Abstract
Previous evidence from tooth agenesis studies suggested IRF6 and TGFA interact. Since tooth agenesis is commonly found in individuals with cleft lip/palate (CL/P), we used four large cohorts to evaluate if IRF6 and TGFA interaction contributes to CL/P. Markers within and flanking IRF6 and TGFA genes were tested using Taqman or SYBR green chemistries for case-control analyses in 1,000 Brazilian individuals. We looked for evidence of gene-gene interaction between IRF6 and TGFA by testing if markers associated with CL/P were overtransmitted together in the case-control Brazilian dataset and in the additional family datasets. Genotypes for an additional 142 case-parent trios from South America drawn from the Latin American Collaborative Study of Congenital Malformations (ECLAMC), 154 cases from Latvia, and 8,717 individuals from several cohorts were available for replication of tests for interaction. Tgfa and Irf6 expression at critical stages during palatogenesis was analyzed in wild type and Irf6 knockout mice. Markers in and near IRF6 and TGFA were associated with CL/P in the Brazilian cohort (p<10−6). IRF6 was also associated with cleft palate (CP) with impaction of permanent teeth (p<10−6). Statistical evidence of interaction between IRF6 and TGFA was found in all data sets (p = 0.013 for Brazilians; p = 0.046 for ECLAMC; p = 10−6 for Latvians, and p = 0.003 for the 8,717 individuals). Tgfa was not expressed in the palatal tissues of Irf6 knockout mice. IRF6 and TGFA contribute to subsets of CL/P with specific dental anomalies. Moreover, this potential IRF6-TGFA interaction may account for as much as 1% to 10% of CL/P cases. The Irf6-knockout model further supports the evidence of IRF6-TGFA interaction found in humans.
Introduction
Oral-facial clefts are common birth defects with an incidence of 1–2 in 1000 live births, thus comprising almost one-half of all craniofacial anomalies. They impose adverse health, social, and economic implications for the affected individuals and their families [1]. Although the mortality and morbidity of an infant born with a cleft lip and or a cleft palate has improved greatly in the last century, it is still elevated for infants born with multiple additional anomalies. Among the consequences of being born with clefts are shorter life span and increased risk for all major causes of death when compared to individuals without clefts [2].
Cleft lip with or without cleft palate (herein called cleft lip/palate) can be classified as nonsyndromic or syndromic based on the presence of other associated congenital defects. Approximately 20–50% of all cleft cases are associated with one of more than 400 syndromes [3]. Syndromic forms usually present Mendelian inheritance patterns, which allow identification of causal genes. Nonsyndromic cleft lip/palate however, is considered a genetically complex trait with no clearly recognizable inheritance pattern [4].
Identifying the key genes responsible for the genesis of cleft lip/palate is fundamental for elucidating the pathogenetic mechanisms and developing measures for its management and prevention. Studies have estimated that 3–14 genes interacting multiplicatively may be involved in the etiology of cleft lip/palate [5], and a variety of genes have been associated and suggested to play a role in the genetic susceptibility to cleft lip/palate [4].
To date, the most consistent finding for the genetic etiology of nonsyndromic cleft lip/palate has been the association of the interferon regulatory factor 6 (IRF6) gene at 1q32 [6], previously identified as etiologic for Van der Woude syndrome which includes cleft lip/palate as part of the clinical spectrum [7]. A particularly strong overtransmission of the ancestral allele V at a V274I polymorphism (rs2235371) was detected in individuals of Asian and South American ancestry from 8,003 individuals representing ten distinct populations. Attributable risk calculations suggested IRF6 could contribute to as much as 12% of all cleft cases [6]. Intriguingly, additional studies with different populations have consistently shown positive association between markers in IRF6 and cleft lip/palate [8]–[22]. The frequency of the V274I risk allele is over 97% in European and African populations making it an unlikely candidate for the etiological mutation.
The association of the transforming growth factor alpha (TGFA) gene at 2p13 with cleft lip/palate has also rendered intriguing results. TGFA was the first gene associated to nonsyndromic cleft lip/palate in a case-control study [23]. Several studies followed with rather discrepant results; in turn, comparison among studies with TGFA has been somewhat difficult due to unaccounted variations in study design, markers tested and percentages of patients with positive family history [24]. Meta-analytic approaches [25], [26] concluded that TGFA plays a small but significant role in cleft lip/palate with odds ratios indicating a modest effect size. Instead of an effector gene, TGFA has been regarded as a]modifier to the clefting phenotype [24].
Evidence from tooth agenesis studies suggested that IRF6 and TGFA genes may interact [27], [28]. Tooth agenesis is a common congenital anomaly where one or more permanent teeth are absent and is a frequent observation in individuals with cleft lip/palate. Therefore interaction between IRF6 and TGFA in tooth agenesis may also be relevant to cleft lip/palate. Since tooth agenesis is commonly found in individuals with cleft lip/palate, we used three large samples of cleft cases to test for interaction between IRF6 and TGFA in the etiology of the cleft phenotype.
Results
Results of Case-control Comparisons
Tables 1 and 2 summarize the studied Brazilian samples and genetic markers. There were no evidences of deviation from Hardy-Weinberg equilibrium for any of the markers in cases and controls (data not shown). Table 3 summarizes the linkage disequilibrium relationships of the markers studied.
Table 1. Baseline clinical characteristics of the Brazilian population.
| Populations | Cleft | Control | ||
| N | % | N | % | |
| Age | ||||
| Range | 4–59 | – | 4–94 | – |
| Mean | 17.32 | – | 36.8 | – |
| Gender | ||||
| Males | 302 | 60 | 165 | 33 |
| Females | 198 | 40 | 335 | 67 |
| Race | ||||
| Caucasian | 406 | 82 | 285 | 58.2 |
| African | 79 | 16 | 38 | 7.8 |
| Asian | 9 | 2 | 167 | 34 |
| Unknown | 6 | 16 | 10 | 18 |
Table 2. Details of the SNPs investigated in this study.
| SNP marker | Base Positiona | Approximate Llocation | Function | BaseChange | Average Heterozygozity | Type of Assay |
| IRF6 | ||||||
| rs4844880 | 207,937,539 | 90 kb 3′ of IRF6 | intron | AT | 0.488+/−0.075 | Taqman ODb |
| rs2235371 (V274I) | 208,030,703 | In IRF6 | missense | CT | 0.247+/−0.250 | Taqman ODb |
| rs2013162 | 208,035,307 | In IRF6 | coding-synonymous | AC | 0.478+/−0.102 | Taqman ODb |
| rs861019 | 208,042,009 | In IRF6 | 5′UTR | AG | 0.474+/−0.111 | Taqman ODb |
| rs2073487 | 208,043,269 | In IRF6 | intron | CT | 0.479+/−0.099 | Taqman ODb |
| rs658860 | 208,057,172 | 11 kb 5′ of IRF6 | unknown | CT | 0.290+/−0.247 | Taqman ODb |
| TGFA | ||||||
| rs1058213 (C3827T) | 70,530,971 | In TGFA | 3′UTR | CT | 0.286+/−0.247 | Kinetic PCR |
| rs2166975 (C3296T) | 70,531,502 | In TGFA | coding-synonymous | GA | 0.340+/−0.233 | Kinetic PCR |
| rs930655 | 70,537,959 | In TGFA | intron | AG | 0.438+/−0.166 | Taqman ODb |
| rs1523305 | 70,552,364 | In TGFA | intron | CT | 0.497+/−0.038 | Taqman ODb |
| rs2902345 | 70,570,107 | In TGFA | intron | CT | 0.471+/−0.118 | Taqman ODb |
| rs377122 | 70,620,533 | In TGFA | intron | CT | 0.485+/−0.085 | Taqman ODb |
According to the USCS Genome Browser on Human March 2006 Assembly (hg18).
