Abstract
cNXL104 is a novel β-lactamase inhibitor with a non-lactam structural scaffold. Our kinetic and mass spectrometric analysis demonstrates that NXL104 quantitatively inhibits BlaC, the only chromosomally encoded β-lactamase from Mycobacterium tuberculosis, by forming a carbamyl adduct with the enzyme. The inhibition efficiency (k2/K) of NXL104 was shown to be more than 100-fold lower than that of clavulanate, a classical β-lactamase inhibitor, probably caused by the bulky rings of NXL104. However, the decarbamylation rate constant (k3) was determined to be close to zero. The BlaC-NXL104 adduct remained stable for at least 48 hours, while the hydrolysis of the BlaC-clavulanate adduct was observed after two days. The three-dimensional crystal structure of the BlaC-NXL104 carbamyl adduct was determined at a resolution of 2.3 A. Interestingly, the sulfate group of NXL104 occupies the position of a phosphate ion in the structure of the BlaC-clavulanate adduct, and is hydrogen bonded to residues Ser128, Thr 237 and Thr239. Favorable interactions are also seen in the electrostatic potential map. We propose that these additional interactions, as well as the intrinsic stability of carbamyl linkage, contribute to the extraordinary stability of the BlaC-NXL104 adduct.
Mycobacterium tuberculosis is the causative agent of tuberculosis (TB), which causes nearly two million deaths each year (1). It is estimated that one third of the world’s population is currently infected with M. tuberculosis, and new infections occur at an alarming rate of approximately one per second (2). Current TB treatments are time-consuming, and not effective against the non-replicating forms of M. tuberculosis that are responsible for the persistence of TB infections. In addition, the increasing emergence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) M. tuberculosis strains (3) makes it important to develop novel therapies against this bacterium.
β-lactam antibiotics are among the most clinically prescribed antibacterial drugs, and act by inhibiting the D,D-transpeptidases involved in the biosynthesis of the bacterial cell wall (4). A major strategy of bacterial resistance to β-lactams is the production of β-lactamases that catalyze the hydrolysis of β-lactams, leading to the inactivation of the antibiotics. β-lactams have not been used in clinical practice to treat TB infections, since an active penicillinase was reported in M. tuberculosis (5). Recently it has been shown that this penicilinase is a chromosomally-encoded β-lactamase (BlaC), rendering M. tuberculosis resistant to β-lactam antibiotics (6).
The Ambler classification divides β-lactamases into four classes based on their amino acid sequences (7). Class A, C and D enzymes employ a nucleophilic serine residue to attack the β-lactam ring, while class B enzymes are metallo-β-lactamases binding one or two zinc ions in the active site. BlaC is a class A β-lactamase that contains a nucleophilic serine residue (Ser70) and shares sequence homology with the penicillin-binding protein (PBP) domain of the ancestral D,D-transpeptidases. Previously we have shown that BlaC hydrolyzes a broad-spectrum of β-lactam antibiotics, including penicillins, cephalosporins, and even carbapenems (8). Our recent structural and kinetic studies on two BlaC mutants (K73A and E166A) suggest that K73 and E166 are essential for acylation and deacylation of β-lactams, respectively (9). Specifically, Lys73 activates Ser70 by deprotonating its hydroxyl group, which then attacks the carbonyl carbon in the β-lactam ring, resulting in the acylated enzyme. During deacylation, Glu166 activates a highly conserved active site water molecule that attacks acylated BlaC and consequently releases the inactive ring-opened compound from the enzyme.
Additionally, we have shown that BlaC is irreversibly inhibited by clavulanate (Figure 1), a FDA-approved β-lactamase inhibitor (8, 10), and the combination of clavulanate and meropenem, a poor β-lactam substrate of BlaC, exhibited potent inhibitory activity against laboratory strains of M. tuberculosis as well as thirteen clinical XDR strains (11), suggesting that the combination of a β-lactam antibiotic and a β-lactamase inhibitor might represent an effective TB chemotherapy.
