Abstract
Background
Treponema pallidum ssp. pallidum (TPA), the causative agent of syphilis, and Treponema pallidum ssp. pertenue (TPE), the causative agent of yaws, are closely related spirochetes causing diseases with distinct clinical manifestations. The TPA Mexico A strain was isolated in 1953 from male, with primary syphilis, living in Mexico. Attempts to cultivate TPA Mexico A strain under in vitro conditions have revealed lower growth potential compared to other tested TPA strains.
Methodology/Principal Findings
The complete genome sequence of the TPA Mexico A strain was determined using the Illumina sequencing technique. The genome sequence assembly was verified using the whole genome fingerprinting technique and the final sequence was annotated. The genome size of the Mexico A strain was determined to be 1,140,038 bp with 1,035 predicted ORFs. The Mexico A genome sequence was compared to the whole genome sequences of three TPA (Nichols, SS14 and Chicago) and three TPE (CDC-2, Samoa D and Gauthier) strains. No large rearrangements in the Mexico A genome were found and the identified nucleotide changes occurred most frequently in genes encoding putative virulence factors. Nevertheless, the genome of the Mexico A strain, revealed two genes (TPAMA_0326 (tp92) and TPAMA_0488 (mcp2-1)) which combine TPA- and TPE- specific nucleotide sequences. Both genes were found to be under positive selection within TPA strains and also between TPA and TPE strains.
Conclusions/Significance
The observed mosaic character of the TPAMA_0326 and TPAMA_0488 loci is likely a result of inter-strain recombination between TPA and TPE strains during simultaneous infection of a single host suggesting horizontal gene transfer between treponemal subspecies.
Author Summary
Treponema pallidum is a Gram-negative spirochete that causes diseases with distinct clinical manifestations and uses different transmission strategies. While syphilis (caused by subspecies pallidum) is a worldwide venereal and congenital disease, yaws (caused by subspecies pertenue) is a tropical disease transmitted by direct skin contact. Currently the genetic basis and evolution of these diseases remain unknown.
In this study, we describe a high quality whole genome sequence of T. pallidum ssp. pallidum strain Mexico A, determined using the ?next generation? sequencing technique (Illumina). Although the genome of this strain contains no large rearrangements in comparison with other treponemal genomes, we found two genes which combined sequences from both subspecies pallidum and pertenue. The observed mosaic character of these two genes is likely a result of inter-strain recombination between pallidum and pertenue during simultaneous infection of a single host.
Introduction
Treponema pallidum ssp. pallidum (TPA) and Treponema pallidum ssp. pertenue (TPE) strains, the causative agents of syphilis [1] and yaws [2], infect more than 12 and 2 million people annually, respectively [3]. Whereas syphilis is a sexually transmitted and congenital disease affecting adults and newborns worldwide, yaws is transmitted predominantly through direct skin contact and affects preferably children in warm, humid, rural areas.
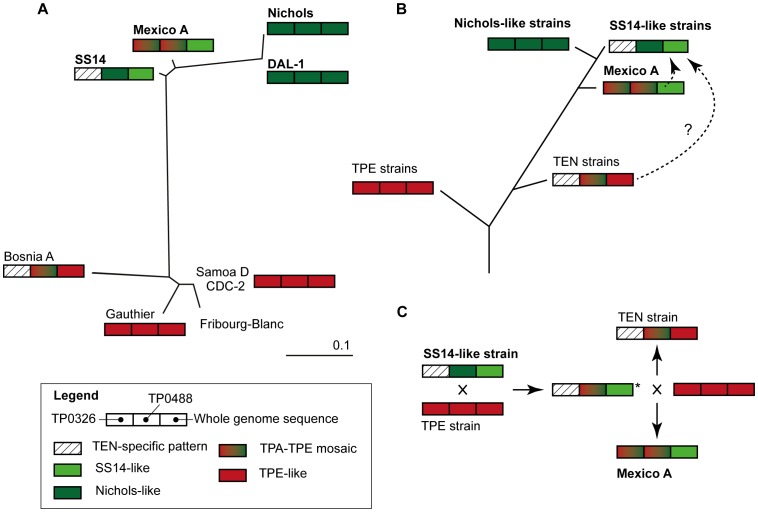
During the last several years, a number of treponemal genomes have been completely sequenced including TPA Nichols (GenBank acc. no. AE000520.1 [4]), TPA SS14 (CP000805.1 [5]), TPA Chicago (CP001752.1 [6]), TPE Samoa D (CP002374.1), TPE CDC-2 (CP002375.1), TPE Gauthier (CP002376.1) [7] and T. paraluiscuniculi strain Cuniculi A (CP002103.1 [8]). In general, when compared to TPE strains, TPA strains differ by less than 1,200 nucleotide positions [7], [9]. Phylogenetic trees constructed from whole genome binary restriction target site data [9], from multilocus sequencing [10] and whole genome sequence alignments [10] showed a distinct clustering of TPA and TPE strains. As shown by Centurion-Lara et al. [11] and Gray et al. [12], the unusual clustering of the Mexico A TP0131 gene with several TPE strains is the result of intra-chromosomal gene conversion events. Three different alleles of the tprD (TP0131) gene (D, D2, and D3) have been identified among TPA and TPE strains [11] and the presence of individual gene alleles determines the cluster patterns [12].
The TPA Mexico A strain was isolated in 1953 from an 18-year-old male, with primary syphilis, living in Mexico [13]. Attempts to cultivate TPA Mexico A strain under in vitro conditions revealed a lower growth rate (compared to other tested TPA strains) and also a decreased percentage of motile treponemes compared to TPA strain Nichols [14]. The lower growth potential of Mexico A is likely to result from genetic differences between this strain and other TPA strains. Our previous study [9] revealed that the Mexico A strain contained the largest genome of all investigated TPA strains.
In this study, we compared the complete genome sequence of TPA Mexico A to complete TPA and TPE genome sequences and found a mosaic character of the Mexico A TPAMA_0326 (tp92) and TPAMA_0488 (mcp2-1) loci, i.e. having both TPA and TPE specific nucleotide sequences.
Materials and Methods
Preparation of chromosomal DNA
The TPA Mexico A strain used in this study was kindly provided by David L. Cox, CDC, Atlanta, GA, USA. The DNA was amplified directly from 1 µl of cells (105 cells per µl) frozen in glycerol using a QIAGEN Whole Genome Amplification REPLI-g Kit (QIAGEN, Valencia, CA, USA). To separate treponemal cells from rabbit testicular cells, the samples were first centrifuged at 100×g for 5 min. Supernatant containing treponemal cells was carefully extracted and centrifuged at 14,100×g for 3 min. The resulting pellet containing treponemal cells was washed 2× in PBS buffer and centrifuged at 14,100×g for 3 min. The supernatant was removed for a final volume of 3 µl and the procedure continued according to the manufacturer's instructions. Amplified DNA was purified using a QIAEX II kit (QIAGEN, Valencia, CA, USA). The resulting DNA concentration was 602 ng/µl in a 30 µl volume.