Assay-on-demand.
Table 3. Results of linkage disequilibrium analyses for the investigated markers in the Brazilian Caucasian population (406 cases and 285 controls).
| Markers | rs4844880 | rs2235371 | rs2013162 | rs861019 | rs2073487 | rs658860 |
| IRF6 | ||||||
| rs4844880 | – | 0.057 | 0.009 | 0.011 | 0.009 | 0.006 |
| rs2235371 | 0.538 | – | 0.091 | 0.005 | 0.093 | 0.007 |
| rs2013162 | 0.221 | 0.858 | – | 0.070 | 0.986 | 0.090 |
| rs861019 | 0.190 | 0.309 | 0.385 | – | 0.066 | 0.030 |
| rs2073487 | 0.223 | 0.860 | 1.000 | 0.375 | – | 0.095 |
| rs658860 | 0.097 | 0.677 | 0.873 | 0.404 | 0.903 | – |
| rs1058213 | rs2166975 | rs930655 | rs1523305 | rs2902345 | rs377122 | |
| TGFA | ||||||
| rs1058213 | – | 0.043 | 0.017 | 0.024 | 0.018 | 0.0001 |
| rs2166975 | 0.434 | – | 0.032 | 0.036 | 0.031 | 0.002 |
| rs930655 | 0.536 | 0.450 | – | 0.223 | 0.232 | 0.008 |
| rs1523305 | 0.742 | 0.413 | 0.536 | – | 0.741 | 0.018 |
| rs2902345 | 0.712 | 0.420 | 0.494 | 0.954 | – | 0.015 |
| rs377122 | 0.065 | 0.074 | 0.092 | 0.160 | 0.160 | – |
r2 is above the diagonal; D’ is below the diagonal.
Table 4 summarizes the results of the association analysis obtained for Brazilian Caucasian cases (N = 406) and controls (N = 285) for each marker studied, according to each cleft subphenotype. When comparing Brazilian cleft cases with controls, we observed an association between the intronic marker rs2902345 with cleft lip/palate (P<0.001). For IRF6, we found significant association between the V274I polymorphism (rs2235371) with complete left cleft lip/palate (P<0.001). An intronic marker in IRF6 (rs2073487) also showed a trend for association with complete left cleft lip/palate (P = 0.0009).
Table 4. Summary of the association analysis in Brazilian Caucasians according to each cleft subphenotype.
| Subphenotype | n | TGFA | |||||||||||
| rs1058213genotypep-value | rs1058213allelep-value | rs2166975genotypep-value | rs2166975allelep-value | rs930655genotypep-value | rs930655allelep-value | rs1523305genotypep-value | rs1523305allelep-value | rs2902345genotypep-value | rs2902345allelep-value | rs377122genotypep-value | rs377122genotypep-value | ||
| CP | 53 | 1.0 | 0.91 | 0.44 | 0.18 | 0.37 | 0.45 | 0.32 | 0.28 | 0.31 | 0.43 | 0.17 | 1.0 |
| CL/P | 324 | 0.68 | 0.86 | 0.34 | 0.09 | 0.45 | 0.81 | 0.71 | 0.63 | 0.00001 | 0.02 | 0.007 | 0.68 |
| Complete | 237 | 0.39 | 0.51 | 0.10 | 0.01 | 0.41 | 0.68 | 0.64 | 0.43 | 0.60 | 0.44 | 0.04 | 0.39 |
| Incomplete | 86 | 0.76 | 0.95 | 0.78 | 0.47 | 0.30 | 0.13 | 0.77 | 0.60 | 0.96 | 0.95 | 0.007 | 0.76 |
| CL/P Unilateral | 200 | 0.37 | 0.95 | 0.21 | 0.06 | 0.16 | 0.85 | 0.59 | 0.99 | 0.52 | 0.55 | 0.005 | 0.37 |
| Complete | 136 | 0.19 | 0.81 | 0.01 | 0.001 | 0.10 | 0.33 | 0.48 | 0.82 | 0.37 | 0.54 | 0.01 | 0.19 |
| Incomplete | 64 | 0.78 | 0.62 | 0.31 | 0.09 | 0.35 | 0.24 | 0.90 | 0.70 | 0.97 | 0.80 | 0.007 | 0.78 |
| CL/P Right | 60 | 0.37 | 0.06 | 0.92 | 0.64 | 0.15 | 0.46 | 0.75 | 0.47 | 0.62 | 0.41 | 0.23 | 0.37 |
| Complete | 43 | 0.08 | 0.001 | 0.50 | 0.19 | 0.40 | 0.36 | 0.54 | 0.32 | 0.73 | 0.46 | 0.46 | 0.08 |
| Incomplete | 17 | 0.96 | 0.50 | 0.49 | 0.18 | 0.16 | 0.97 | 0.54 | 0.80 | 0.80 | 0.65 | 0.30 | 0.96 |
| CL/P Left | 140 | 0.19 | 0.37 | 0.13 | 0.04 | 0.39 | 0.83 | 0.35 | 0.67 | 0.30 | 0.79 | 0.006 | 0.19 |
| Complete | 93 | 0.05 | 0.11 | 0.008 | 0.001 | 0.17 | 0.51 | 0.31 | 0.35 | 0.18 | 0.75 | 0.01 | 0.05 |
| Incomplete | 47 | 0.84 | 0.87 | 0.55 | 0.23 | 0.39 | 0.17 | 0.57 | 0.56 | 0.96 | 0.96 | 0.01 | 0.84 |
| CL/P Bilateral | 123 | 0.59 | 0.36 | 0.40 | 0.34 | 0.69 | 0.41 | 0.67 | 0.40 | 0.90 | 0.67 | 0.18 | 0.59 |
| Complete | 101 | 0.44 | 0.13 | 0.46 | 0.64 | 0.89 | 0.64 | 0.44 | 0.26 | 0.76 | 0.53 | 0.54 | 0.44 |
| Incomplete | 22 | 0.72 | 0.31 | 0.42 | 0.14 | 0.09 | 0.03 | 0.73 | 0.56 | 0.79 | 0.82 | 0.06 | 0.72 |
| CL/P + CP | 393 | 0.66 | 0.51 | 0.28 | 0.07 | 0.82 | 0.88 | 0.80 | 0.53 | 0.64 | 0.48 | 0.09 | 0.66 |
Genotype p-values are listed first, followed by allele p-values.
P≤0.0002 indicates statistical difference (in bold) in comparison to controls (n = 285). Unknown cleft types (n = 16) were not considered for analyses.
For both genotype and allele.
We also compared cleft subphenotypes with tooth agenesis and other dental anomalies and controls. Table 5 summarizes the results of the association analysis obtained in the Brazilian cases (N = 406) and controls (N = 285) for TGFA and IRF6 markers, according to each cleft subphenotype with dental anomalies. Although genotype/allele frequencies did not significantly differ between cases presenting with tooth agenesis and controls, we found an association between the V274I marker in IRF6 and cleft palate in the presence of impaction of permanent teeth (P<0.0001).