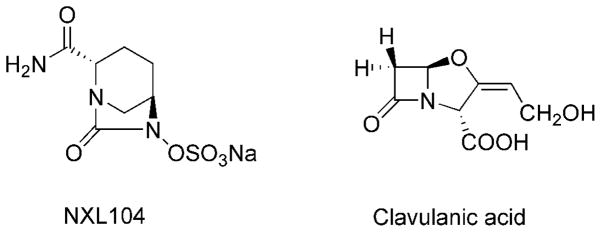
Figure 1.
Chemical structures of NXL104 and clavulanic acid.
NXL104 (trans-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octan-2-carboxamide sodium salt, Avibactam) is a novel non-lactam inhibitor of β-lactamases (Figure 1). Previous studies show that NXL104 inhibits both Class A and Class C β-lactamases (12–14), making the bacterial strains expressing these β-lactamases susceptible to β-lactam antibiotics (15–17). The combination of NXL104 and ceftazidime, a cephasplorin antibiotic is currently in Phase II clinical trial to treat complicated urinary tract infections (cUTIs), which are caused by various pathogenic bacteria (18).
In this work, we conducted kinetic and mass spectrometric analysis of NXL104, and showed that it quantitatively inactivates BlaC by forming a carbamyl linkage with the enzyme. In addition, we determined the three-dimensional structure of the BlaC-NXL104 adduct, providing molecular insight into the slow decarbamylation mechanism.
EXPERIMENTAL PROCEDURES
Materials
NXL104 was a generous gift from from Anacor Pharmaceuticals (Palo Alto, CA). BlaC was expressed and purified as described previously (8). Buffer reagents for crystallography were purchased from Hampton Research (Aliso Viejo, CA). Unless noted, other chemicals were from Sigma-Aldrich (St. Louis, MO).
Inhibition assays
1.5 μM of BlaC was incubated with various inhibitor to enzyme ratios (0 – 2.5) at room temperature for 30 minutes (clavulanate), and for 2 hours or 10 hours (NXL104), respectively. The activity of BlaC was measured by following the hydrolysis of 100 μM nitrocefin at 486 nm (ε = 20500 M−1 cm−1) after a 500-fold dilution. The fractional enzyme activity was then plotted against the inhibitor/enzyme ratio.
To measure the rate constants of carbamylation and decarbamylation, hydrolysis of 100 μM nitrocefin was continuously monitored with 0.6 nM BlaC in the presence of 72 to 540 μM NXL104. The progress curve was fitted into the equation 1,
| (1) |
where [P] is the concentration of the product, vi and vs represent the initial and final reaction velocities, respectively, and kiso is the apparent first-order rate constant.
The general inhibition mechanism is modeled as,
| (2) |
where k1 and k−1 represent the rate constants of the binding and dissociation of NXL104 from BlaC, respectively, while k2 and k3 correspond to the rate constants of carbamylation and decarbamylation, respectively.
For this model, kiso determined from equation 1 was fitted into the equation 3 to obtain the values of k2 and k3, where K equals k−1/k1.
| (3) |
When k3 is close to zero and K is much larger than [I], equation 3 could be simplified as equation 4,
| (4) |
Re-activation assay
2 μM of BlaC was completely inactivated by incubating with 5 μM of NXL104 for 24 hours or 5 μM of clavulanate for 2 hours at room temperature. The excess inhibitors were removed by ultracentrifugation using an Amicon 3K cutoff filter device (Millipore). Then the re-activation of the acylated or carbamylated enzyme was monitored by measuring the BlaC activity with the nitrocefin activity assay described above.