DNA sequencing
The chromosomal DNA was sequenced using the Illumina (Illumina, San Diego, CA, USA) technique. Several chromosomal regions of the Mexico A strain, representing sequentially related and repetitive components of treponemal genome, were amplified using a GeneAmp XL PCR kit (Applied Biosystems, Foster City, CA, USA) using the previously described TPI amplicons [9], [15]. These regions comprised the following TPI amplicons (genes): TPI-11 (tprC), TPI-12 (tprD), TPI-13B (TP0136), TPI-17A (5S, 16S and 23S rRNA [rRNA operon 1]), TPI-21B (5S, 16S and 23S rRNA [rRNA operon 2]), TPI-25A (tprE), TPI-25B-A (tprF), TPI-25B-B (tprG), TPI-26 (TP0326), TPI-32B (arp), TPI-34 (TP0470), TPI-38 (TP0488), TPI-42A (TP0548), TPI-48 (tprI, tprJ), TPI-66A (TP0868) and TPI-67 (tprK). From these XL-PCR amplicons, small insert libraries were prepared and the resulting clones were sequenced as previously described [5]. Alternatively, amplified DNA was Sanger sequenced directly using specific primers.
A set of 639 Illumina contigs (100–69,908 bp in length) and 16 Sanger contigs, resulting from sequencing of XL-PCR products, were assembled using the TPA SS14 reference genome [5]. This assembly contained 122 gaps (8.9 kb in length) in the TPA Mexico A sequence. Altogether, 117 DNA regions (containing all 122 gaps) were additionally PCR amplified and sequenced using the Sanger method.
The TP0326 (tp92) and TP0488 (mcp2-1) loci of Treponema pallidum subsp. endemicum (TEN), strain Bosnia A, were amplified using GeneAmp XL PCR kit (Applied Biosystems, Foster City, CA, USA) and Sanger sequenced using specific primers.
Whole genome fingerprinting
The resulting genome assembly was verified using the previously described fingerprinting technique [15], [16]. The experimentally identified DNA fragments (resulting from DNA digestion at 1774 restriction target sites; [7]) were compared to the corresponding in silico restriction fragment lengths. The 1774 restriction target sites corresponded to a total sequence length of 10.6 kb. The average error rate of WGF was calculated previously [8] and corresponded to 27.9 bp (1.6% of the average fragment length) with a variation range between 0 and 132 bp.
Genome annotation and G+C content calculations
Considering the close relatedness of the Mexico A and SS14 genomes (99.99% identity at the nucleotide level), the Mexico A genome was annotated according to the SS14 genome [5] with minimal gene length of 150 bp. Genes identified in the Mexico A genome were denoted with the prefix TPAMA followed by four numbers to indicate gene number. Putative virulence factors were defined as those previously described by Čejková et al. [7] and comprised 31 genes (including tpr, arp, and TPAMA0136 genes). All of these genes are listed in Table S1.
The G+C content was calculated in 501 bp windows using CLC Bio software (CLC Bio Katrinebjerg, Denmark).
Nucleotide sequences accession numbers
The whole genome sequence of TPA strain Mexico A was placed in the GenBank under accession number CP003064.1. Sequences of TP0326 (tp92) and TP0488 (mcp2-1) of TEN strain Bosnia A were deposited in the GenBank under accession numbers JX392330.1 and JX392331.1, respectively.
Results
Whole genome sequence of the Mexico A strain and genome annotation
The genome of the Mexico A strain was determined to be 1,140,038 bp with 1,035 predicted ORFs. The final assembled genome sequence was verified using a fingerprinting technique [15], [16] where 1774 experimentally identified DNA fragments were compared to in silico restriction fragment lengths. No differences in fragment lengths were identified indicating correct overall assembly of the Mexico A genome. The 1774 restriction target sites corresponded to a total sequence length of 10.6 kb. Since no discrepancies between the in silico and the experimental restriction analysis were found (i.e. in 10.6 kb of the genome sequence out of 1,140 kb), the sequencing error rate was estimated to 10−4 or less.
From all annotated ORFs, 161 (15.6%) are involved in general metabolism, 125 (12.1%) in cell structure and cell processes, 51 (4.9%) in DNA replication, repair and recombination, 173 (16.7%) in regulation, transcription and translation, 113 (10.9%) in transport, and 31 (3%) in virulence. 327 ORFs (31.6%) had unknown function. In addition, 54 (5.2%) genes encoded RNAs. Coding regions represented 93.5% of the Mexico A genome. As in the SS14 (CP000805.1 [5]) and Chicago (CP001752.1 [6]) genomes, the tprK gene (TPAMA_0897) is represented by a number of variable sequences and the consensus sequence, therefore, contains unidentified nucleotides in these regions.
Gene fusions and authentic frameshifts
Altogether, six genes (pseudogenes) were annotated to contain authentic frameshifts (AF) in the Mexico A genome (TPAMA_0009, TPAMA_0146, TPAMA_0316, TPAMA_0520, TPAMA_0532 and TPAMA_0812) compared to 9 genes with AF annotated in the Nichols and SS14 genomes, where 3 additional genes with AF were described (TP0217, TP0575 and TP0866). In an additional 21 cases, frameshift mutations identified in the Mexico A genome resulted in gene fusions (Table S2).
Genomic differences between Mexico A and other syphilis treponemes
Whole genome sequence of the TPA strain Mexico A has been compared with other sequenced genomes of TPA strains including the Nichols strain (AE000520.1 [4]), SS14 (CP000805.1 [5]), and Chicago (CP001752.1 [6]) using the Lasergene software package (DNASTAR, Madison, WI, USA) and Crossmatch (P. Green, unpublished). Because of high sequence diversity, TP0131 (tprD) and TP0897 (tprK) were excluded from our calculations. The Mexico A genome differed from the SS14 genome in 175 substitutions, 85 insertions and 28 deletions, from the Chicago genome in 419 substitutions, 18 insertions and 20 deletions, and from the Nichols genome in 438 substitutions, 94 insertions and 38 deletions (ambiguously identified bases present in the Nichols genome were not counted). Changes differentiating Mexico A and Nichols genomes were found in 206 ORFs listed in Table S3. Since it is known that the Nichols and SS14 genomes contain about 200 nt errors ([10], Pospíšilová, unpublished results), we also compared the Mexico A genome with the improved version of the Nichols genome (Pospíšilová, unpublished results). From 206 ORFs originally identified as sequentially different, 138 ORFs (67%) also showed differences when compared to the improved Nichols genomic sequence. The originally identified nucleotide changes in the remaining 68 ORFs (33%) were considered to be Nichols sequencing errors. However, in the case of 14 Nichols ORFs (1.3% of the total Nichols ORFs), only partial or no sequencing data were available.