Table 5. Summary of the association analysis in Brazilian Caucasians according to each cleft subphenotype with dental anomalies.
| Cleft subphenotypes with dental anomalies | n | TGFA | |||||||||||||||
| rs1058213genotypep-value | rs1058213genotypep-value | rs2166975genotypep-value | rs2166975allelep-value | rs930655genotypep-value | rs930655allelep-value | rs1523305genotypep-value | rs1523305allelep-value | rs2902345genotypep-value | rs2902345allelep-value | rs377122genotypep-value | rs377122genotypep-value | ||||||
| CP with agenesis | 12 | 0.90 | 0.67 | 0.06 | 0.04 | 0.15 | 0.07 | 0.04 | 0.08 | 0.06 | 0.09 | 0.41 | 0.21 | ||||
| CP with impaction of permanent teeth | 2 | – | – | – | – | – | 0.006 | – | 0.01 | – | 0.008 | – | 0.01 | ||||
| Right CL/P with agenesis | 24 | 0.24 | 0.02 | 0.94 | 0.69 | 0.78 | 0.57 | 0.97 | 0.83 | 0.32 | 0.16 | 0.95 | 0.60 | ||||
| Left CL/P with agenesis | 36 | 0.01 | 0.48 | 0.06 | 0.03 | 0.38 | 0.20 | 0.51 | 0.72 | 0.21 | 0.16 | 0.38 | 0.29 | ||||
| Bilateral CL/P with agenesis | 34 | 0.88 | 0.97 | 0.29 | 0.17 | 0.73 | 0.96 | 0.81 | 0.88 | 0.09 | 0.03 | 0.71 | 0.50 | ||||
| Unsuccessful bilateral CL/P* | 26 | 0.02 | 0.31 | 0.93 | 0.96 | 0.62 | 0.94 | 0.20 | 0.60 | 0.87 | 0.97 | 0.33 | 0.26 | ||||
| Cleft subphenotypes with dental anomalies | n | IRF6 | |||||||||||||||
| rs4844880 genotype p-value | rs4844880 genotype p-value | rs2235371genotype p-value | rs2235371allele p-value | rs2013162genotype p-value | rs2013162 allele p-value | rs861019 genotype p-value | rs861019 allele p-value | rs2073487 genotype p-value | rs2073487 allele p-value | rs658860genotype p-value | rs658860genotype p-value | ||||||
| CP with agenesis | 12 | 0.47 | 0.87 | 0.16 | 0.12 | 0.93 | 0.75 | 0.24 | 0.90 | 0.76 | 0.53 | 0.001 | 0.01 | ||||
| CP with impaction of permanent teeth | 2 | 0.79 | 0.88 | 0.00001 | 0.00001 | 0.002 | 0.003 | – | – | – | – | – | – | ||||
| Right CL/P with agenesis | 24 | 0.27 | 0.58 | 0.20 | 0.81 | 0.94 | 0.83 | 0.67 | 0.69 | 0.93 | 0.79 | 0.30 | 0.41 | ||||
| Left CL/P with agenesis | 36 | 0.75 | 0.78 | 0.56 | 0.32 | 0.54 | 0.36 | 0.10 | 0.25 | 0.50 | 0.33 | 0.19 | 0.39 | ||||
| Bilateral CL/P with agenesis | 34 | 0.57 | 0.28 | 0.78 | 0.74 | 0.57 | 0.34 | 0.29 | 0.35 | 0.44 | 0.23 | 0.80 | 0.52 | ||||
| Unsuccessful bilateral CL/P* | 26 | 0.54 | 0.33 | 0.03 | 0.75 | 0.46 | 0.53 | 0.86 | 0.70 | 0.47 | 0.57 | 0.62 | 0.54 | ||||
Unilateral CL/P with agenesis or microdontia of the maxillary lateral incisor on the noncleft side.
P≤0.0008 indicates significant difference (in bold).
For both genotype and allele.
Results of Attributable Fraction Calculations for IRF6-TGFA Interaction
We calculated the attributable fraction (AF) for the high-risk alleles at IRF6 V274I and TGFA C3827T (P = 0.03) for the Brazilian sample, and the estimated contribution of the interaction between these two genes in this population was found to be approximately 1% (Table 6).
Table 6. Results for interaction of TGFA C3827T and IRF6 V274I marker alleles in the Brazilian Caucasian cases (N = 406) and controls (N = 285).
| TGFA rs1058213 (C3827T) | IRF6 rs2235371 (V274I) | P-value* | ||
| allele 1 (C) | allele 2 (T) | |||
| allele 1 (C) | cases | 355 | 3 | 0.49 |
| controls | 256 | 1 | ||
| allele 2 (T) | cases | 16 | 0 | 0 |
| controls | 15 | 0 | ||
| allele 1 (C) | allele 2 (T) | |||
| allele 1 (C) | cases | 327 | 31 | 0.35 |
| controls | 229 | 28 | ||
| allele 2 (T) | cases | 13 | 3 | 0.08 |
| controls | 15 | 0 | ||
| allele 1 (C) | allele 2 (T) | |||
| allele 1 (C) | cases | 351 | 3 | 0.49 |
| controls | 255 | 1 | ||
| allele 2 (T) | cases | 20 | 0 | 0 |
| controls | 16 | 0 | ||
| allele 1 (C) | allele 2 (T) | |||
| allele 1 (C) | cases | 325 | 29 | 0.25 |
| controls | 228 | 28 | ||
| allele 2 (T) | cases | 15 | 5 | 0.03 |
| controls | 16 | 0 | ||
Mantel-Haenszel test; p≤0.05 indicates statistical difference (in bold).
We also tested for IRF6-TGFA interaction in the ECLAMC samples by observing the transmission of the high-risk alleles at IRF6 V274I and TGFA C3827T in the 142 case-parent trios and detected significant overtransmission of these alleles to the affect child (P = 0.001). The attributable fraction for these samples (AF = 0.04) suggests this interaction may account for ∼4% of the cases of cleft lip/palate in this particular population. Genotypes used for these calculations are included as Supplemental Material (Table S1).
Analysis of genotypes in additional 7,047 people from seven distinct populations provides suggestive evidence of interaction between two TGFA markers (rs3732253 and rs377122) with a polymorphism in IRF6 (rs2013162) among Caucasians (P = 0.02) and Asians (P = 0.03) (although these p-values are nominal and would not be significant under strict Bonferroni correction). Analysis of the pooled samples indicates statistical interaction between a marker at TGFA (rs1807968) and another marker at IRF6 (rs2013162) in the cleft lip only group (P = 0.003) (Table 7). Attributable fraction calculations (AF = 0.10) further suggest ∼10% of cleft lip cases may be attributed to such interaction in the general population.
Table 7. Results of interaction analyses of associated TGFA and IRF6 marker alleles in the family dataset (861 simplex and multiplex families) comprising 7047 people stratified by population origin and cleft types.
| SNPs | All Samples | ASIA | PHILIPPINES | CAUCASIANS | COLOMBIA | INDIA | ||||||||
| TGFA | IRF6 | Initial FBAT p-value | Corrected p-value | Nominal p-value | Corrected p-value | Nominal p-value | Corrected p-value | Nominal p-value | Corrected p-value | Nominal p-value | Corrected p-value | Nominal p-value | Corrected p-value | Nominal p-value |
| rs3732253 | rs2013162 | 0.87 | 0.25 | 0.12 | 0.14 | 0.03 | 0.19 | 0.06 | 0.81 | 0.74 | 0.47 | 0.45 | 0.91 | 0.44 |
| rs1807968 | rs2013162 | 0.83 | 0.59 | 0.5 | 0.81 | 0.52 | 0.39 | 0.24 | 0.32 | 0.34 | 0.77 | 0.74 | 0.93 | 0.87 |
| rs374640 | rs2013162 | 0.96 | 0.86 | 0.65 | 0.63 | 0.77 | 0.51 | 0.57 | 0.68 | 0.64 | 0.26 | 0.19 | 0.98 | 0.24 |
| rs377122 | rs2013162 | 0.44 | 0.16 | 0.12 | 0.45 | 0.45 | 0.94 | 0.79 | 0.04 | 0.03 | 0.64 | 0.98 | 0.09 | 0.11 |
Cleft types, CL: cleft lip only; CLP: cleft lip with or without cleft palate; CLCLP: cleft lip only + cleft lip with or without cleft palate; MX: mixed cleft types; CP: cleft palate only.