Mass spectrometric analysis
The mass spectra were collected as described previously with minor modifications (8, 19). BlaC was dialyzed into 20 mM ammonium bicarbonate (pH 6.5) to minimize salt inference. Then 50 μM BlaC was incubated with 50 μM NXL104 at room temperature. 1 μL of the reaction mixture was withdrawn at various incubation times (0, 10, 120, and 600 minutes), mixed with 9 μL of 50% acetonitrile, 0.1% formic acid solution, and then injected into 9.6 T Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer (Ionspec, Lake Forest, CA). The molecular weight of the protein sample was determined for the +25 charge state using the equation m = (m/z × 25) − 25 on the isotopic centroid.
Crystallization
Hanging drop vapor diffusion was used for crystallization of BlaC. The composition of the well consists of 0.1 M HEPES, pH 7.5 and 2 M NH4H2PO4, which makes the final pH of the well solution 4.1. Protein at a concentration of 12 mg/ml was mixed 1:1 with the well solution and incubated at 10 °C. Initial crystals grew within 4–5 days but were small, needle shaped and thousands in number. Repeated micro-seeding was performed which resulted in efficient crystal growth as well as improved morphology to produce diffraction quality crystals of the active enzyme.
Data collection and refinement
BlaC crystals were soaked with 40 mM NXL104 in mother liquor. Before freezing, 20% glycerol was added to the solution as a cryo-protectant. Data were collected at Brookhaven National Laboratory on crystals frozen on 120 minute soaks with NXL104. Beam lines used were X12C and X29, in which various resolutions of diffraction were obtained dependent on the soaking times and beam line. The data were processed using HKL2000 (20). Our previous structure of clavulanate-bound BlaC (10) (PDB entry 3CG5) was used to phase the data, using the CCP4 software suite (21). Multiple rounds of structural refinement and model building were performed in Refmac5 (22, 23) and Coot (24). Table 1 lists the data collection statistics for the structures as well as the final refinement statistics.
Table 1.
Data collection and refinement statistics
| Data Collection statistics | ||
| X-ray source | NSLS | Beamline |
| X29A | ||
| Wavelength (Å) | 1.0 | |
| Temperature (K) | 100 | |
| Resolution Range (Å) | 2.29–75.0 | |
| Reflectiona | 11647 (598) | |
| Completeness (%) | 95.44(93.62) | |
| Rmerge | 0.14(0.81) | |
| I/σ(I) | 11.9 (3.5) | |
| Redundancy | 7.0 (7.2) | |
| Space group | P212121 | |
| Unit cell (Å) | ||
| a | 49.960 | |
| b | 68.004 | |
| c | 75.705 | |
| α = β = γ | 90.0° | |
| Molecules per a.u. | 1 | |
| Refinement statistics | ||
| Rwork | 0.170 | |
| Rfree | 0.261 | |
| Number of atoms | ||
| Protein | 1989 | |
| NXL104 | 17 | |
| PO4 | 10 | |
| Water | 236 | |
| RMS deviation | ||
| Bond length (Å) | 0.020 | |
| Bond angles (°) | 1.667 | |
| Average B-factors (Å2) | ||
| Overall | 22.31 | |
| Protein main chain | 21.53 | |
| Protein side chain | 23.20 | |
| NXL104 | 41.38 | |
| PO4 | 42.60 | |
| Water | 36.80 | |
| Ramachandran | ||
| Favored | (91.4 +7.6)% | |
| Outlired | 0.0% | |
| PDB accession code | 4DF6 | |
values for the highest resolution shell are in parenthesis.
RESULTS AND DISCUSSION
Inhibition studies
Inhibition studies were performed by measuring the residual BlaC activity after incubation with various concentrations of the inhibitor, revealing that only one equivalent of NXL104 is needed to inactivate BlaC (Figure 2A) after a 10-hour incubation. Complete inactivation of BlaC by one equivalent of NXL104 was not realized after 2-hour incubation, while clavulanate achieved 100% inhibition of BlaC within 30 minutes (Figure 2A), indicative of slow onset inhibition by NXL104 compared to clavulanate. NXL104-inhibited BlaC remained inactive after extensive dialysis, suggesting the formation of a covalent adduct via carbamylation, followed by a slow decarbamylation step (Figure 2B).