In general, the identified changes were more frequently found among genes encoding putative virulence factors and among genes involved in cell structure and processes and in genes coding for DNA replication, repair and recombination. In contrast, genes encoding components associated with general metabolism, transcription, translation, gene regulation and transport contained nucleotide changes less frequently (Table S4).
Mosaic character of TPAMA_0326 (tp92) and TPAMA_0488 loci (mcp2-1)
In addition to TPA strains, the Mexico A genome sequence was also compared with whole genome sequences of three TPE strains including Samoa D (GenBank acc. no. CP002374.1), CDC-2 (CP002375.1) and Gauthier (CP002376.1) [7]. Of all the annotated genes, two (TPAMA_0326 (tp92) and TPAMA_0488 (mcp2-1)) showed a mosaic character, which combined sequences from both TPA and TPE strains (Fig. 1). The complete set of nucleotide changes found in the TPA and TPE regions for TP0326 and TP0488 loci are shown in Table 1 and Table 2, respectively. In the TP0326 locus, there were 8 single nucleotide positions and one 15 bp deletion that differentiated TPE strains (Samoa D, CDC-2 and Gauthier) from TPA strains (Nichols, SS14, Chicago). Out of these 9 positions, the TPAMA_0326 locus contained 5 nucleotide positions with an identical sequence to the TPA strains and 4 regions that were identical to the TPE regions, including 3 nucleotide positions and the 15 bp deletion (Fig. 1, Table 1). Similarly, the TP0488 locus contained 30 nucleotide positions that were found to be different for all analyzed TPA and TPE strains. In addition, two nucleotide positions (584, 1655) differentiated the Nichols and Chicago strains from TPE strains and from the SS14 strain. In TPAMA_0488, 12 of these 30 positions contained sequences identical to TPA strains, whereas 18 positions corresponded to sequences of TPE strains (Fig. 1, Table 2). In the remaining part of the Mexico A genome, similarities to the TPE sequences were only found in the tprC sequence and at two additional nucleotide positions (present in TP0314 locus and TPAMA_0319, respectively).
Figure 1. A schematic representation of the TPAMA_0326 and TPAMA_0488 loci.
The Mexico A sequence was compared to three TPA (Nichols, SS14, and Chicago) and three TPE CDC-2, Gauthier, Samoa D) strains. Black vertical lines represent nucleotide positions differentiating all TPA and TPE strains. Green and red vertical lines represent nucleotide changes present in all analyzed TPA and TPE strains where the TPA Mexico A sequence is identical to TPA and TPE strains, respectively. Two nucleotide positions (584, 1655) in the TP0488 locus were found only in the Nichols and Chicago strains and not in the SS14 genome (open vertical lines). The symbol Δ stands for 15-bp deletion present in the TPE and Mexico A strains in the TP0326 gene.
Table 1. A list of changes found in the TPAMA_0326 locus.
| Mexico A coordinate (GenBank acc. no. CP003064.1) | TPAMA_0326 coordinate | Mexico A | TPE strains | TPA strains | Domain1) |
| 345746 | 270 [90] | T (Y) | C (Y) | T (Y) | POTRA 1 |
| 346359 | 883 [295] | G (G) | A (N) | G (G) | POTRA 4 |
| 346360 | 884 [295] | G (G) | A (N) | G (G) | POTRA 4 |
| 347302 | 1826 [609] | A (Q) | A (Q) | T (L) | β-barrel domain |
| 347603 | 2127 [709] | C (N) | G,T (R, E, N)2) | C (N) | β-barrel domain |
| 347610 | 2134 [712] | G (G) | A (S) | G (G) | β-barrel domain |
| 347849 | 2373 [791] | C (S) | C (S) | T (S) | β-barrel domain |
| 347859–347860 | 2383–2384 [794–795] | deletion of 15 bp (del of 5 AA) | deletion of 15 bp (del of 5 AA) | GTAGMACCACCAGCT 3) (SSTTS, SRTTS)3) | β-barrel domain (serine residue) |
| 347955 | 2479 [823] | G (A) | G (A) | C (P) | β-barrel domain |
Three TPA (Nichols, SS14, and Chicago) and three TPE (CDC-2, Gauthier, and Samoa D) strains were analyzed. The numbers in square brackets correspond to amino acid position in the TPAMA_0326 protein. The amino acid sequence of the encoded protein is shown in brackets.
Domain prediction according to Desrosiers et al. [41].
G (R) was found in TPE CDC-2 strain, G (E) in TPE Gauthier, while T (N) was found in TPE Samoa D strain.
15 bp (GTAGAACCACCAGCT) encode SRTTS in TPA Nichols and Chicago strain and 15 bp (GTAGCACCACCAGCT) encode SSTTS in TPA SS14 strain.
Table 2. A list of changes found at the TPAMA_0488 locus.
| Mexico A coordinate (GenBank acc. no. CP003064.1) | TPAMA_0488 coordinate | Mexico A | TPE strains | TPA strains | Domain1) |
| 523342 | 523 [175] | A (S) | A (S) | G (G) | - |
| 523375 | 556 [186] | A (T) | A (T,I)2) | G (V) | - |
| 523378 | 559 [187] | A (R) | A (R) | G (G) | - |
| 523392 | 573 [191] | C (S) | C (S) | T, A (S,R)3) | - |
| 523403 | 584 [195] | A (D) | A (D) | G, A (G,D)4) | - |
| 523421 | 602 [201] | G (C) | G (C) | C (S) | - |
| 523507 | 688 [230] | G (G) | G (G) | A (R) | - |
| 523564 | 745 [249] | A (T) | A (T) | G (A) | - |
| 523652 | 833 [278] | A (Q) | A (Q) | G (R) | - |
| 523669 | 850 [284] | C (R) | C (R) | T (C) | - |
| 523691 | 872 [291] | G (R) | G (R) | C (P) | Cache |
| 523741 | 922 [308] | A (N) | A (N) | G (D) | Cache |
| 523756 | 937 [313] | A (I) | A (I) | G (V) | Cache |
| 523772 | 953 [318] | T (V) | C (A) | T (V) | Cache |
| 523829 | 1010 [337] | A (E) | A (E) | G (G) | Cache |
| 523831 | 1012 [338] | G (A) | A (T) | G (V) | Cache |
| 523832 | 1013 [338] | C (A) | C (T) | T (V) | Cache |
| 523838 | 1019 [340] | G (R) | A (K) | G (R) | Cache |
| 523841 | 1022 [341] | G (S) | G (S) | T (I) | Cache |
| 523846 | 1027 [343] | A (I) | A (I) | G (V) | Cache |
| 523849 | 1030 [344] | T (F) | T (F) | A (I) | Cache |
| 523906 | 1087 [363] | G (A) | G (A) | A (T) | - |
| 523972 | 1153 [385] | A (R)5) | G (K) | A (E) | - |
| 523994 | 1175 [392] | C (A)5) | T (T) | C (I) | - |
| 524003 | 1184 [395] | A (R) | G (H) | A (R) | - |
| 524005 | 1186 [396] | G (V) | A (T) | G (V) | - |
| 524006 | 1187 [396] | T (V) | C (T) | T(V) | - |
| 524014 | 1195 [399] | T (Y) | C (H) | T (Y) | - |
| 524024 | 1205 [402] | T (L) | C (S) | T (L) | - |
| 524040 | 1221 [407] | A (L) | G (S) | A (S) | - |
| 524042 | 1223 [408] | C (S) | A (Y) | C (S) | - |
| 524474 | 1655 [552] | A (Q) | A (Q) | G, A (R,Q)6) | - |
Three TPA (Nichols, SS14, and Chicago) and three TPE (CDC-2, Gauthier, and Samoa D) strains were analyzed. Numbers in square brackets correspond to amino acid position in TPAMA_0488 protein. Amino acid sequence in the encoded protein is shown in brackets.
