Abstract
Recent phase III trial results have demonstrated the effectiveness of sipuleucel-T, a therapeutic cancer vaccine, in the treatment of metastatic prostate cancer. Yet despite the survival benefit of sipuleucel-T, questions remain about how immunologic agents can be employed in the treatment of metastatic prostate cancer. The primary issue confounding researchers and practitioners about the benefits of sipuleucel-T is the lack of effect on time to progression. It may be helpful to note that recent phase II data from a different therapeutic prostate cancer vaccine (Prostvac), as well as phase III data from an anti-CTLA-4 blocking agent in metastatic melanoma, also show improved survival without short-term changes in disease progression. Furthermore, mathematical tumor growth models provide some insight into the fact that immunologic therapies do allow for continued tumor growth, but at a slower rate, thus prolonging survival. This understanding can help to clarify the role of the newly approved sipuleucel-T in the treatment of metastatic prostate cancer. It is also possible that appropriate sequencing of therapies could further improve the clinical course for such patients. Additional clinical trials will further our understanding of the role of therapeutic cancer vaccines and add new agents to the armamentarium of therapy for patients with prostate cancer.
Keywords: Prostate Cancer, Immunotherapy, Cancer Vaccine, Treatment
Introduction
In 2004, docetaxel plus prednisone became the first regimen approved by the U.S. Food and Drug Administration (FDA) for the treatment of mCRPC, based on data showing improved overall survival. But in the years that followed, many phase III trials failed to meet their endpoints in mCRPC.1,2 Now, however, treatment options for patients with mCRPC are increasing thanks to promising outcomes in recent trials. In April 2010, sipuleucel-T vaccine (Provenge®; Dendreon Corp., Seattle, WA) received FDA approval for use in mCRPC, followed in June by FDA approval of carbazitaxel (Jevtana®; Sanofi-aventis, Paris, France) for patients with mCRPC who have progressed on docetaxel-based therapy.3,4 In addition, two novel hormonal therapies have also generated considerable interest in preliminary clinical trials and are in phase III testing.5,6 The advent of these new therapies brings alternative therapeutic options for providers and hope for patients, but the emergence of immunotherapeutic agents also brings new questions and challenges. A review of clinical experiences with several immune-stimulating therapies provides valuable insight into their potential role in the treatment of mCRPC.
Therapeutic Cancer Vaccines
Standard approaches to treating prostate cancer have been either hormonal therapies that target the androgen receptor or the endocrine axis, or conventional cytotoxic chemotherapy.1,2,7 Therapeutic cancer vaccines represent an entirely different approach. The goal of therapeutic cancer vaccines is to generate a targeted immune response that ultimately curtails tumor growth. Accomplishing this goal requires enhanced immune recognition of specific tumor-associated antigens (TAAs), which are expressed within the major histocompatability complex (MHC) of tumor cells. Once identified by immune effector cells such as cytolytic T cells, the tumor cells are selectively destroyed.8 The destruction of tumor cells by way of an active immune response results in the recognition of additional TAAs that can also be targeted, resulting in a diversified immune response targeting multiple TAAs.9
Sipuleucel-T is a therapeutic cancer vaccine generated from peripheral blood mononuclear cells obtained from individual patients via leukapheresis. The primary target of this vaccine is the prostate cancer TAA prostatic acid phosphatase (PAP). The active component of the vaccine includes antigen-presenting cells (APCs; e.g., dendritic cells), which are enriched by density centrifugation and stimulated in vitro by exposure to a PAP/GM-CSF fusion protein.10 In addition to APCs, the final product contains T cells, B cells, natural killer (NK) cells, and other cells, dependent on the composition of cells obtained from the patient's leukapheresis. The potency of the vaccine is measured by the level of CD54, a marker of immune activation, with a minimum of 50 million CD54+ cells in each vaccine.10 At the end of this process, the activated cellular product is reinfused into the patient. A full course of therapy repeats this process 3 times every 2 weeks for 1 month.11,12
After promising early clinical trials of sipuleucel-T (Table 1), 2 phase III studies were launched in mCRPC, each randomizing patients 2:1 in favor of the vaccine. The initial trial was fully accrued, with 82 patients on the sipuleucel-T arm and 45 patients receiving placebo, but did not achieve its primary endpoint of time to progression (sipuleucel-T: 16.6 weeks vs. placebo: 10 weeks; P = 0.052).13–16 These results led to discontinuation of the second trial (n = 98), which also showed no benefit in terms of time to progression upon interval analysis.12,17 At long-term follow-up, however, the initial phase III trial demonstrated a 4.5-month improvement in overall survival favoring sipuleucel-T (25.9 months vs. 21.4 months; P = 0.01).16 Based on these findings, a larger (n = 512) definitive overall survival endpoint study was initiated. The outcome of that trial showed a similar survival benefit for the vaccine (25.8 months vs. 21.7 months; P = 0.032), and a similar lack of change in time to progression.18 Based on these overall survival findings, the FDA approved sipuleucel-T, making it the first FDA-approved therapeutic cancer vaccine for the treatment of any malignancy.4,16,19
Table 1.