Figure 2.
Inhibition characterizations of NXL104 against BlaC. (A) Inhibition of BlaC with NXL104 and clavulanate. BlaC was incubated with various concentrations of NXL104 or clavulanate. Then the fractional BlaC activity was plotted against the ratio of inhibitor/BlaC. (B) Proposed inhibition mechanism of BlaC by NXL104. Upon deprotonation of Ser70 by Lys73, the hydroxyl group of Ser70 attacks the carbonyl carbon of NXL104, resulting in an inactive carbamyl BlaC intermediate. The decarbamylation of this intermediate mediated by Glu166 and a catalytic water molecule is much slower than carbamylation, which leads to the inactivation of BlaC.
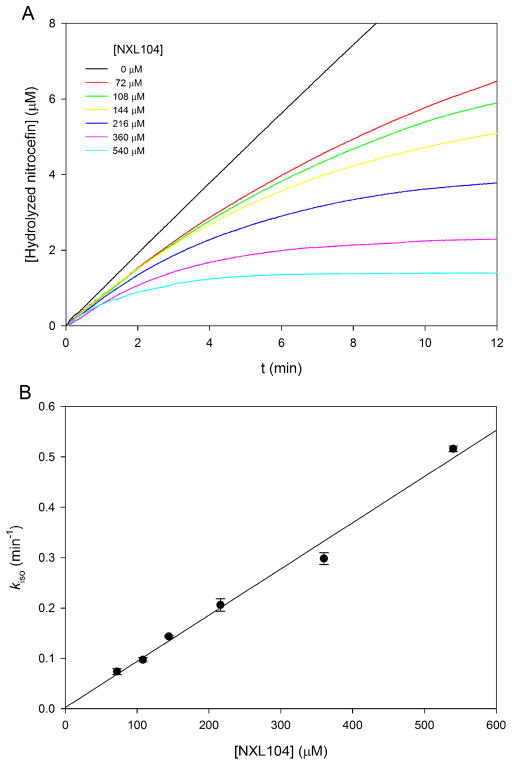
To measure the rate constants of carbamylation (k2) and decarbamylation (k3), the reporter substrate assay was conducted by monitoring the hydrolysis of nitrocefin by BlaC (8), in the presence of various concentrations of NXL104. As shown in Figure 3A, NXL104 displayed time-dependent inhibition against BlaC. The apparent first order rate constants (kiso) were calculated from the progress curves in Figure 3A, and then plotted against NXL104 concentrations (Figure 3B), revealing an intercept indistinguishable from zero, which suggests that the value of rate of decarbamylation, k3, is close to zero. The plot is quite linear at the concentrations of NXL-104 used, indicating that the dissociation constant K (defined as k−1/k1) is much higher than 500 μM and k2 is greater than 0.5 min−1. The poor reversible binding of NXL104 to BlaC is probably caused by the bulky fused rings and the lack of β-lactam feature of NXL104. Although the precise values of k2 and K could not be determined, we were able to measure the value of k2/K (0.92 ± 0.03 min−1 mM−1) based on the slope of the plot. The fact that this value is more than 100-fold lower than that of clavulanate (k2/K, 132 ± 4 min−1 mM−1, 8) suggests that NXL104 is less efficient than clavulanate in converting BlaC into the acylated or carbamylated species.
Figure 3.
Determination of the inhibition rate constants. (A) Time-dependent hydrolysis of nitrocefin in the presence of various concentrations of NXL104. The apparent first-order rate constants (kiso) were calculated by fitting the curves to equation 1. (B) Plot of kiso versus NXL104 concentration. The data were fitted to the equation 4 to obtain k2/K and k3 values.