Domain prediction according to the NCBI Conserved Domain Database.
A (T) was identified in TPE CDC-2 strain while A (I) in TPE Gauthier and Samoa D strains.
T (S) was identified in TPA Nichols and Chicago strains while A (R) in TPA SS14 strain.
G (G) was identified in TPA Nichols and Chicago strains while A (D) in TPA SS14 strain.
Mexico A-specific nucleotide change in the vicinity of TPA-like change causes amino acid change specific for Mexico A.
G (R) was identified in TPA Nichols and Chicago strain while A (Q) in TPA SS14 strain.
G+C content in treponemal genomes
The average G+C content of the Mexico A genome was found the same as for other treponemal species, 52.8%. Based on an analysis of G+C content, codon and amino acid usage, and gene positions, 77 (8.32%) of the TPA genes were predicted to be horizontally transferred [17]. To identify chromosomal regions with horizontal transfer potential, G+C content was calculated in 501 bp windows in TPA Mexico A, TPA SS14 [5], TPE Samoa D [7] and Treponema paraluiscuniculi strain Cuniculi A (CP002103.1 [8]) (Fig. 2). The chromosomal regions showing different G+C content (defined as G+C content higher than 63% or lower than 41%) showed a similar pattern in all four tested genomes. We compared regions with higher/lower G+C content with 5 kb-long chromosomal regions containing 40 or more nucleotide changes differentiating TPA and TPE strains which were previously identified by Čejková et al. [7]. From 11 such regions [7] (Fig. 2), only 3 showed significant differences in G+C content. Similarly, no clear association was found in regions with different G+C content and tpr-containing DNA regions.
Figure 2. G+C content variation in selected Treponema strains.
TPA Mexico A, TPA SS14, TPE Samoa D and Treponema paraluiscuniculi strain Cuniculi A were analyzed. Colored vertical lines represent 501 bp-long windows with G+C content above the 63% or below the 41% threshold. Black vertical lines represent genome locations of tpr genes. Stars denote 5 kb-long DNA regions with 40 or more nucleotide positions differentiating TPA and TPE strains [7]. Please note that there is no clear association of DNA regions with different G+C content and regions differentiating TPA and TPE strains [7] or locations of tpr genes.
Discussion
Complete genome sequences of the TPA Mexico A strain was revealed. The genome size, G+C content and gene order was identical with other already sequenced TPA genomes [4]–[6]. The Mexico A genome was most closely related to SS14 genome and differed in less than 300 hundred substitutions and indels. Since it has been published that the Nichols and the SS14 genomes contain about 200 nt errors [10] a lower number of nucleotide changes differentiating the Mexico A and SS14 genome can be expected. In fact, the number of nucleotide differences between Mexico A and SS14 genomes (except of differences present in the tprD and tprK genes) is probably lower than one hundred (Pětrošová, unpublished results). In any of these comparisons, the identified differences were more frequently present in (i) genes encoding putative virulence factors, (ii) genes involved in cell structure and processes and (iii) genes coding for DNA replication, repair and recombination. In contrast, genes encoding components of general metabolism, transcription, translation, gene regulation and transport appear to be conserved.
The observed mosaic character of the Mexico A TPAMA_0326 (tp92) and TPAMA_0488 (mcp2-1) loci, combining both TPA- and TPE-specific nucleotide sequences, can be, in principle, explained by six independent mechanisms including i) an ancestral position of the Mexico A strain with respect to both TPA and TPE strains, ii) rapid accumulation of nucleotide changes during evolution of TPA strains from TPE strains with the Mexico A as an intermediate, iii) intra-strain recombination between paralogous sequences, iv) artifacts during PCR amplification (as a result of contamination with TPE genomic DNA) and/or contamination with TPE-amplified DNA, v) convergent evolution and vi) inter-strain recombination between TPA and TPE strains during simultaneous infection of one host.
i) The first explanation can be ruled out because only two chromosomal loci (TPAMA_0326 and TPAMA_0488) showed demonstrable similarity to TPE strains. Moreover, the number of Mexico A-specific mutations (i.e., mutations that are only present in the Mexico A genome and not in other sequenced TPA genomes) is not significantly different from the number of specific mutations in other TPA genomes (data not shown). In a predicted common ancestor, one would expect a considerably higher number of ancestor-specific mutations in comparison to progenies. ii) The second hypothesis is illustrated in Fig. 3B. The hypothetical evolution scheme comprises TPA, TPE and TEN strains arranged according to their relatedness to other TP strains [18] (see also Fig. 3A). We sequenced TP0326 (tp92) and TP0488 (mcp2-1) loci in TEN strain Bosnia A (GenBank acc.no. JX392330.1 and JX392331.1, respectively; our TP0326 sequence is identical to partial tp92 sequence of Bosnia A published by Harper et al. [19]). The sequencing data showed that TEN strain Bosnia A contains the same nucleotide mosaic in the TP0488 (mcp2-1) locus as Mexico A (with the exception of 2 single nucleotide substitutions) and similarly, some TPA isolates belonging to the SS14-like group of TPA strains show a TEN-specific pattern in the TP0326 (tp92) locus. It was impossible to propose an evolutionary model based only on accumulation or loss of nucleotide changes (see Fig. 3B), and this fact supports recombination hypothesis. iii) The third hypothesis was rejected when we failed to identify potential recombinant (donor) sites for the TPAMA_0326 and TPAMA_0488 genes in the Mexico A genome, despite several attempts to identify such regions using several computer programs and algorithms (RDP3, EditSeq (DNASTAR), BLAST). iv) While it is known that PCR amplification of two sequentially related templates can result in the production of chimeric DNA amplicons [20], contamination of the Mexico A genomic DNA with TPE genomic DNA can be ruled out because recombinant genes were only found for two genes of the genome. Contamination with TPE-amplified DNA (corresponding to TPAMA_0326 and TPAMA_0488 genes) was excluded based on careful analysis of Illumina reads, where no TPA- or TPE-specific Illumina reads were found in any of these regions. In fact, the presence of 15 bp-deletions in the TPAMA_0326 gene was found in all 169 individual Illumina reads covering this region. Similar analysis of the TPAMA_0488 region revealed no TPA- or TPE-specific Illumina reads; and all 37 reads, covering regions with both TPA and TPE