Early trials in the clinical development of sipuleucel-T
| Phase | n | Outcome | (Ref) |
|---|---|---|---|
| I/II | 31 | In phase I portion (n = 12), the agent was well tolerated, with fever a side effect in < 15% of patients. Six patients had a > 25% PSA decline, 3 of which were greater than 50%. All patients had increased T-cell proliferation in response to the PAP/GM-CSF construct within the vaccine. | 13 |
| II | 21 | In 19 evaluable patients, median time to progression was 118 days. Three patients had a PSA decline > 25%. Notably, one patient had an undetectable PSA after treatment (on-study = 221 ng/dL) and resolution of retroperitoneal disease. His T-cell stimulation in response to the PAP/GM-CSF construct remained elevated at 96 weeks. | 14 |
| II | 18 | Patients with rising PSA after definitive therapy were treated with sipuleucel-T. In 13 of 18 patients, median PSA doubling time was prolonged after therapy, increasing from 4.9 months prior to enrollment to 7.9 months after therapy. | 15 |
PSA-TRICOM (Prostvac™; developed by the National Cancer Institute [NCI] and licensed to BN Immunotherapeutics, Mountain View, CA) represents an alternative therapeutic cancer vaccine strategy. To target prostate-specific antigen (PSA), PSA-TRICOM vaccine employs genetically altered poxviruses to deliver targeting information to immune cells and generate an immune response. Administered subcutaneously, the poxviruses deliver the transgenes for the TAA (PSA) to APCs through cellular infection. Once these poxviruses are within the cellular cytoplasm, the transgenes are processed. The end result is an APC expressing a PSA peptide within the MHC, resulting in PSA-specific T-cell activation.20,21
After obtaining preliminary clinical data on poxviral vaccines targeting PSA, transgenes for 3 T-cell costimulatory molecules (TRICOM) were included with PSA in the second generation of the vaccine (PSA-TRICOM), resulting in enhanced T-cell activation (Table 2).22–28 This vaccine was then investigated in 2 phase II trials in mCRPC, dosing the vaccine at monthly intervals until disease progression. One study was an industry-sponsored, placebo-controlled, multicenter trial in 125 mCRPC patients with Gleason scores of ≤ 7. Patients were randomized 2:1 in favor of PSA-TRICOM; the placebo was an empty poxviral vector containing no transgenes. As was seen in the sipuleucel-T studies, patients receiving vaccine showed no change in time to progression (the primary endpoint), yet had an overall survival benefit (25.1 months with PSA-TRICOM vs. 16.6 months with placebo; P = 0.0061).29
Table 2.