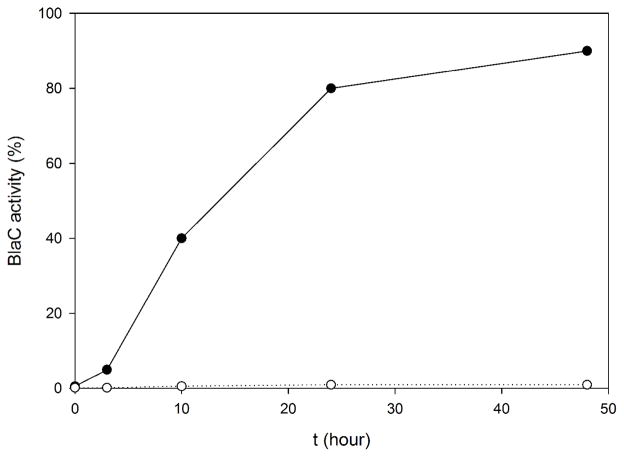
To further investigate the adduct stability, we followed the regain of β-lactamase activity of the carbamylated BlaC using the nitrocefin hydrolysis assay, which revealed that more than 98% of BlaC remained inactive after 48 hours (Figure 4). In contrast, BlaC activity was almost completely restored 2 days after inactivation by clavulanate. Therefore, the BlaC-NXL104 adduct is much more stable than the BlaC-clavulanate adduct and the k3 value of NXL104 much smaller than that of clavulanate.
Figure 4.
Recovery of NXL104- or clavulanate-inhibited BlaC. BlaC activity was monitored for 48 hours using nitrocefin hydrolysis assay, after complete inactivation of BlaC by NXL104 (○) or clavulanate (●).
Mass spectrometry
To identify the NXL104 adduct formed during the incubation, Fourier transform ion-cyclotron resonance (FT-ICR) mass spectrometry was performed by incubating BlaC with an equal amount of NXL104. Free BlaC showed an experimental molecular weight of 28784.95 (peak A in Figure 5). 10 minutes after incubation, peak B with a molecular weight of 29050.12 was observed (Figure 5). The molecular mass increase of 265.18 compared to the free enzyme corresponds to the addition of a ring-opened NXL104 molecule (predicted, 265.04), which further supports that NXL104 inhibits BlaC by forming a covalent carbamyl linkage with the enzyme. The free enzyme was still observed after 2 hours (Figure 5), confirming the unusually slow reaction of apo-BlaC and NXL104.
Figure 5.
FT-ICR mass spectra of BlaC-NXL104 covalent adduct. Only the +25 charge states are shown. Peak A is the free BlaC enzyme with an experimental molecular weight of 28784.95, while peak B corresponds to the BlaC-NXL104 adduct with an experimental molecular weight of 29050.12.
X-ray Crystallography
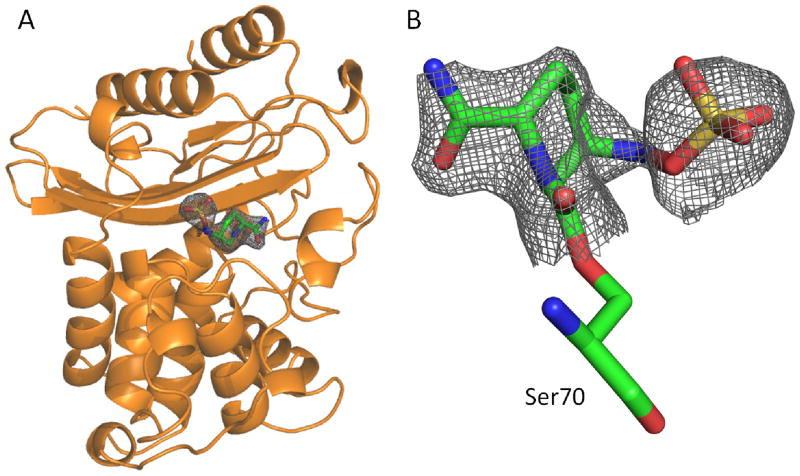
The extremely slow decarbamylation of the adduct allowed us to trap the carbamylated BlaC intermediate by soaking the apo BlaC crystal with NXL104. The three-dimensional structure was resolved at a resolution of 2.3 A with an Rwork of 0.170 and an Rfree of 0.261 (Figure 6A, Table 1). In the structure, the nucleophilic active residue Ser70 is covalently linked to the ring opened form of NXL104 (Figure 6B), substantiating the inhibition mechanism in Figure 2B. The quality of the electron density of the ligand is shown under the calculated Fo − Fc omit maps contoured at 2.2 σ (Figure 6B).