molecular signatures, revealed the Mexico A consensus sequence. Since Illumina technology sequences individual DNA molecules, contamination of Mexico A genomic DNA with TPE PCR product can be excluded. To exclude artifacts during REPLI-g kit amplification of the Mexico A genomic DNA, three different REPLI-g amplifications were used for TPAMA_0326 and TPAMA_0488 sequencing. No discrepancies were identified during analysis of Sanger reads in these regions. Moreover, Harper et al. [19] sequenced partial tp92 locus of the Mexico A strain (obtained directly from CDC, Atlanta) and the sequenced region (960 nt, GenBank acc. no. EU102088.1, containing TPE-like sequence in three nucleotide positions and a 15-bp deletion) was identical to our sequence. Sequences of TP0326 (tp92) from various TPE isolates published by Harper et al. [19] contained the 15 bp TPE-like deletion and also corresponded to TPE-like changes in the South Africa treponemal isolate. All 21 South Africa partial nucleotide sequences available in the GenBank [19] were 100% identical to the corresponding sequences of Mexico A published by Harper et al. [19]. Therefore, the South Africa strain appears to be another strain that is identical, or very closely related, to the Mexico A strain. Nevertheless, we found 3 nucleotide changes differentiating South Africa and Mexico A sequences published by Harper et al. [19] from our own sequences of Mexico A. Two of these differences were found in homopolymeric stretches (in fliG-tp0027 and tp0347 regions) and one SNP (C→T) was found in the rpiA-tp0617 region. Since both Mexico A strains came from the same laboratory (D. L. Cox, CDC Atlanta), the data suggest that possible sequencing errors in sequences published by Harper et al. [19] may explain these differences. To further asses the frequency of strains similar to Mexico A/South Africa, we investigated clinical samples published by Flasarová et al. [21] for Mexico A-specific mutations. No such nucleotide changes were found in 49 genotyped samples, indicating that the Mexico A/South Africa group of strains is not prevalent in central Europe. v) Since convergent evolution assumes acquisition of the same biological trait in unrelated lineages (operating on the level of biological function), it is extremely unlikely that it would result in exactly the same amino acid sequence of the relevant proteins. Due to degeneration of the genetic code, it is even more unlikely that convergent evolution would end up in two identical nucleotide sequences. vi) In contrast to previous alternatives, inter-strain recombination cannot be ruled out despite the fact that the probability of such event is relatively low. Moreover, the mosaic character of the TPAMA_0326 and TPAMA_0488 loci, combining both TPA- and TPE-specific nucleotide sequences, is a typical result of a recombination event after horizontal gene transfer [22]–[24]. Also, patterns found in TEN strains indicate that observed mosaics in the Mexico A genome are not artifacts, but rather the results of recombination events in the common ancestor of TPA and TEN strains (see Fig. 3C).
Figure 3. Evolutionary relationships among TP strains and hypothetical evolutionary schemes.
A. Unrooted tree constructed from restriction target site data from 25.6% of the analyzed TP genomes (modified according to Šmajs et al. [18]). TPA (Dallas, Nichols, Mexico A and SS14), TPE (CDC-2, Gauthier and Samoa D), TEN (Bosnia A) strains and unclassified simian isolate Fribourg-Blanc were analyzed. TPA strains are shown in bold. Patterns shown for the Bosnia A strain are based on the sequences of TP0326 (GenBank acc. no. JX392330.1, this sequence is identical to partial sequence published by Harper et al. [19]) and TP0488 (GenBank acc. no. JX392331.1) determined by us, and on the whole genome fingerprinting data [18]. B. Scheme illustrating hypothetical evolution of TP strains. Please note that the ancestral position of Mexico A related to other TPA strains does not explain presence of TEN-specific pattern in TP0326 (tp92) locus in the SS14-like group of TPA strains. C. Scheme illustrating hypothetical recombinations among TP strains. A hypothetical ancestor of TEN and Mexico A strains is marked by star.
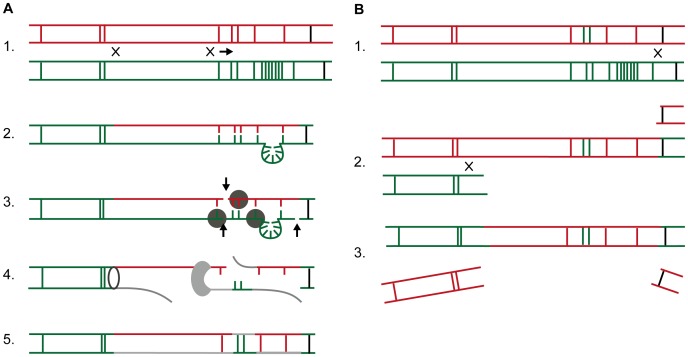
There are several possible molecular mechanisms that could lead to the formation of the mosaic structure seen at the TPAMA_0326 and TPAMA_0488 loci. We propose two models (Fig. 4) that are based on the incorporation of TPE double stranded DNA. In the first model, dsDNA was integrated into the chromosome of the Mexico A ancestor through homologous recombination. The resulting DNA heteroduplex was block-repaired via mismatch repair mechanisms. Similar reparation patterns have been observed after DNA transformation of Escherichia coli [25] and Helicobacter pylori [24]. In other bacteria, mismatch repair involves the cleavage of a daughter strand by MutH, which recognizes methylated cytosine in the GATC sequence. Since TPA does not contain a MutH orthologue and no methyltransferases, the mechanism of DNA cleavage remains unknown. Both mutS and mutL have been annotated to the TPA genome.
Figure 4. Two possible molecular mechanisms resulting in formation of the mosaic structure of the TPAMA_0326 locus.
Green lines are nucleotide sequences specific for TPA strains (Nichols, SS14, Chicago), red ones for TPE strains (CDC-2, Gauthier, Samoa D). Vertical black lines denote corresponding positions of the TPAMA_0326 loci. A) Homologous recombination. 1. Homologous recombination between TPA and TPE loci with suggested possible recombination sites (x); arrow indicates direction of branch migration. 2. The resulting heteroduplex contains both TPA and TPE sequences; green loop denotes a 15 bp insertion present in all investigated TPA strains. 3. Recognition of mismatched DNA by MutS-MutL complex and subsequent cleavage of DNA strands by endonucleases (marked by arrows). 4. Unwinding of cleaved strands by DNA helicase (open elipse) and DNA polymerase-mediated (grey) gap filling. 5. Reparation of DNA breaks with DNA ligase. Alternatively, there is a possibility of an active DNA uptake across the cell membrane. Although no gene orthologs involved in natural competence have been identified in the TPA genomes, one cannot exclude this activity in one or several genes with unknown function. B) Gene conversion. Successive half crossing-over model. 1. First half crossing-over with suggested recombination site (x). 2. Successive second half crossing-over and the second recombination site (x). 3. Duplex recovery and degradation of both displaced ends. Please note that model B) requires the presence of two TPA-like nucleotide sequences in the donor TPE sequence.