Populations: Caucasians from USA and Europe.
We used genotypes for 154 cases from Latvia and genotype frequencies from the HapMap Project as a replication panel for this interaction between TGFA marker rs3732253 and IRF6 rs2013162. The results also suggest IRF6 and TGFA may interactively contribute to the risk for having an affected child (Table 8). The attributable fraction for these samples (AF = 0.04) suggests such interaction may account for ∼4% of the cases of cleft lip/palate in this particular population.
Table 8. Summary of the analysis with the Latvian cases (N = 154) with cleft lip/palate and 30 case-parent trios (90 individuals) from CEPH.
| IRF6-TGFA Marker Genotypes* | Expected Frequency | Observed Frequency in Cleft Cases | p-value |
| CC-CC | 0.2332 | 0.074074 | 0.002 |
| CC-CT | 0.176808 | 0.055556 | 0.005 |
| CC-TT | 0.013992 | 0.009259 | 0.5 |
| AC-CC | 0.26125 | 0.166667 | 0.1 |
| AC-CT | 0.198075 | 0.194444 | 0.02 |
| AC-TT | 0.015675 | 0.083333 | 0.03 |
| AA-CC | 0.0561 | 0.166667 | 0.005 |
| AA-CT | 0.042534 | 0.222222 | 0.0000001 |
| AA-TT | 0.003366 | 0.027778 | 0.27 |
The IRF6 marker used was rs2013162. The TGFA marker used was rs3732253.
Results of Gene Expression Analysis
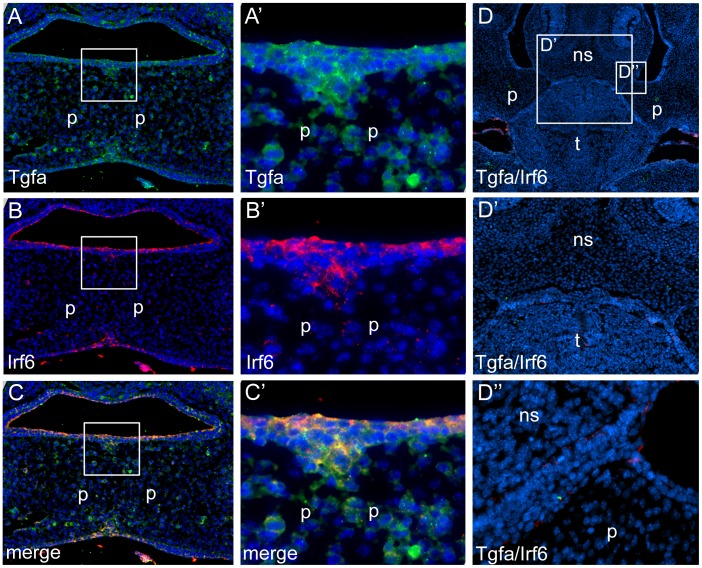
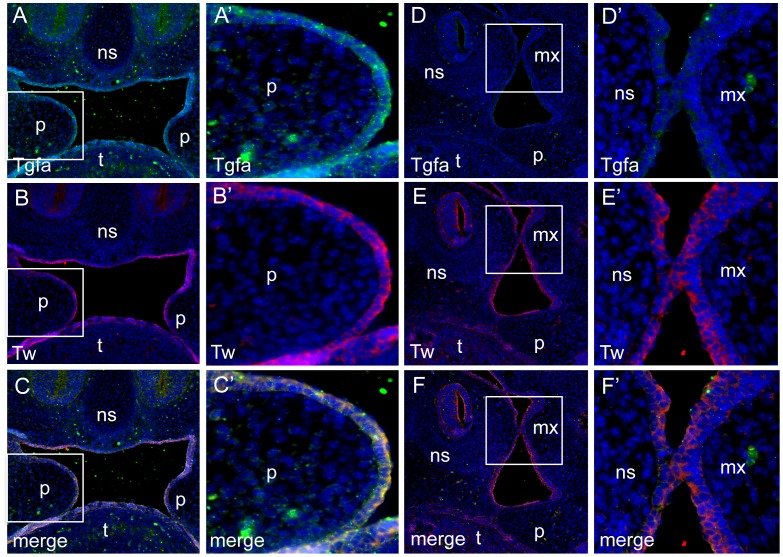
We investigated localization of Tgfa and Irf6 in wild type and Irf6-null mice at critical stages during palate development. We observed similar expression patterns for Tgfa and Irf6 in wild-type mouse craniofacial tissues at embryonic days E13 through E15. Positive immunoreactivity was observed in both epithelial and mesenchymal tissues of the mouth and nasal cavities. Expression was also detected in the brain. At day E14.5, when palatal fusion takes place, both epithelial and mesenchymal cells in the palate were intensely stained for Tgfa and Irf6 (Figure 1). In Irf6 knockout mice, however, we did not see Tgfa expression at E14.5 (Figure 2).
Figure 1. Loss of Tgfa expression in embryos that lack Irf6.
Expression of Tgfa (A,A′), Irf6 (B, B′) and merge (C, C′) in coronal sections of E14.5 wild type murine embyos. Tgfa and Irf6 expression colocalized to oral and nasal epithelium and remaining medial edge epithelium. Magnification was 10× (A–C) and 40× (A′–C′) for boxed regions in panels A–C. No expression was observed for Tgfa and Irf6 in coronal sections of E14.5 embryos that lack Irf6 (D). Regions of higher magnification are indicated (D′, D′′). Abbreviations are palate (p), tongue (t), nasal septum (ns).
Figure 2. Loss of Tgfa, but not Twist, expression in embryos that lack Irf6.
Expression of Tgfa (A,A′), Twist (B, B′) and merge (C, C′) in coronal sections of E13.5 wild type murine embyos. Tgfa and Twist expression colocalized to all oral epithelial surfaces of palate (p), tongue (t) and nasal septum (ns). Magnification was 10× (A–C) and 40× (A′–C′) for boxed regions in panels A–C. No expression was observed for Tgfa in coronal sections of E13.5 embryos that lack Irf6 (D, F), but Twist expression was not affected (E, F). Regions of higher magnification are indicated (D′–F′)). Abbreviations are palate (p), tongue (t), nasal septum (ns).
Discussion
Of all the genetic studies with cleft lip/palate, the association with IRF6 is certainly the most consistent [6], and has been consistently replicated in multiple populations [8]–[22]. IRF6 belongs to a gene family (IRFs) of transcription factors that regulate expression of interferons-α and –β after viral infections, and is a causal gene for Van der Woude’s syndrome which includes cleft lip/palate, pits on the lower lip, and tooth agenesis as part of the clinical phenotype [7]. Although the exact function of this gene remains unknown, polymorphisms in IRF6 may account for ∼12% of all cleft cases in the background of other genes, with an association of a particular V274I allele with isolated cleft lip/palate among Filipinos [6].
Efforts are being made to unravel the specific role of IRF6 in cleft lip/palate. Although direct sequencing of the coding regions of IRF6 did not detect potential causative mutations, the causative variant(s) could be in linkage disequilibrium with V274I but reside in the regulatory element(s) of IRF6 [32]. An additional possibility is that the V274I variant may be in linkage disequilibrium with a marker in another gene and such interaction might influence development of CL/P.