Early trials in the clinical development of PSA-TRICOM
| Phase | n | Outcome | (Ref) |
|---|---|---|---|
| I | 42 | This study established the safety of a first-generation poxviral vaccine (rV-PSA) targeting PSA. The most common adverse events were local injection-site reaction and fever. | 22 |
| II | 64 | This trial demonstrated that patients treated with a vaccinia priming dose (rV-PSA) and subsequent boosting doses of fowlpox (rF-PSA) had the greatest clinical response, establishing vaccinia prime and fowlpox boost as the optimal treatment strategy. | 23 |
| I | 15 | A second-generation poxviral vaccine (PSA-TRICOM) that contains 3 T-cell costimulatory molecules that enhance T-cell activation had no additional toxicity. Nine of 15 patients had decreases in PSA velocity after enrollment. | 24 |
| II | 69 | Preliminary data on PSA-TRICOM in patients with rising PSA after definitive therapy suggest a delay in time to PSA progression. | 25 |
A second phase II study of PSA-TRICOM was conducted at the NCI in 32 patients with mCRPC, regardless of Gleason score, all of whom received vaccine. The median overall survival was 26.6 months, which was similar to the multicenter PSA-TRICOM study. In addition, correlative immunologic analyses demonstrated an increase in PSA-targeting T cells in some patients, along with a trend associating greatest magnitude of immune response with improved overall survival (P = 0.055).30 Based on the findings in these trials, a phase III trial of PSATRICOM in mCRPC is planned for late 2010, with overall survival as a primary endpoint.31
CTLA-4 Blockade: An Alternative Immunologic Approach
Ipilimumab (Bristol-Myers Squibb, New York, NY) represents a different approach to immune stimulation as a means of developing antitumor immunologic activity. CTLA-4 is a molecule expressed by effector T cells after they have been activated, providing a mechanism for immune system autoregulation. Within 48 hours, CTLA-4 expression and subsequent binding with APCs results in down-regulation of the effector cell-mediated immune response. The role of CTLA-4 is highlighted in CTLA-4 knockout mice, who only survive a few weeks until multiorgan failure results from infiltrating autoreactive T cells.32 A blocking antibody such as ipilimumab prevents CTLA-4 binding, potentially prolonging and enhancing immune activity against tumors.33–35
Ipilimumab has been extensively evaluated in melanoma, where it recently demonstrated an overall survival advantage in metastatic disease. As expected, there was an increased incidence of autoimmune-related toxicity typical of this drug, but not seen with therapeutic cancer vaccines.36 Ipilimumab has also been studied in the treatment of prostate cancer, alone and in combination with therapeutic cancer vaccines.37–39 A phase III trial currently enrolling 800 patients will evaluate the benefits of ipilimumab with or without radiation in mCRPC following treatment with docetaxel.40 A second trial will accrue chemo-naïve mCRPC patients, who will receive either ipilimumab or placebo.41 The primary endpoint of both trials will be overall survival.
Survival Benefit Without Initial Change in Disease Progression
Although the immunologic agents discussed above represent different approaches to stimulating an immunologic antitumor response, they share one perplexing commonality: they all extend overall survival without altering time to progression. As discussed above, multiple trials with sipuleucel-T and PSA-TRICOM in prostate cancer have demonstrated prolonged survival benefit, with no change in short-term disease progression.16,19,29,30 In addition, ipilimumab in metastatic melanoma demonstrated an overall survival benefit without any improvement in median time to progression relative to an active control (GP100).36
As vexing as this may be to clinicians who are accustomed to seeing at least stable disease after implementing a successful treatment, a growing body of evidence suggests that a lack of short-term change in disease progression is an attribute of this new and growing class of therapeutic agents. Although there are strategies for maximizing the benefits of vaccine in prostate cancer, the prevailing belief that increased time to progression is the sine qua non of improved survival means that the mounting data showing delayed clinical response with immunotherapies need to be further investigated and better understood. Emerging data suggest that this theory is likely true for conventional therapeutics, but modern therapeutics may require a more contemporary understanding.