The second mechanism is based on gene conversion events following internalization of dsDNA. Gene conversion is a common mechanism for producing antigenic variability in TPA [26]. Since TPA possesses only the RecF recombination pathway, gene conversion in TPA is likely to follow the successive half crossing-over model [27], as shown in Fig. 4. However, the mosaic structure observed at the TPAMA_0326 and TPAMA_0488 loci would require multiple successive gene conversion events in both loci, which is unlikely. One possible explanation would presume a partial mosaic structure (Fig. 4) in both loci in the TPE donor DNA prior to crossing-over. Assuming this, the observed mosaic sequence at the TPAMA_0326 and TPAMA_0488 loci could result from a single gene conversion/recombination event.
Alternatively, there is a possibility of active DNA uptake across the cell membrane, which is more efficient, compared to natural competence of bacteria. Although no gene orthologs involved in natural competence have been identified in the TPA genomes, one cannot exclude this activity in one or more genes with unknown function. Internalization of TPE ssDNA would follow the model of mismatch repair.
TPAMA_0326 and TPAMA_0488 are mosaics resulting from interchromosomal recombination/gene conversion between TPA and TPE strains, while tprC and tprD alleles are the results of intrachromosomal recombination in tprC and tprD loci [12]. Therefore, similarities to TPE strains seen in tprC locus and TPAMA_0326 and TPAMA0488 loci arose via different mechanisms. Except for the TPAMA_0326 and TPAMA_0488 loci, two additional nucleotide positions (2 out of 1,192 single nucleotide changes differentiating TPA and TPE strains [7]; i.e. 0.168%) were found in the TP0314 locus and TPAMA_0319 gene. In these cases the Mexico A sequence was identical to the TPE sequences. These two nucleotide differences appear to represent differences that occurred by chance. For a single nucleotide position, the theoretical probability is 1,192/1,140,038*1/3 (i.e. 0.035%), where 1/3 is the probability that a particular nucleotide would be changed into a TPE nucleotide. Moreover, since the set of 1,192 single nucleotide changes that differentiate TPA and TPE strains is only based on comparisons of three TPA and three TPE strains, it is likely that the number of nucleotide positions differentiating all TPA and TPE strains will decrease with the newly reported whole genome sequences from other TPA and TPE strains.
Horizontal gene transfer (HGT) is an important process in bacterial evolution and the most frequently transferred genes usually bring selective advantage to the host cell. The TPA genome contains no prophages or IS-elements [28] or plasmids [29]. Nevertheless, the absences of modification and restriction systems together with the presence of genes for homologous recombination in TPA strains [4] appear to allow incorporation of foreign DNA molecules with subsequent integration into the chromosomal DNA. DNA transformation is commonly used in cultivable Treponema denticola [30] and related Borrelia burgdorferi strains [31]. Moreover, natural gene transfer among Borrelia burgdorferi has been observed [32]. In fact, 77 (8.32%) TPA genes were identified to be horizontally transferred by analysis of G+C contents, codon and amino acid usage, and gene position [17]. In our analysis, we did not find DNA regions of different G+C content to be associated with regions that differentiate TPA and TPE strains [7], nor were such associations found in tpr regions, indicating that the genome rearrangements took place before the diversification of these strains. It is therefore likely that the diversification of TPA and TPE strains was due to an accumulation of more subtle changes.
As shown by Centurion-Lara et al. [11], recombination mechanisms are more active during treponemal infection and gene conversion events represent important mechanisms for avoiding the host immune response. Therefore, uptake of TPE DNA by TPA strain, during a simultaneous TPA and TPE infection of a single host, with subsequent integration into TPA chromosome, appears to be a plausible explanation. Simultaneous infection with TPA and TPE is certainly possible during the early stages of syphilis infection. It has been shown that experimental infection with either TPA or TPE strains did not result in complete cross-protection, which suggests differences in the pathogenesis of syphilis and yaws [33], [34]. Although syphilis is preferentially transmitted sexually among adults, and yaws is preferentially transmitted via direct skin contact among children, simultaneous infection in a single host cannot be ruled out. The Haiti B strain, originally classified as a TPE strain due to having been isolated from “typical yaws lesions” in an 11-year-old child [13], has been recently reclassified as a TPA strain [19], [35], [36]. Moreover, Mexico A strain was isolated in a geographic region where both TPA and TPE infections occurred [37]–[39]. Nevertheless, recombination could also take place outside Mexico.
The mosaic TPAMA_0326 protein (Tp92) belongs to a relatively small group of treponemal outer membrane proteins [40] and is an ortholog of the BamA protein involved in outer membrane biogenesis [41]. BamA protein was identified as a TPA antigen exhibiting reactivity with sera from patients with syphilis [42], [43], and antibodies against this protein have opsonized living treponemes [44]. The 15 bp (TPE-like) deletion in the TPAMA_0326 influences the polyserine tract in a predicted large extracellular loop of TPAMA_0326 protein, which serves as a potential site for attachment to the host cells [44].
TPAMA_0488 encodes the methyl-accepting chemotaxis protein (Mcp2-1) [45]. Mcp2-1 is strongly expressed during experimental rabbit infections [46] and elicits a humoral response [45]. In the Mcp2-1 protein, there are 18 TPE-like changes, 8 of which are localized in the Cache domain [47], which binds small molecules during chemotaxis. All of these TPE-like changes cause amino acid changes, 7 non-conservative and 1 conservative. Taken together, due to described changes in extracellular/sensoring protein domains, both proteins can exhibit different antigenic epitopes and/or ligand binding activities.
Both TPAMA_0326 and TPAMA_0488 genes are under positive selection within TPA strains, as well as between TPA and TPE strains (genes were tested using codon-based testing by Čejková et al. [7]). The recombinant TPA strain (Mexico A) can thus possess a selective advantage in an infected host and could provide evasion from the host's immune system. However, it was recently shown that β-barrel structures, including surface-exposed loops of TPAMA_0326, where the TPE-like deletion is present, do not induce antibody response in humans [41], [48] On the other hand, positive selection need not be driven solely by the production of antibodies and may also comprise T-cell mediated cellular response, similar to the case of TprK [49]. In addition, positive selection operating on the periplasmic Cache domain of TPAMA_0488, recognizing small molecules, could reflect changed tissue tropism of TPE bacteria in comparison to TPA.
Despite selective advantage in the infected host (evasion from immune response, changed tissue tropism), these changes could result in the observed lower growth ability of the Mexico A strain compared to the Nichols strain under in vitro conditions [14]. Under positive selection, such a change can still have a growth advantage relative to the selective pressure on the host's immune system.
In summary, the mosaic character of the TPA Mexico A genome is likely the result of interstrain recombination between TPA and TPE strains during simultaneous infection in one host and similar patterns can be observed among other TP strains. These findings suggest the importance of horizontal gene transfer in the evolution of pathogenic treponemes.