For many of the other genes previously associated with cleft lip/palate, including TGFA, a variety of positive and negative results have been reported, and TGFA has been largely ignored in the recent years. TGFA was the first gene associated to isolated cleft lip/palate in a case-control study [13] and was selected as a candidate gene because of its expression on palatal tissue in culture and its presence at high levels in the medial epithelial edge of the palatal shelves at the time of palatal fusion [42]. More recently, evidence of an excess of maternal transmission and possible interactions with maternal exposures to cigarette smoking, alcohol consumption and vitamin supplementation have been suggested to underlie the influence of TGFA in human clefting [43].
For this study, we used a collection of 1000 samples from cleft and control individuals from the Southeast region of Brazil [29]. Although the majority of residents of this region are descendants of Portuguese who migrated during the colonization years of Brazil, there is a substantial level of admixture confounding due to population stratification. To avoid biased results due to population stratification, the reported results reflect the analyses with individuals of self-reported Caucasian ethnicity only.
Markers located within and flanking the IRF6 and TGFA genes were tested for association with cleft of the lip or palate under a case-control design. We found an association between a single nucleotide polymorphism in the intron of the TGFA gene with cleft lip/palate. There is no evidence suggesting this intronic variant is etiologic, however introns seem to affect virtually any step of mRNA maturation, including transcription initiation, transcription elongation, transcription termination, polydenylation, nuclear export, and mRNA stability [44]. Rs2902345 can potentially affect transcription, and is coincidently located flanking a region we suggested involved in segmental uniparental isodisomy in a case of maxillary lateral incisor and mandibular second premolar agenesis [45].
For IRF6, we found a positive association between the V274I polymorphism and complete left cleft lip/palate. In contrast to other studies [6], [8]–[22], all of which showed association of IRF6 comparing only the three major cleft categories (cleft lip, cleft lip/palate and cleft palate), we only found positive association when comparing cleft subphenotypes with controls. Maybe IRF6 is not a strong risk factor for clefting in Brazil, or maybe it has specific contributions, e.g. controlling the side of unilateral cleft. We also found an association between the V274I polymorphism with cases of cleft palate with impaction of permanent teeth. Tooth impaction occurs when, for some reason, the permanent teeth do not erupt and remain inside the alveolar bone. The reported prevalence in the general population is about 1 to 2.5%, and it has also been reported in children with clefts [46].
The frequency of the V274I polymorphism varies greatly depending on geographic origin [6]. For instance, rs642961 was found to be more useful in studies with populations of European origin, since V274I frequency in these groups is remarkably low [32]. But even this marker has distinct frequencies even populations originating from north Europe are compare with groups coming from the south (i.e., Hispanics versus non-Hispanics) [47]. Although rs642961 is located at a site suggested to be an AP-2a binding site promoter, we studied V274I in the Brazilians due to the expected allele frequency differences. Evidence however suggest that there is a contribution to cleft susceptibility at the IRF6 locus but multiple genetic variants, rather than a single one, may have etiological roles in this defect [47], [48].
A common type of dental anomaly, tooth agenesis, has also been reported in association with this same IRF6 variant by our group [28], [49]. Tooth agenesis is a common congenital anomaly where one or more permanent teeth are absent and a frequent observation in individuals with cleft lip/palate [29]. Previous evidence from tooth agenesis studies suggested IRF6 and TGFA genes may interact [28]. Therefore, we hypothesized interaction between IRF6 and TGFA may also be relevant to cleft lip/palate. We speculated on the attributable fraction for the interaction of IRF6 and TGFA genes to the risk of cleft lip/palate using the Brazilian case-control sample and three additional family or case series data sets comprising a total of 8,717 individuals. We found statistical evidence of gene-gene interaction in all of these data sets and estimate such interaction could contribute from 1 percent to as much as 10 percent of cleft cases. These findings are in accordance with Zucchero et al. [6] who reported an attributable risk of cleft lip or palate of about 12 percent for IRF6. Those authors further stated the risk of recurrence is 9 percent among siblings in families with a history of cleft lip/palate where the child could have inherited the common risk allele. Taken together, these findings suggest individual IRF6 status may be an important tool to revisit recurrence risk estimates for cleft lip/palate.
Our expression assays showed Tgfa and Irf6 expression patterns are similar at critical stages for mouse palate development. However, Tgfa was not expressed in Irf6 knock out mice, which suggests that Tgfa and Irf6 may share common pathways and Tgfa may ultimately depend upon the Irf6 expression status. Mice deficient for Irf6 have abnormal skin, limb and craniofacial development, resultant from a primary defect in keratinocyte differentiation and proliferation. Furthermore, mice homozygous for the Irf6 null allele have a cleft palate which seems to be caused by a defect in elevation, either as a primary defect or secondary to crowding of the craniofacial structures owing to the constrictive action of the skin or oral adhesions [40]. Deficiency of Tgfa has been shown to affect skin, hair and eye development although the presence of a cleft phenotype has not yet been described, and Tgfa has been regarded thus as a modifier gene [24]. Tgfa is expressed in a variety of developing and adult tissues and the majority of expression studies have assayed for the presence of mRNA, the levels of which may not correlate with the production and processing of the protein. Alternatively, there may be physiological redundancy among the ligands of the EGFR in some tissues. This hypothesis has gained some validity since the demonstration by bioinformatics approaches that the EGFR pathway contains regions of functional redundancy in its upstream parts that may alleviate the consequences of low EGF stimulus [50]. Although in theory these two genes have antagonizing functions - Tgfa as a growth factor capable of stimulating cellular proliferation and cellular differentiation, and Irf6 as a regulator of keratinocyte proliferation and differentiation – our biological results further support the genetic interaction findings and warrant additional investigations.
The data presented here together with other evidence that suggest p63 and AP-2a cooperate to regulate IRF6 [32], [51]–[53] make us believe that a regulatory loop to coordinate epithelial proliferation and differentiation exist. Disruption of this loop by insufficient expression of TGFA, p63, AP-2a or any combination of these genes could lead to disruption in epithelial development. This disruption could lead to alterations such as clefts of the lip and palate and arresting of dental development, leading to tooth agenesis. Since TGFA, IRF6, and p63 are known to be involved in cancer [24], [54], [55], and in the view of our recent findings that cleft lip and palate families report more cancer [56]–[60], these gene-gene interactions might not only explain susceptibility to oral clefts, but also cancer.
Cleft lip/palate is a complex and heterogeneous disorder and a likely scenario is that variation in more than one gene underlies the isolated cleft lip/palate [61]. Additional studies should be realized regarding IRF6-TGFA interaction in other populations and if confirmed, these results could be used to revisit estimates of the recurrence rates of clefting.
Materials and Methods
Subjects
The subjects of this study, their cleft subphenotypes and characteristics of dental anomalies have been previously described in detail [29]. Written informed consent was obtained from all participants in the study. Parents or legal guardians provided written consent on behalf of the minors/children participants involved in the study. The sample consisted of 500 individuals with clefts in treatment at the Hospital of Rehabilitation and Craniofacial Anomalies of the University of São Paulo, Bauru, Brazil. Of these, 400 had cleft lip with cleft palate (168 with left cleft lip, 154 with bilateral cleft lip, 76 with right cleft lip, and 2 median clefts), six had cleft lip only (two on the right side and four on the left side), 66 had cleft palate only and 28 had unknown cleft types. The control group comprised 500 healthy, non-related individuals with no history of syndromic clefting, whom were mostly patients and students at Bauru Dental School.
We detected evidence of confounding due to population stratification within this Brazilian sample and therefore, for this study, we have included only the individuals of Caucasian ethnicity (hereby defined as Brazilians of Caucasian descent to the third generation and without any African or Japanese descent). Hence, 406 individuals with clefts and 285 control individuals were included in the current analysis (Table 1).
