière A, Cayrel A, Vagner S, Millevoi S. Essential role for the interaction between hnRNP H/F and a G quadruplex in maintaining p53 pre-mRNA 3′-end processing and function during DNA damage. Genes Dev 2011;25:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veraldi KL, Arhin GK, Martincic K, Chung-Ganster LH, Wilusz J, Milcarek C. hnRNP F influences binding of a 64-kilodalton subunit of cleavage stimulation factor to mRNA precursors in mouse B cells. Mol Cell Biol 2001;21:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida T, Makino Y, Tamura T. Association of the rat heterogeneous nuclear RNA-ribonucleoprotein F with TATA-binding protein. FEBS Lett 1999;457:251–254 [DOI] [PubMed] [Google Scholar]
- 32.Gamberi C, Izaurralde E, Beisel C, Mattaj IW. Interaction between the human nuclear cap-binding protein complex and hnRNP F. Mol Cell Biol 1997;17:2587–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Schnetz MP, Irarrazabal CE, et al. Proteomic identification of proteins associated with the osmoregulatory transcription factor TonEBP/OREBP: functional effects of Hsp90 and PARP-1. Am J Physiol Renal Physiol 2007;292:F981–F992 [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46:887–894 [DOI] [PubMed] [Google Scholar]
- 35.Haseyama T, Fujita T, Hirasawa F, et al. Complications of IgA nephropathy in a non-insulin-dependent diabetes model, the Akita mouse. Tohoku J Exp Med 2002;198:233–244 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez AP, Zhao J, You Y, Declèves AE, Diamond-Stanic M, Sharma K. Role of the USF1 transcription factor in diabetic kidney disease. Am J Physiol Renal Physiol 2011;301:F271–F279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohl M, Kaminski H, Castrop H, et al. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 2010;285:41935–41946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006;55:225–233 [PubMed] [Google Scholar]
- 39.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 1990;346:371–374 [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A 1993;90:1814–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian JF, Wang TT, Wu XH, et al. Angiotensinogen gene expression is stimulated by the cAMP-responsive element binding protein in opossum kidney cells. J Am Soc Nephrol 1997;8:1072–1079 [DOI] [PubMed] [Google Scholar]
- 42.Awazu M, Omori S, Ishikura K, Hida M, Fujita H. The lack of cyclin kinase inhibitor p27(Kip1) ameliorates progression of diabetic nephropathy. J Am Soc Nephrol 2003;14:699–708 [DOI] [PubMed] [Google Scholar]
- 43.Wolf G, Schanze A, Stahl RA, Shankland SJ, Amann K. p27(Kip1) Knockout mice are protected from diabetic nephropathy: evidence for p27(Kip1) haplotype insufficiency. Kidney Int 2005;68:1583–1589 [DOI] [PubMed] [Google Scholar]