The clinical picture is further complicated by the absence of any valid, standardized biomarkers to predict response with immune-based treatments. While some trials discussed here have demonstrated antigen-specific T-cell response after vaccine therapy, such methods are not standardized and may vary from institution to institution and reader to reader.30 To further complicate the matter, the intricacy of an immune response may result in the targeting of an antigen not specified in the vaccine, and this secondary response may become the most relevant anti-tumor response.42,43 In such circumstances, the absence of immune response to a given antigen could be misleading and would not necessarily indicate an absence of response to the immune therapy. As modern immunotherapeutics enter the clinic, the need for biomarkers as intermediate markers will become greater, which is why this is a field of active investigation.9
In the absence of valid biomarkers, alternative methods may be required to better understand the delayed clinical benefits of immunotherapeutics. Mathematical models of tumor growth can provide insight into novel treatment strategies that may be required for immunologic and other noncytotoxic agents, such as targeted molecular inhibitors. These models suggest that conventional measurements, such as radiographic imaging, may belie the underlying therapeutic benefits of a given agent. Ultimately, the relative rate of tumor growth may be a better indicator of overall survival than absolute measurements, such as arbitrary RECIST cut-offs for disease progression. In other words, slow disease progression while on therapy may not warrant discontinuing an agent that may still provide long-term clinical benefit.44
Using mathematically derived models, tumor growth rates can be calculated and associated with survival times based on enlarging tumors on scans, M-spikes in multiple myeloma, and PSA in prostate cancer.44–46 A review of recent prostate cancer clinical trials at the NCI noted a fundamental difference between trials employing chemotherapy or second-line hormonal therapies, compared to the randomized NCI phase II study of PSA-TRICOM. Unlike other nonvaccine trials, where off-treatment growth rates predicted survival, the PSA-TRICOM study showed no correlation between tumor growth rate while on vaccine treatment and overall survival. Patients from that study survived longer than would have been predicted by the tumor growth trajectory while they received vaccine.47 These findings suggest that there may be subtle, vaccine-induced changes in tumor biology, or interactions between the immune system and the tumor or its microenvironment, that have a potentially delayed, yet sustained effect on tumor growth after vaccine treatment has been discontinued. Prospective monitoring of future trials of therapeutic cancer vaccines may be required to explain the clinical observation of overall survival benefit without changes in time to progression.
Who Should Be Treated with Sipuleucel-T?
Mathematical tumor growth models suggest that it takes time to derive clinical benefit from therapeutic cancer vaccines such as sipuleucel-T—time for the tumor growth rate to decline, leading to longer-than-expected overall survival.47 The NCI phase II study of PSA-TRICOM provided clinical corroboration of this concept. Patients in this study were retrospectively stratified into those with aggressive disease and those with indolent disease using the Halabi nomogram.30 This prognostic tool was derived from an analysis of mCRPC patients in Cancer and Leukemia Group B (CALGB) trials in the pre-docetaxel era. Based on prostate cancer characteristics (PSA, performance status, LDH, alkaline phosphatase, hemoglobin, Gleason score, and presence/absence of visceral disease), the Halabi nomogram can be used to project survival of patients treated with second-line hormonal therapy or chemotherapy.48 For the NCI study analysis, indolent disease was defined as a projected survival greater than 18 months, and aggressive disease was a projected survival less than 18 months. The results suggested that patients with indolent disease characteristics are likely to derive the greatest benefit from vaccine therapy. Patients with indolent disease survived a median of > 37.3 months, compared to a median Halabi nomogram-predicted survival of 20.9 months. For patients with aggressive disease and a projected survival of < 18 months, the median overall survival was 14.6 months relative to a median predicted survival of 12.3 months.30
Taken together, these data suggest that patients with slower-growing, more indolent prostate cancer are most likely to benefit from therapeutic cancer vaccines and, thus, a clinical assessment is the best way to identify the optimal treatment for individual patients. The pace of disease growth could be determined by progression of lesions on scans, rather than strictly PSA-based parameters. Volume of disease will probably influence these prognostic factors and should be taken into account during clinical assessment. Furthermore, there is an immunologic basis for disease burden that could affect response to therapeutic cancer vaccines. The magnitude of tumor burden has been associated with an increased number of regulatory immune cells that may suppress the immune response, thereby diminishing potential response to therapeutic cancer vaccines.49–53 Again, data from the NCI trial of PSA-TRICOM vaccine may provide insight. In patients whose survival times that were longer than predicted by the Halabi nomogram, there was an association between decreased numbers of regulatory T cells (relative to cytotoxic T cells) and overall survival.54
Beyond the metrics of tumor volume and lab values, the subjective complaints of patients with mCRPC may also factor into the choice of treatment. The CALGB evaluated 599 patients from 3 phase III studies and noted that magnitude of baseline pain could have prognostic value in patients with mCRPC. For patients with the highest magnitude of pain, median overall survival was 10.2 months compared to those with low levels of pain, who had a median overall survival of 17.6 months (P < 0.001).55 In the context of this discussion, patients with severe pain symptoms and anticipated survival of less than a year would not be identified as having indolent disease, and thus would not be likely to benefit from a therapeutic cancer vaccine.