The study was conducted with the consent of the participants and approved by the Research and Ethics Committee of the University of São Paulo, Bauru and University of Pittsburgh. In the case of children under 15 years of age, authorization was also requested from their parents or from their legal guardian. Buccal epithelial cells were collected from each individual as source of genomic DNA. Procedures for buccal cell collection and DNA extraction were performed as described elsewhere [30], [31].
Genotyping
Genotyping was performed using Taqman or SYBR® Green chemistries (Applied Biosystems) on an automatic sequence-detection instrument (ABI Prism 7900HT, Applied Biosystems). Reactions were carried out with the use of standard conditions as suggested by the manufacturer.
SNP selection was based on previous reports. Six markers were genotyped in/nearby the IRF6 gene [6], [32]. In addition, six intragenic markers were used for TGFA [23]. Details of the markers are presented in Table 2. For the C3296T (rs2166975) and C3827T (rs1058213) variants in TGFA, we used allele-specific primers according to previously published protocols [33]. The primer sequences were: for C3296T, forward 5′CTTATTTTCCCAACGTGGCC 3′; reverse (for C allele), 5′CTCCTCTGGGCTCTTCTG 3′, reverse (for T allele), 5′TCCTCCTCTGGGCTCTTCTA 3′; for C3827T, forward 5′CTTATTTTCCCAACGTGGCC 3′; reverse (for C allele) 5′CTCCTCTGGGCTCTTCTG 3′, reverse (for T allele) 5′TCCTCCTCTGGGCTCTTCTA 3′. PCR conditions were the same for both variants: 95°C for 10 min (1 cycle), 95°C for 15 sec, and 56°C for 40 sec (55 cycles).
Since some of these markers had not yet been previously genotyped in Brazilians, we calculated linkage disequilibrium between all markers using the Graphical Overview of Linkage Disequilibrium (GOLD) software using both the squared correlation coefficient (r2, above the diagonal) and Lewontin’s standardized disequilibrium coefficient (D′, below the diagonal) [34] (Table 3).
All SNP markers were first tested on a collection of samples from the Centre d’Étude du Polymorphisme Humain (CEPH).
Statistical Analysis
Hardy-Weinberg equilibrium and case-control analyses
Chi-square statistics were used to assess adherence to Hardy-Weinberg equilibrium between cases and controls. There was no evidence of deviation from Hardy-Weinberg equilibrium for both groups (data not shown).
For case-control comparisons in the Brazilian samples, Chi-square test was used to assess association of markers with each cleft subphenotype. Bonferroni correction was applied considering the number of variables and tests performed, and P-values below 0.0002 (12 SNPs, and 17 phenotypes: 0.05/204) were considered significant.
IRF6-TGFA interaction and attributable fraction
The IRF6 and TGFA markers yielding the most significant associations were used to infer the overall contribution of their interaction to nonsyndromic cleft lip/palate in Brazilian cases and controls. We calculated the attributable fraction (AF) for the associated IRF6 and TGFA alleles as the proportion of cleft cases in a population that could be attributed to the interaction terms, assuming true causality. We calculated AF = f (R-1)/R where f is the frequency of the risk factor in the population and R is the measure of relative risk (35). To obtain f, we used the number of cases presenting at least one copy of overrepresented alleles compared to controls for both markers and divided by the total number of cases. We used the relative risk values for heritability of clefting in Brazilians in the State of São Paulo (RR = 4.96) as reported by Lofredo et al. [36].
We also studied three distinct data sets to replicate evidence of interaction between IRF6 and TGFA. Genotypes available for an additional 142 case-parent trios from ECLAMC (Latin American Collaborative Study of Congenital Malformations) were also included in the analysis for interaction between the IRF6 V274I variant (rs2235371) and TGFA C3827T (rs1058213) by observing transmission of the associated alleles at each gene from parents heterozygous for both of the markers using the parental haplotypes not present in the affected child as controls [37]. ECLAMC is a hospital-based birth defects registry study that included sites in Argentina, Bolivia, Brazil, Chile, Ecuador, Paraguay, Uruguay and Venezuela. Genotypes and alleles at each IRF6 and TGFA marker were tested for association with cleft lip/palate using of Family-Based Association Test (FBAT) [38] software in the ECLAMC cohort. The study was conducted with the consent of the participants and approved by the Research and Ethics Committee of the CEMIC (Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno”), Buenos Aires, Argentina and University of Pittsburgh. Written informed consent was obtained from all participants in the study. Parents or legal guardians provided written consent on behalf of the minors/children participants involved in the study.
In addition, data from a population pool consisting of 7,047 people from family studies of CL/P sampled from Colombia, USA, India, Spain, Philippines, China and Turkey were also analyzed. Details regarding these are provided elsewhere [17]. In brief, most of these families were extended multiplex kindreds, i.e. multigenerational families with two or more affected individuals. The phenotype was CL/P, i.e. for families to be included, it was necessary that the proband have CL/P (i.e. no other anomalies) and that no other family member have an indication of an orofacial syndrome (e.g. lip pits). Each study population included evaluations of family members by clinical geneticists to rule out syndromic forms of CL/P. For this analysis, we considered the per-family Z-scores for each SNP from FBAT and performed correlation and logistic regression between each IRF6 and TGFA SNPs to generate interaction p-values. We used the odds ratio of the associated alleles as a measure of the relative risk in this pooled population. The study was conducted with the consent of the participants and approved by the Research and Ethics Committee of the University of Pittsburgh. Written informed consent was obtained from all participants in the study. Parents or legal guardians provided written consent on behalf of the minors/children participants involved in the study.
As a replication panel for these interaction analyses, we used genotypes of 154 cases with isolated cleft lip/palate from Latvia to test for interaction between markers in the IRF6 and TGFA genes. Since no Latvian control samples were available, comparison data from this analysis was drawn from genotype frequency in 30 U.S. trios (mother/father/offspring), which were collected in 1980 from U.S. residents with northern and western European ancestry by the Centre d’Etude du Polymorphisme Humain (CEPH) available at the HapMap Project (http://hapmap.ncbi.nlm.nih.gov/). Although these control samples are presumed to be of Northern Europe origin that is the potential that population substructures are not matched in this set of cases and controls. We did the same calculations used for the Brazilian and ECLAMC datasets. To obtain f, we used the frequency of the over-represented haplotype among the CEPH controls. We used a relative risk value of 1.0 in the Latvians due to the very high frequency of maternal smoking in the population (67% to 81%) as reported by Patla et al. [39]. Maternal smoking is known to increase the susceptibility to clefts approximately 1.5 times [61] and in a population that have a frequency of smokers as high as the Latvians, we decided that would be appropriate to not input relative risk values higher than 1.0 for this calculation. The study was conducted with the consent of the participants and approved by the Research and Ethics Committee of the Riga Stradins University and University of Pittsburgh. Written informed consent was obtained from all participants in the study. Parents or legal guardians provided written consent on behalf of the minors/children participants involved in the study.
Overall, 8,717 individuals were used in this test of interaction between IRF6 and TGFA.
Fluorescent Immunostaining
Expression of Tgfa and Irf6 proteins was performed on paraffin sections from heads of wild type and Irf6 null embryos at E13.5 and 14.5. Maintenance and handling of mice were approved by the Animal Care Unit at Michigan State University. Tissues were deparaffinized and rehydrated in a series of ethanol dilutions. Slides were boiled for 5 min in 0.08% saponin in BPS for antigen retrieval. Sections were blocked with 10% normal goat serum in 1% PBS-BSA for 1 hr, then incubated overnight at 4 šC with the following primary antibodies: monoclonal mouse anti-Tgfa (1∶150, clone 213-4.4, GeneTex, Irvine, CA) and polyclonal rabbit anti-Irf6 (1∶500, Irf6-SPEA). After rinsing in PBS, sections were incubated with secondary antibodies conjugated to Alexa Fluorophore 488 or 555 (Molecular Probes, Invitrogen, CA). The nuclei were counterstained with DAPI in PBS (1∶1000). The images were taken using a Nikon Eclipse 90i fluorescent microscope.