Figure 6.
Crystal structure of the BlaC-NXL104 adduct. (A) Overall structure of BlaC with the NXL104 adduct displayed in gray mesh. (B) Fo−Fc omit density (2.3 A) of the BlaC-NXL104 adduct formed at the Ambler active site residue Ser70. The figure was produced with Pymol and contoured at 2.2 σ.
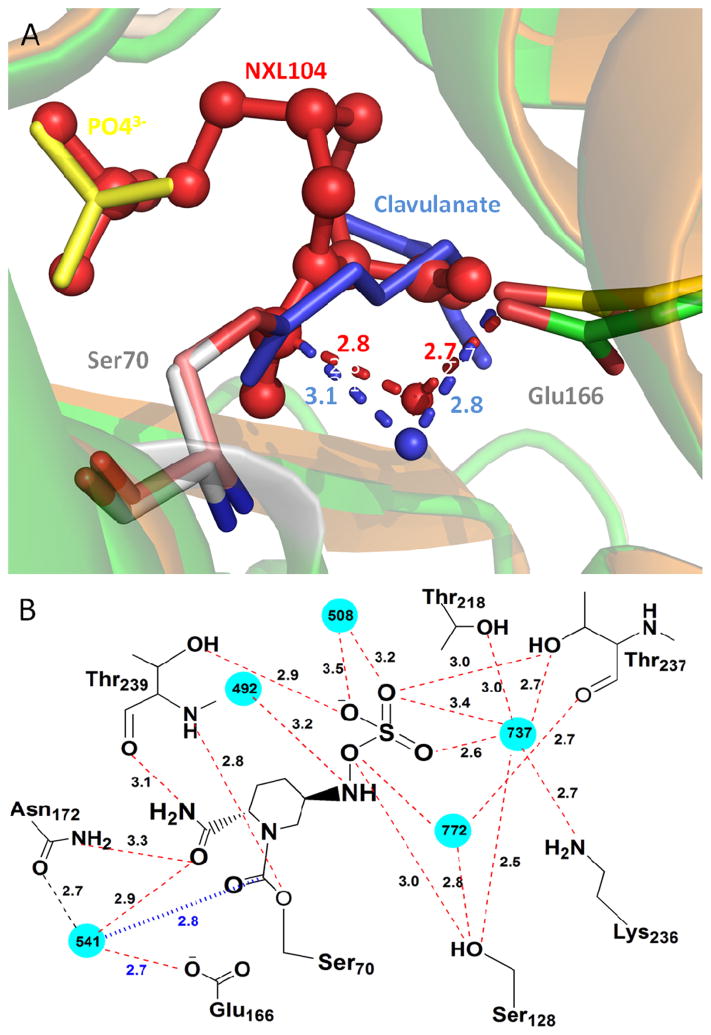
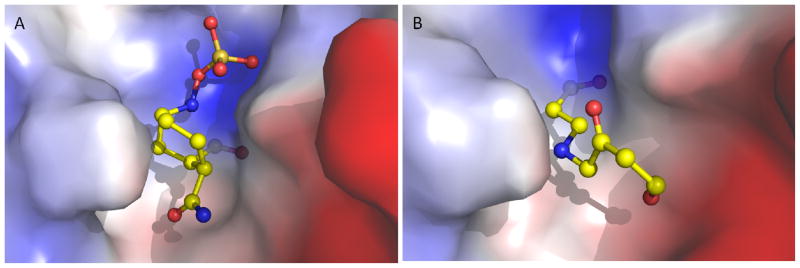
To provide molecular insight into the unusually slow decarbamylation of the BlaC-NXL104 covalent intermediate, we superimposed the adduct structures of BlaC-NXL104 and BlaC-clavulanate (PDB entry 3CG5). The two BlaC structures are quite similar with an RMSD of 0.2 A. The conserved active site hydrolytic water molecule is seen in the BlaC-NXL104 adduct (water541 in Figure 7A). In addition, the distance between the carboxylate oxygen of E166 and the catalytic water in BlaC-NXL104 (2.7 A) is quite close to that in the BlaC-clavulanate adduct (2.8 A, Figure 7A), as well as the distance between the water molecule and the carbonyl carbon of the adduct (2.8 A in BlaC-NXL104 versus 3.1 A in BlaC-clavulanate, Figure 7A), ruling out the possibility that the slow decarbamylation is due to the exclusion of the hydrolytic water molecule from the active site or the misalignment of E166 and the water molecule. The sulfate group of NXL104 occupies the position of a nearby phosphate ion in the structure of BlaC-clavulanate adduct, and is hydrogen bonded to Ser128, Thr237 and Thr239 (Figure 7B, Table S1). In addition, a number of favorable interactions are seen in the calculated electrostatic interaction map for BlaC-NXL104 complex (Figure 8A). The piperidine ring of NXL104 orients towards the hydrophobic residues in the active site of BlaC. Moreover, the negatively charges sulfate moiety is oriented towards a region of positively charge on the enzyme, and these interactions are absent in BlaC-clavunalate complex (Figure 8B). We believe that these interactions that are absent in the BlaC-clavulanate adduct, together with the intrinsic stability of carbamyl linkage, may contribute to the exceptional stability of the BlaC-NXL104 adduct. This information may be useful in designing NXL104 derivatives as novel BlaC inhibitors.