Supporting Information
List of genes encoding putative virulence factors annotated in the Mexico A genome. Putative virulence factors were defined as those previously described by Čejková et al. [7] and comprised 31 genes (including tpr, arp, and TPAMA0136 genes).
(XLS)
Gene fusions identified in the Mexico A genome. Frameshift mutations identified in the Mexico A genome resulted in gene fusions of 43 ORFs originally annotated in the SS14 genome. The resulting 21 ORFs annotated in the Mexico A genome are listed in this table. In stands for insertion and del for deletion.
(XLS)
ORFs containing sequence changes in comparison of the Mexico A genome with the Nichols genome. Mexico A genome was compared with the Nichols genome (AE000520.1) and with the improved version of the Nichols genome (Pospíšilová, unpublished results). No change means that ORF sequence in the improved Nichols version was identical to the ORF in the Mexico A genome and the originally identified change was considered to be Nichols sequencing error. In the case of 14 Nichols ORF sequences, only partial (partial data) or no (N/A) sequencing data were available. Sub stands for substitution, in for insertion, del for deletion and MCS stands for multiple sequence changes.
(XLS)
Number of ORFs containing sequence changes in comparison of Mexico A with Nichols and SS14. The Mexico A genome was compared with the Nichols genome (AE000520.1) and with the improved version of the Nichols genome (Pospíšilová, unpublished results). Numbers in brackets represent percentage of ORFs with sequence changes in each functional category.
(XLS)
Acknowledgments
The authors thank Dr. David Cox for providing the Mexico A strain.
Accession Numbers (GenBank)
Genomes: TPA Mexico A CP003064.1, TPA Nichols AE000520.1, TPA SS14 CP000805.1, TPA Chicago CP001752.1, TPE Samoa D CP002374.1, TPE CDC-2 CP002375.1, TPE Gauthier CP002376.1, T. paraluiscuniculi strain Cuniculi A CP002103.1.
Genes: TPA Mexico A: tp92 EU102088.1, fliG-tp0027 EU101896.1, tp0347 EU102160.1, rpiA-tp0617 EU101922.1; TPA South Africa: tp92 EU102098.1, fliG-tp0027 EU101902.1, tp0347 EU102170.1, rpiA-tp0617 EU101910.1; TEN Bosnia A: tp92 EU102075.1, TP0326: JX392330.1, TP0488 JX392331.1.
Funding Statement
This work was supported by a grant from the Grant Agency of the Czech Republic (310/04/0021 and P302/12/0574) and a grant from the Ministry of Health of the Czech Republic (NT11159-5/2010) and Ministry of Education of the Czech Republic (VZ MSM0021622415) to D.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schaudin FR, Hoffmann E (1905) Vorläufiger Bericht über das Vorkommen von Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen. Arb K Gesund 22: 527–534. [PubMed] [Google Scholar]
- 2. Castellani A (1905) Further Observations on Parangi (Yaws). Br Med J 2: 1330–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (1998) The world health report - Life in the 21st Century: A vision for all. [Google Scholar]
- 4. Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, et al. (1998) Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281: 375–388. [DOI] [PubMed] [Google Scholar]
- 5. Matejkova P, Strouhal M, Smajs D, Norris SJ, Palzkill T, et al. (2008) Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giacani L, Jeffrey BM, Molini BJ, Le HT, Lukehart SA, et al. (2010) Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J Bacteriol 192: 2645–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cejkova D, Zobanikova M, Chen L, Pospisilova P, Strouhal M, et al. (2012) Whole Genome Sequences of Three Treponema pallidum ssp. pertenue Strains: Yaws and Syphilis Treponemes Differ in Less than 0.2% of the Genome Sequence. PLoS Negl Trop Dis 6: e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smajs D, Zobanikova M, Strouhal M, Cejkova D, Dugan-Rocha S, et al. (2011) Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One 6: e20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mikalova L, Strouhal M, Cejkova D, Zobanikova M, Pospisilova P, et al. (2010) Genome analysis of Treponema pallidum subsp. pallidum and subsp. pertenue strains: most of the genetic differences are localized in six regions. PLoS One 5: e15713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smajs D, Norris SJ, Weinstock GM (2012) Genetic diversity in Treponema pallidum: Implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws. Infect Genet Evol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centurion-Lara A, Sun ES, Barrett LK, Castro C, Lukehart SA, et al. (2000) Multiple alleles of Treponema pallidum repeat gene D in Treponema pallidum isolates. J Bacteriol 182: 2332–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray RR, Mulligan CJ, Molini BJ, Sun ES, Giacani L, et al. (2006) Molecular evolution of the tprC, D, I, K, G, and J genes in the pathogenic genus Treponema. Mol Biol Evol 23: 2220–2233. [DOI] [PubMed] [Google Scholar]
- 13. Turner TB, Hollander DH (1957) Biology of the treponematoses based on studies carried out at the International Treponematosis Laboratory Center of the Johns Hopkins University under the auspices of the World Health Organization. Monogr Ser World Health Organ 3–266. [PubMed] [Google Scholar]
- 14. Cox DL, Moeckli RA, Fieldsteel AH (1984) Cultivation of pathogenic treponema in tissue cultures of SflEp cells. In Vitro 20: 879–883. [DOI] [PubMed] [Google Scholar]
- 15. Strouhal M, Smajs D, Matejkova P, Sodergren E, Amin AG, et al. (2007) Genome differences between Treponema pallidum subsp. pallidum strain Nichols and T. paraluiscuniculi strain Cuniculi A. Infect Immun 75: 5859–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinstock GM, Norris SJ, Sodergren E, Smajs D (2000) Identification of virulence genes in silico: Infection disease genomics. Virulence mechanisms of Bacterial Pathogens 378. [Google Scholar]
- 17. Garcia-Vallve S, Romeu A, Palau J (2000) Horizontal gene transfer in bacterial and archaeal complete genomes. Genome Res 10: 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smajs D, Mikalova L, Cejkova D, Strouhal M, Zobanikova M, et al.. (2011) Whole Genome Analyses of Treponemes: New Targets for Strain- and Subspecies-Specific Molecular Diagnostics. In: Sato NS, editor. Syphilis - Recognition, Description and Diagnosis: InTech. pp. 130. [Google Scholar]
- 19. Harper KN, Ocampo PS, Steiner BM, George RW, Silverman MS, et al. (2008) On the origin of the treponematoses: a phylogenetic approach. PLoS Negl Trop Dis 2: e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang GC, Wang Y (1996) The frequency of chimeric molecules as a consequence of PCR co-amplification of 16S rRNA genes from different bacterial species. Microbiology 142 (Pt 5) 1107–1114. [DOI] [PubMed] [Google Scholar]
- 21. Flasarova M, Pospisilova P, Mikalova L, Valisova Z, Dastychova E, et al. (2012) Sequencing-based Molecular Typing of Treponema pallidum Strains in the Czech Republic: All Identified Genotypes are Related to the Sequence of the SS14 Strain. Acta Derm Venereol [DOI] [PubMed] [Google Scholar]
- 22. Smith JM, Dowson CG, Spratt BG (1991) Localized sex in bacteria. Nature 349: 29–31. [DOI] [PubMed] [Google Scholar]