To investigate if Tgfa was influenced by Irf6, we also investigated Tgfa expression in the Irf6 knockout mice generated by Ingraham et al. [40] using the methods and reagents as described above.
Skin tissue sections were used as positive controls for Tgfa [41] and Irf6 [40]. To confirm the specificity of the immunostaining, primary antibodies were substituted with PBS (for Tgfa) and normal rabbit serum (for Irf6). No immunoreactivity was detected in any of the negative control sections.
Supporting Information
Genotyping data of ECLAMC cleft lip and palate trios.
(PDF)
Acknowledgments
We are very grateful to the individuals who participated in this study. Thanks to Dr. Gustavo Garlet for help with immunohistochemistry results. Sarah Vinski provided administrative support.
Funding Statement
This study was supported by grants from the National Institutes of Health: K99 018954-01 to AL; K99 018913-02 to RM; R21-DE016718 to ARV, R01-DE14899 and R01-DE16148 to MLM. Support also came from grants from the National Research Council of Brazil (CNPq # 308885/2006-0 and 401467/2004-0) to IMO; a grant from FAPERJ (E-26/152.831/2006) to IMO; by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina, Grant number: PICTO-CRUP 2005 # 31101; by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina; and for ECLAMC financial support from CNPq (National Research council of Brazil), process # 573993/2008-4; INAGEMP. In Latvia, support came from the Latvian Science Council grant No. 09.1115 and the European Social Fund project, which supports the doctoral study program and PhD degree qualification in Riga Stradins University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gorlin RJ, Cohen MM, Hennekam RCM (2001) Orofacial clefting syndromes: general aspects. In: Gorlin, RJ, Cohen MM, editors. Syndromes of the Head and Neck, 4th ed. New York: Oxford University Press.
- 2. Hujoel PP, Bollen AM, Mueller BA (1992) First-year mortality among infants with facial clefts. Cleft Palate Craniofac J 29: 451–455. [DOI] [PubMed] [Google Scholar]
- 3. Christensen K, Juel K, Herskind AM, Murray JC (2004) Long term follow up study of survival associated with cleft lip and palate at birth. BMJ 328: 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lidral AC, Moreno LM (2005) Progress toward discerning the genetics of cleft lip. Curr Opin Pediatr 17: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schliekelman P, Slatkin M (2002) Multiplex relative risk and estimation of the number of loci underlying an inherited disease. Am J Hum Genet 71: 1369–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, et al. (2004) Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. New Engl J Med 351: 769–780. [DOI] [PubMed] [Google Scholar]
- 7. Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, et al. (2002) Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet 32: 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanton SH, Cortez A, Stal S, Mulliken JB, Finnell RH, et al. (2005) Variation in IRF6 contributes to nonsyndromic cleft lip and palate. Am J Med Genet 137A: 259–262. [DOI] [PubMed] [Google Scholar]
- 9. Ghassibé M, Bayet B, Revencu N, Verellen-Dumoulin C, Gillerot Y, et al. (2005) Interferon regulatory factor-6: a gene predisposing to isolated cleft lip with or without palate in the Belgian population. Eur J Hum Genet 13: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 10. Scapoli L, Palmieri A, Martinelli M, Pezzetti F, Carinci P, et al. (2005) Strong evidence of linkage disequilibrium between polymorphisms at the IRF6 locus and nonsyndromic cleft lip with or without cleft palate, in an Italian population. Am J Hum Genet 76: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srichomthong C, Siriwan P, Shotelersuk V (2005) Significant association between IRF6 820G/A and non-syndromic cleft lip with or without cleft palate in the Thai population. J Med Genet 42: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JW, McIntosh I, Hetmanski JB, Jabs EW, Kolk CAV, et al. (2007) Association between IRF6 and nonsyndromic cleft lip with or without cleft palate in four populations. Genet Med 9: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vieira AR, Cooper ME, Marazita ML, Orioli IM, Castilla EE (2007) Interferon regulatory factor 6 (IRF6) is associated with oral-facial cleft in individuals that originate in South America. Am J Med Genet A 143: 2075–2078. [DOI] [PubMed] [Google Scholar]
- 14. Jugessur A, Rahimov F, Lie RT, Wilcox AJ, Gjessing HK, et al. (2008) Genetic variants in IRF6 and the risk of facial clefts: single-marker and haplotype-based analyses in a population-based case-control study of facial clefts in Norway. Genet Epidemiol 32: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia ZL, Li Y, Li L, Wu J, Zhu LY, et al. (2009) Association among IRF6 polymorphism, environmental factors, and nonsyndromic orofacial clefts in western China. DNA Cell Biol 28: 249–257. [DOI] [PubMed] [Google Scholar]
- 16. Jugessur A, Shi M, Gjessing HK, Lie RT, Wilcox AJ, et al. (2009) Genetic determinants of facial clefting: analysis of 357 candidate genes using two national cleft studies from Scandinavia. PLoS One 4: e5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marazita ML, Lidral AC, Murray JC, Field LL, Maher BS, et al. (2009) Genome scan, fine-mapping, and candidate gene analysis of non-syndromic cleft lip with or without cleft palate reveals phenotype-specific differences in linkage and association results. Hum Hered 68: 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Y, Wu J, Ma J, Beaty TH, Sull JW, et al. (2009) Association between IRF6 SNPs and oral clefts in West China. J Dent Res 88: 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mostowska A, Hozyasz KK, Wojcicki P, Biedziak B, Paradowska P, et al. (2010) Association between genetic variants of reported candidate genes or regions and risk of cleft lip with or without cleft palate in the polish population. Birth Defects Res A Clin Mol Teratol 88: 538–545. [DOI] [PubMed] [Google Scholar]
- 20. Nikopensius T, Jagomägi T, Krjutskov K, Tammekivi V, Saag M, et al. (2010) Genetic variants in COL2A1, COL11A2, and IRF6 contribute risk to nonsyndromic cleft palate. Birth Defects Res A Clin Mol Teratol 88: 748–756. [DOI] [PubMed] [Google Scholar]
- 21. Pan Y, Ma J, Zhang W, Du Y, Niu Y, et al. (2010) IRF6 polymorphisms are associated with nonsyndromic orofacial clefts in a Chinese Han population. Am J Med Genet A 152: 2505–2511. [DOI] [PubMed] [Google Scholar]
- 22. Larrabee YC, Birkeland AC, Kent DT, Flores C, Su GH, et al. (2011) Association of common variants, not rare mutations, in IRF6 With nonsyndromic clefts in a Honduran population. Laryngoscope 121: 1756–1759. [DOI] [PubMed] [Google Scholar]
- 23. Ardinger HH, Buetow KH, Bell GI, Bardach J, Van Demark DR, et al. (1989) Association of genetic variation of the transforming growth factor alpha gene with cleft lip and palate. Am J Hum Genet 45: 348–353. [PMC free article] [PubMed] [Google Scholar]
- 24. Vieira AR (2006) Association between the transforming growth factor alpha gene and nonsyndromic oral clefts: a HuGE review. Am J Epidemiol 163: 790–810. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell LE (1997) Transforming growth factor alpha locus and nonsyndromic cleft lip with or without cleft palate: a reappraisal. Genet Epidemiol 14: 231–240. [DOI] [PubMed] [Google Scholar]