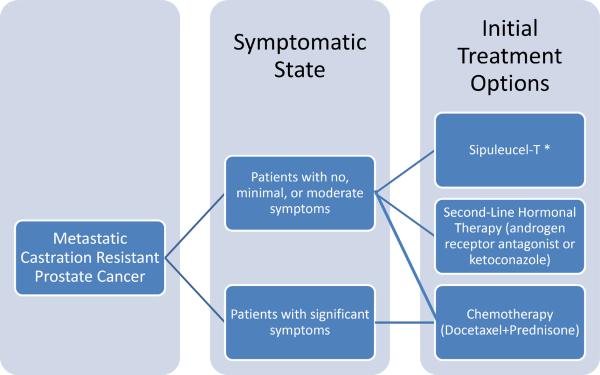
These findings suggest that sipuleucel-T would be an appropriate option for patients with mCRPC who have relatively indolent disease characteristics, low tumor volume, and relatively less severe symptoms (Figure 1). For these patients, sipuleucel-T could be given in place of second-line hormonal therapies.7 Although chemotherapy is not precluded as an option for this patient population, it is clearly most appropriate, and may provide symptomatic relief, for patients with advanced symptomatic disease. In addition, there are no data suggesting that chemotherapy given earlier in minimally symptomatic mCRPC influences the course of disease in the long term. Furthermore, given the side-effect profile of docetaxel relative to sipuleucel-T, it would be appropriate to allow patients with minimal symptoms to receive vaccine first, and perhaps withhold chemotherapy, with its accompanying toxicities, until symptoms become more severe.
Figure 1.
Emerging Treatment Paradigm for Patients with Metastatic Prostate Cancer
*See Table 2 for Options after Sipuleucel-T
Follow-Up for Patients Treated with Sipuleucel-T
The issue of follow-up for patients treated with sipuleucel-T is unique in oncology for 2 reasons: 1) sipuleucel-T is administered for a relatively short period of time (one month), and 2) it has not been shown to alter time to disease progression in the majority of patients. Follow-up may therefore vary from patient to patient and provider to provider, but there are several common considerations, foremost of which is the recommendation of the PSA Working Group II that PSA alone should not determine progressive disease in mCRPC.56 This advisory is based on the likelihood that subtle or even moderate rises in PSA while on treatment may not indicate a failure of that treatment. This is particularly relevant in the case of therapeutic cancer vaccines, where PSA responses are seen less frequently than with the use of hormonal therapies and chemotherapy. (It is also important to note that although the mathematical modeling discussed previously suggests that PSA trajectory may predict survival, such measures need to be confirmed in prospective trials before they can be employed in clinical practice. Nonetheless, implicit within that model is the fact that rises in PSA may not be as important as significant changes in trajectory.47)
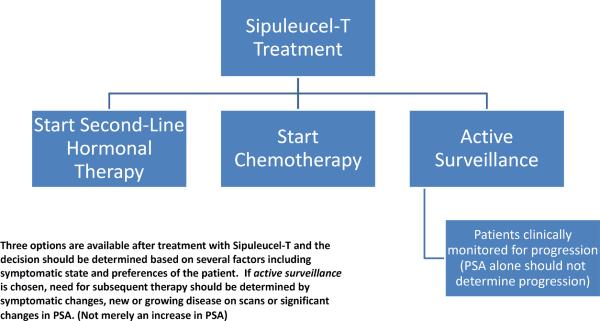
Clinicians ultimately have 3 options for follow-up treatment for patients receiving sipuleucel-T. (Figure 2) The first is to monitor patients clinically after treatment with the vaccine. While patients may appreciate the lack of toxic side effects with sipuleucel-T, they may be overwhelmed with anxiety about the short course of treatment followed by a period of surveillance without therapy. In this circumstance, clinicians should bear in mind phase III trial data showing that approximately 18% of patients received no treatment after sipuleucel-T and approximately 43% did not go on to receive docetaxel. In addition, median time to objective progression was 3.7 months.19 Patients could be monitored at periodic follow-up visits for changes in symptoms or abrupt changes in PSA, either of which could signal a need for repeat imaging to evaluate for progressive disease, and a discussion of the risks and benefits of additional therapy.
Figure 2.