- 23. Feavers IM, Heath AB, Bygraves JA, Maiden MC (1992) Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol Microbiol 6: 489–495. [DOI] [PubMed] [Google Scholar]
- 24. Kulick S, Moccia C, Didelot X, Falush D, Kraft C, et al. (2008) Mosaic DNA imports with interspersions of recipient sequence after natural transformation of Helicobacter pylori. PLoS One 3: e3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abastado JP, Cami B, Dinh TH, Igolen J, Kourilsky P (1984) Processing of complex heteroduplexes in Escherichia coli and Cos-1 monkey cells. Proc Natl Acad Sci U S A 81: 5792–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centurion-Lara A, LaFond RE, Hevner K, Godornes C, Molini BJ, et al. (2004) Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol Microbiol 52: 1579–1596. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi I (1992) Mechanisms for gene conversion and homologous recombination: the double-strand break repair model and the successive half crossing-over model. Adv Biophys 28: 81–133. [DOI] [PubMed] [Google Scholar]
- 28. Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, et al. (2004) Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A 101: 5646–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker EM, Arnett JK, Heath JD, Norris SJ (1991) Treponema pallidum subsp. pallidum has a single, circular chromosome with a size of approximately 900 kilobase pairs. Infect Immun 59: 2476–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuramitsu HK, Chi B, Ikegami A (2005) Genetic manipulation of Treponema denticola. Curr Protoc Microbiol Chapter 12: Unit 12B 12. [DOI] [PubMed] [Google Scholar]
- 31. Hyde JA, Weening EH, Skare JT (2011) Genetic transformation of Borrelia burgdorferi. Curr Protoc Microbiol Chapter 12: Unit 12C 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tilly K, Elias AF, Bono JL, Stewart P, Rosa P (2000) DNA exchange and insertional inactivation in spirochetes. J Mol Microbiol Biotechnol 2: 433–442. [PubMed] [Google Scholar]
- 33. Miller JN (1973) Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol 110: 1206–1215. [PubMed] [Google Scholar]
- 34. Chan JK, Schell RF, Le Frock JL (1982) Mitogenic responses of hamsters infected with Treponema pertenue Lack of correlation with passive transfer of resistance. Br J Vener Dis 58: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centurion-Lara A, Castro C, Castillo R, Shaffer JM, Van Voorhis WC, et al. (1998) The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J Infect Dis 177: 1036–1040. [DOI] [PubMed] [Google Scholar]
- 36. Cameron CE, Castro C, Lukehart SA, Van Voorhis WC (1999) Sequence conservation of glycerophosphodiester phosphodiesterase among Treponema pallidum strains. Infect Immun 67: 3168–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mansilla J, Rothschild BM, Pijoan C, Rothchild C (2000) Transitions among treponematoses in ancient Mexico. Chungará (Arica) 32. [Google Scholar]
- 38. de Melo FL, de Mello JC, Fraga AM, Nunes K, Eggers S (2010) Syphilis at the crossroad of phylogenetics and paleopathology. PLoS Negl Trop Dis 4: e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perine PL, Hopkins DR, Niemel PLA, St John RK, Causse G, et al.. (1984) Handbook of endemic treponematoses. Geneva: World Health Organization. [Google Scholar]
- 40. Cox DL, Luthra A, Dunham-Ems S, Desrosiers DC, Salazar JC, et al. (2010) Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect Immun 78: 5178–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Desrosiers DC, Anand A, Luthra A, Dunham-Ems SM, LeDoyt M, et al. (2011) TP0326, a Treponema pallidum beta-barrel assembly machinery A (BamA) orthologue and rare outer membrane protein. Mol Microbiol 80: 1496–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Voorhis WC, Barrett LK, Lukehart SA, Schmidt B, Schriefer M, et al. (2003) Serodiagnosis of syphilis: antibodies to recombinant Tp0453, Tp92, and Gpd proteins are sensitive and specific indicators of infection by Treponema pallidum. J Clin Microbiol 41: 3668–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brinkman MB, McKevitt M, McLoughlin M, Perez C, Howell J, et al. (2006) Reactivity of antibodies from syphilis patients to a protein array representing the Treponema pallidum proteome. J Clin Microbiol 44: 888–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, et al. (2000) Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 181: 1401–1413. [DOI] [PubMed] [Google Scholar]
- 45. Greene SR, Stamm LV (1998) Molecular characterization of Treponema pallidum mcp2, a putative chemotaxis protein gene. Infect Immun 66: 2999–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smajs D, McKevitt M, Howell JK, Norris SJ, Cai WW, et al. (2005) Transcriptome of Treponema pallidum: gene expression profile during experimental rabbit infection. J Bacteriol 187: 1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anantharaman V, Aravind L (2000) Cache - a signaling domain common to animal Ca(2+)-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci 25: 535–537. [DOI] [PubMed] [Google Scholar]
- 48. Anand A, Luthra A, Dunham-Ems S, Caimano MJ, Karanian C, et al. (2012) TprC/D (Tp0117/131), a Trimeric, Pore-Forming Rare Outer Membrane Protein of Treponema pallidum, Has a Bipartite Domain Structure. J Bacteriol 194: 2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morgan CA, Lukehart SA, Van Voorhis WC (2002) Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect Immun 70: 6811–6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of genes encoding putative virulence factors annotated in the Mexico A genome. Putative virulence factors were defined as those previously described by Čejková et al. [7] and comprised 31 genes (including tpr, arp, and TPAMA0136 genes).
(XLS)
Gene fusions identified in the Mexico A genome. Frameshift mutations identified in the Mexico A genome resulted in gene fusions of 43 ORFs originally annotated in the SS14 genome. The resulting 21 ORFs annotated in the Mexico A genome are listed in this table. In stands for insertion and del for deletion.
(XLS)
ORFs containing sequence changes in comparison of the Mexico A genome with the Nichols genome. Mexico A genome was compared with the Nichols genome (AE000520.1) and with the improved version of the Nichols genome (Pospíšilová, unpublished results). No change means that ORF sequence in the improved Nichols version was identical to the ORF in the Mexico A genome and the originally identified change was considered to be Nichols sequencing error. In the case of 14 Nichols ORF sequences, only partial (partial data) or no (N/A) sequencing data were available. Sub stands for substitution, in for insertion, del for deletion and MCS stands for multiple sequence changes.
(XLS)
Number of ORFs containing sequence changes in comparison of Mexico A with Nichols and SS14. The Mexico A genome was compared with the Nichols genome (AE000520.1) and with the improved version of the Nichols genome (Pospíšilová, unpublished results). Numbers in brackets represent percentage of ORFs with sequence changes in each functional category.
(XLS)