- 26. Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (2001) Replication validity of genetic association studies. Nat Genet 29: 306–309. [DOI] [PubMed] [Google Scholar]
- 27. Vieira AR, Meira R, Modesto A, Murray JC (2004) MSX1, PAX9, and TGFA contribute to tooth agenesis in humans. J Dent Res 83: 723–727. [DOI] [PubMed] [Google Scholar]
- 28. Vieira AR, Modesto A, Meira R, Barbosa ARB, Lidral AC, et al. (2007) Interferon regulatory factor 6 (IRF6) and fibroblast growth factor receptor 1 (FGFR1) contribute to human tooth agenesis. Am J Med Genet A 143: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Letra A, Menezes R, Granjeiro JM, Vieira AR (2007) Defining cleft subphenotypes based on dental development. J Dent Res 86: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trevilatto PC, Line SRP (2000) Use of buccal epithelial cells for PCR amplification of large DNA fragments. J Forensic Odonto Stomatol 18: 6–9. [PubMed] [Google Scholar]
- 31. Aidar M, Line SRP (2007) A simple and cost-effective protocol for DNA isolation from buccal epithelial cells. Braz Dent J 18: 148–152. [DOI] [PubMed] [Google Scholar]
- 32. Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, et al. (2008) Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet 40: 1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi M, Caprau D, Dagle J, Christiansen L, Christensen K, et al. (2004) Application of kinetic polymerase chain reaction and molecular beacon assays to pooled analyses and high-throughput genotyping for candidate genes. Birth Defects Res 70: 65–74. [DOI] [PubMed] [Google Scholar]
- 34. Abecasis GR, Cookson WOC (2000) GOLD – Graphical Overview of Linkage Disequilibrium. Bioinformatics 16: 182–183. [DOI] [PubMed] [Google Scholar]
- 35. Miettinen OS (1974) Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 99: 325–332. [DOI] [PubMed] [Google Scholar]
- 36. Loffredo LCM, Souza JMP, Yunes J, Freitas JAS, Spiri WC (1994) Fissuras labio-palatais: estudo caso-controle. Rev Saude Publica 28: 213–217. [DOI] [PubMed] [Google Scholar]
- 37. Falk CT, Rubinstein P (1987) Haplotype relative risks: an easy reliable way to construct a proper control sample for risk calculations. Ann Hum Genet 51: 227–233. [DOI] [PubMed] [Google Scholar]
- 38. Horvath S, Xu X, Laird NM (2001) The family based association test method: strategies for studying general genotype-phenoytpe associations. Eur J Hum Genet 9: 301–306. [DOI] [PubMed] [Google Scholar]
- 39. Patja K, Hakala S, Prättälä R, Ojala K, Boldo E, et al. (2009) Adult smoking as a proxy for environmental tobacco smoke exposure among children – Comparing the impact of the level of information in Estonia, Finland and Latvia. Prev Med 49: 240–244. [DOI] [PubMed] [Google Scholar]
- 40. Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, et al. (2006) Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nat Genet 38: 1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshikawa M, Kojima H, Wada K, Tsukidate T, Okada N, et al. (2006) Identification of specific gene expression profiles in fibroblasts derived from middle ear cholesteatoma. Arch Otolaryngol Head Neck Surg 132: 734–742. [DOI] [PubMed] [Google Scholar]
- 42. Dixon MJ, Garner J, Ferguson MWJ (1991) Immunolocalization of epidermal growth factor (EGF), EGF receptor and transforming growth factor alpha (TGFα) during murine palatogenesis in vivo and in vitro. Anat Embryol (Berl) 184: 83–91. [DOI] [PubMed] [Google Scholar]
- 43. Sull JW, Liang KY, Hetmanski JB, Wu T, Fallin MD, et al. (2009) Evidence that TGFA influences risk to cleft lip with/without cleft palate through unconventional genetic mechanisms. Hum Genet 126: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chorev M, Carmel L (2012) The function of introns. Front Genet 3: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Callahan N, Modesto A, Deeley K, Meira R, Vieira AR (2009) Transforming growth factor-alpha gene (TGFA), human tooth agenesis, and evidence of segmental uniparental isodisomy. Eur J Oral Sci 117: 20–26. [DOI] [PubMed] [Google Scholar]
- 46. Takahama Y, Aiyama Y (1982) Maxillary canine impaction as a possible microform of cleft lip and palate. Eur J Orthod 4: 275–277. [DOI] [PubMed] [Google Scholar]
- 47. Blanton SH, Burt A, Garcia E, Mulliken JB, Stal S, et al. (2010) Ethnic heterogeneity of IRF6 AP-2a binding site promoter SNP association with nonsyndromic cleft lip and palate. Cleft Palate Craniofac J 47: 574–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Birnbaum S, Ludwig KU, Reutter H, Herms S, Assis NA, et al. (2010) IRF6 gene variants in Central European patients with non-syndromic cleft lip with or without cleft palate. Eur j Oral Sci 117: 766–799. [DOI] [PubMed] [Google Scholar]
- 49. Vieira AR, Seymen F, Patir A, Menezes R (2008) Evidence of linkage disequilibrium between polymorphisms at the IRF6 locus and isolate tooth agenesis, in a Turkish population. Arch Oral Biol 53: 780–784. [DOI] [PubMed] [Google Scholar]
- 50. Wang DY, Cardell L, Phillips A, Piterman N, Fisher J (2009) Computational modeling of the EGFR network elucidates control mechanisms regulating signal dynamics. BMC Syst Biol 22: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomason HA, Zhou H, Kouwenhoven EN, Dotto GP, Restivo G, et al. (2010) Cooperation between the transcriptionfactors p63 and IRF6 is essential to prevent cleft palate in mice. J Clin Invest 120: 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moretti F, Marinari B, Iacono NL, Botti E, Giunta A, et al. (2010) A regulatory feedback loop involving p63 and IRF6 links the pathogenesis of 2 genetically different human ectodermal dysplasias. J Clin Invest 120: 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gritti-Linde A (2010) p63 and IRF6: brothers in arms against cleft palate. J Clin Invest 120: 1386–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rinne T, Bruner HG, van Bokhoven H (2007) P63-associated disorders. Cell Cycle 6: 262–268. [DOI] [PubMed] [Google Scholar]
- 55. Bailley CM, Hendrix MJC (2008) IRF6 in development and disease: A mediator of quiescence and differentiation. Cell Cycle 7: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Menezes R, Marazita ML, McHenry TG, Cooper ME, Bardi K, et al. (2009) AXIN2, orofacial clefts and positive family history for cancer. J Am Dent Assoc 140: 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taioli E, Ragin C, Robertson L, Linkov F, Thurman NE, et al. (2010) Cleft lip and palate in family members of cancer survivors. Cancer Invest 28: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jindal A, Vieira AR (2012) Family history of cleft lip and palate in subjects diagnosed with leukemia. Am J Med Genet Part A 158: 678–679. [DOI] [PubMed] [Google Scholar]
- 59. Vieira AR, Lace B, Khaliq S (2012) Risk of cancer in relatives of children born with isolated cleft lip and palate. Am J Med Genet A 158A(6): 1503–1504. [DOI] [PubMed] [Google Scholar]
- 60. Yildirim M, Seymen F, Deeley K, Cooper ME, Vieira AR (2012) Defining predictors of cleft lip and palate risk. J Dent Res 91(6): 556–561. [DOI] [PubMed] [Google Scholar]
- 61. Vieira AR (2008) Unraveling human cleft lip and palate research. J Dent Res 87: 119–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genotyping data of ECLAMC cleft lip and palate trios.
(PDF)