Options After Treatment with Sipuleucel-T
The remaining options are to immediately follow one month of treatment with sipuleucel-T with either second-line hormonal therapy or docetaxel-based chemotherapy. The potential benefits of this sequential therapy are discussed below.
Potential Benefits of Subsequent Therapies Following Immunotherapy
Therapeutic cancer vaccines and immunotherapeutic agents such as ipilimumab are fundamentally different from standard therapies, in that immune-based therapies can induce an anticancer immune response that may persist after the therapy itself has been discontinued.57 This may have implications for patients treated with sipuleucel-T who go on to receive additional therapy. Furthermore, subsequent hormonal therapy or chemotherapy may enhance the immunologic response through immunologically relevant tumor-cell killing acting as a vaccine boost, as well as increased recognition of tumor cells, ultimately leading to enhanced tumor-cell killing.58–62
Clinical trials have suggested that a regimen of initial immunotherapy followed by a standard therapy may prolong survival.63,64 A retrospective evaluation of patients with mCRPC who had completed a previous trial of sipuleucel-T vs. placebo and had gone on to receive chemotherapy showed that the 51 patients treated with vaccine followed by docetaxel had a median overall survival of 34.5 months, compared to 25.4 months for the 31 patients treated with placebo followed by docetaxel (P = 0.023).65 Another study in patients with nonmetastatic CRPC suggested that patients were associated with a prolonged survival if they received a therapeutic cancer vaccine earlier in their disease course, followed by the addition of an androgen receptor antagonist.66 Two larger studies will further evaluate this hypothesis: a randomized Eastern Cooperative Oncology Group study of PSA-TRICOM followed by chemotherapy vs. chemotherapy alone,67 and an NCI study of androgen blockade with or without PSA-TRICOM.68
Combining Immunotherapy with Radiation Therapy
Radiation therapy can enhance the antitumor effect of immunotherapeutic agents by inducing phenotypic changes in cancer cells, including up-regulation of MHC class I, Fas, ICAM-1, and several other TAAs.69–71 Preclinical murine models have demonstrated improved antitumor activity with radiation plus a vaccine vs. radiation alone.72 A small clinical trial has also suggested that local radiation therapy combined with a therapeutic cancer vaccine generates a more effective systemic tumor-specific immunologic response than radiation alone.9
An ongoing phase III trial of ipilimumab plus radiation has enrolled patients with mCRPC who have been previously treated with docetaxel. Patients receive radiation to an area of metastatic disease, and then are given either ipilimumab or placebo.40 A phase II trial is employing radiation in the form of samarium-153 lexidronam (Quadramet), an intravenously administered bone-seeking radionuclide that is FDA-approved for relief of pain related to bone metastasis.73 This trial randomizes mCRPC patients with progressive disease on docetaxel to receive samarium-153 lexidronam either alone or in combination with PSA-TRICOM vaccine.74
Future Directions
In 2010, immuno-oncology has evolved from an academic curiosity to a clinical alternative for patients with cancer. However, the lack of short-term markers of response with these new treatments calls for the development of new treatment paradigms. For prostate cancer patients in particular, clinical evaluation will be crucial to determining which patients might benefit the most from sipuleucel-T and which will require conventional chemotherapy. Ongoing and pending trials are evaluating immunotherapies in combination with hormonal agents and radiation, each of which may enhance the effects of immune-based therapies. A trial of sequential vaccine plus chemotherapy will also provide insight into the benefits of such an approach in prostate cancer. These and similar trials may ultimately demonstrate that therapeutic cancer vaccines such as sipuleucel-T may be of greatest benefit when given earlier in the disease course, allowing for time to generate an immune response against a smaller tumor. With more clinical experience and broader understanding, therapeutic cancer vaccines will someday be included among standard treatments for prostate cancer.
Acknowledgements
Supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health. The authors thank Bonnie L. Casey for her editorial assistance in the preparation of this manuscript.
References
- [1].Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- [2].Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- [3].Sartor A, Oudard S, Ozguroglu M, et al. Cabazitaxel or mitoxantrone with prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel: final results of a multinational phase III trial (TROPIC). Presented at: ASCO Genitourinary Cancers Symposium; San Francisco, California. March 5–7, 2010; Abstract 9. [Google Scholar]
- [4].FDA Approves a Cellular Immunotherapy for Men with Advanced Prostate Cancer. 2010 Apr 29; [cited June 7, 2010]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm210174.htm.
- [5].Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Madan RA, Arlen PM. Abiraterone. Cougar Biotechnology. IDrugs. 2006;9:49–55. [PubMed] [Google Scholar]
- [7].Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- [8].Rotow J, Gameiro SR, Madan RA, et al. Vaccines as monotherapy and in combination therapy for prostate cancer. Clin Transl Sci. 2010;3:116–22. doi: 10.1111/j.1752-8062.2010.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- [10].FDA Prescribing Information for Provenge. [cited October 1, 2010]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/Approve dProducts/UCM210031.pdf.
- [11].Rini BI. Technology evaluation: APC-8015, Dendreon. Curr Opin Mol Ther. 2002;4:76–9. [PubMed] [Google Scholar]
- [12].Patel PH, Kockler DR. Sipuleucel-T: a vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42:91–8. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- [13].Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- [14].Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- [15].Beinart G, Rini BI, Weinberg V, et al. Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin Prostate Cancer. 2005;4:55–60. doi: 10.3816/cgc.2005.n.013. [DOI] [PubMed] [Google Scholar]
- [16].Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- [17].FDA Sipuleucel-T Briefing Document (BLA STN 125197/0): Sipuleucel-T is an autologous active cellular immunotherapy indicated for the treatment of men with asymptomatic metastatic androgen independent prostate cancer. [cited July 23, 2008]. Available from: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4291B1_01.pdf.
- [18].Sharifi N, Hamada A, Sissung T, et al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008;102:617–21. doi: 10.1111/j.1464-410X.2008.07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kantoff H, Berger E, Shore N, et al. Updated survival results of the IMPACT trial of sipuleucel-T for metastatic castration-resistant prostate cancer. Presented at: ASCO Genitourinary Cancers Symposium; San Francisco, California. March 5–7, 2010; Abstract 8. [Google Scholar]
- [20].Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther. 2004;4:575–88. doi: 10.1517/14712598.4.4.575. [DOI] [PubMed] [Google Scholar]
- [22].Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–17. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- [23].Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- [24].Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–20. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- [25].DiPaola R, Chen Y, Bubley G, et al. A phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: Results of ECOG 9802. Presented at: ASCO Genitourinary Cancers Symposium; Orlando, Florida. February 26–28, 2009; Abstract 108. [Google Scholar]
- [26].Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- [27].Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–7. [PubMed] [Google Scholar]
- [28].Grosenbach DW, Barrientos JC, Schlom J, et al. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–505. [PubMed] [Google Scholar]
- [29].Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].PROSTVAC: Therapeutic vaccine candidate for the treatment of advanced prostate cancer. [cited July 20, 2010]. Available from: http://www.bavarian-nordic.com/pipeline/prostvac.aspx.
- [32].Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- [33].Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- [34].Allison JP, Chambers C, Hurwitz A, et al. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? Novartis Found Symp. 1998;215:92–8. doi: 10.1002/9780470515525.ch7. discussion 8–102, 86–90. [DOI] [PubMed] [Google Scholar]
- [35].Hodge JW, Chakraborty M, Kudo-Saito C, et al. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Jun 14; doi: 10.1056/NEJMoa1003466. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–5. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- [38].Gerritsen W, van den Eertwegh A, de Gruijl T, et al. Expanded phase I combination trial of GVAX immunotherapy for prostate cancer and ipilimumab in patients with metastatic hormone-refractory prostate cancer (mHPRC) J Clin Oncol. 2008;26(suppl 15):285. Abstract 5146. [Google Scholar]
- [39].Madan R, Mohebtash M, Arlen P, et al. Overall survival analysis of a phase l trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab in the treatment of metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(suppl 15):216. Abstract 2550. [Google Scholar]
- [40].Study of Immunotherapy to Treat Advanced Prostate Cancer. [cited July 17, 2010]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00861614?term=ipilimumab+prostate&rank=6.
- [41].Phase 3 Study of Immunotherapy to Treat Advanced Prostate Cancer. [cited August 5, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT01057810?term=ipilimumab+prostate&rank=3.
- [42].Ryan JE, Ovsyannikova IG, Dhiman N, et al. Inter-operator variation in ELISPOT analysis of measles virus-specific IFN-gamma-secreting T cells. Scand J Clin Lab Invest. 2005;65:681–9. doi: 10.1080/00365510500348252. [DOI] [PubMed] [Google Scholar]
- [43].Cox JH, Ferrari G, Kalams SA, et al. Results of an ELISPOT proficiency panel conducted in 11 laboratories participating in international human immunodeficiency virus type 1 vaccine trials. AIDS Res Hum Retroviruses. 2005;21:68–81. doi: 10.1089/aid.2005.21.68. [DOI] [PubMed] [Google Scholar]
- [44].Stein WD, Figg WD, Dahut W, et al. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist. 2008;13:1046–54. doi: 10.1634/theoncologist.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fojo A, Stein W, Wilkerson J, et al. Kinetic analysis of breast tumor decay and growth following ixabepilone plus capecitabine (IXA + CAP) versus capecitabine alone (CAP) to discern whether the superiority of the combination is a result of slower growth, enhanced tumor cell kill, or both. J Clin Oncol. 2010;28(suppl 15):137. Abstract 1096. [Google Scholar]
- [46].Wilkerson J, Stein W, Kim S, et al. Validation of a kinetic analysis of renal cancer regression and growth following treatment with sunitinib and interferon-alfa (IFN-alpha): analysis of the pivotal randomized trial. J Clin Oncol. 2010;28(suppl 15):366. Abstract 4597) [Google Scholar]
- [47].Gulley J, Stein W, Schlom J, et al. A retrospective analysis of intramural NCI prostate cancer trials: progress made and insights gleaned. J Clin Oncol. 2010;28(suppl 15):380. Abstract 4657. [Google Scholar]
- [48].Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- [49].Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- [50].Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- [51].Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- [52].Morse MA, Clay TM, Mosca P, et al. Immunoregulatory T cells in cancer immunotherapy. Expert Opin Biol Ther. 2002;2:827–34. doi: 10.1517/14712598.2.8.827. [DOI] [PubMed] [Google Scholar]
- [53].Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- [54].Madan R, Vergati M, Cereda P, et al. Changes in regulatory T cell function and overall survival in metastatic castration resistant prostate cancer (mCRPC) patients treated with a vector-based vaccine [poster presentation]. Presented at: AACR Annual Meeting; Denver, Colorado. April 18–22, 2009; Abstract 726. [Google Scholar]
- [55].Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–9. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- [56].Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang S, Hodge JW, Grosenbach DW, et al. Vaccines with enhanced costimulation maintain high avidity memory CTL. J Immunol. 2005;175:3715–23. doi: 10.4049/jimmunol.175.6.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–71. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- [60].Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–5. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–6. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- [64].Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- [65].Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel-T (Provenge) followed by docetaxel derive greatest survival benefit [abstract]. Presented at: Chemotherapy Foundation Symposium 14th Annual Meeting; New York, NY. November 8–11, 2006. [Google Scholar]
- [66].Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–31. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Randomized phase II trial of docetaxel with or without PSA-TRICOM vaccine in patients with castrate-resistant metastatic prostate cancer. doi: 10.1080/21645515.2015.1062190. [cited August 16, 2010]. Available from: http://ecog.dfci.harvard.edu/active_reports/Genitourinary.html. [DOI] [PMC free article] [PubMed]
- [68].Vaccine Therapy with PROSTVAC/TRICOM and Flutamide Versus Flutamide Alone to Treat Prostate Cancer. [cited July 21, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT00450463?term=Prostvac&rank=2.
- [69].Quarmby S, Hunter RD, Kumar S. Irradiation induced expression of CD31, ICAM-1 and VCAM-1 in human microvascular endothelial cells. Anticancer Res. 2000;20:3375–81. [PubMed] [Google Scholar]
- [70].Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–80. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- [71].Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- [72].Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- [73].Petersen LJ, Lund L, Jonler M, et al. Samarium-153 treatment of bone pain in patients with metastatic prostate cancer. Dan Med Bull. 2010;57:A4154. [PubMed] [Google Scholar]
- [74].153Sm-EDTMP With or Without a PSA/TRICOM Vaccine To Treat Men With Androgen-Insensitive Prostate Cancer. [cited August 5, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT00450619?term=samarium-153&rank=13.