Figure 7.
Structures of the BlaC active site. (A) Overlay of BlaC-NXL104 and BlaC-clavulanate structures (PDB entry 3CG5). NXL104 is shown in red, clavulanate in blue, and phosphate ion from the clavulanate structure in yellow. The distance between the carboxylate oxygen and catalytic water as well as the distance between the catalytic water and carbonyl carbon of the adduct is indicated in blue for BlaC-clavulanate and red for BlaC-NXL104. (B) Interactions between BlaC and NXL104. Water molecules are shown in cyan. The distance between the catalytic water and carbonyl carbon of the adduct is indicated in blue.
Figure 8.
Calculated electrostatic interaction maps of BlaC-NXL104 (A) and BlaC-clavulanate (B). Positive electrostatic potentials are drawn in blue, and negative in red. Hydrophobic surfaces are represented in white.
This work demonstrates that NXL104 quantitatively inactivates BlaC by forming a carbamyl adduct with the enzyme. The carbamyl adduct exhibits extraordinary stability beyond that observed with clavulanate. Unfortunately, the very low binding affinity of NXL104 to BlaC will likely preclude its use as a β-lactamase inhibitor that can be partnered with a β-lactam antibiotic for the treatment of tuberculosis.
Supplementary Material
Acknowledgments
We thank Anacor Pharmaceuticals for providing NXL104, Brookhaven National Lab X12C and X29 beamline staff for their support, and Dr. Hui Xiao at Albert Einstein College of Medicine for his assistance in the FT-ICR analysis.
Abbreviations
- BlaC
Mycobacterium tuberculosis β-lactamase
- FT-ICR
Fourier transform ion cyclotron resonance
- PBP
penicillin-binding protein
- RMSD
root-mean-square deviation
- TB
tuberculosis
Footnotes
This work was supported by NIH grant NIH Grant AI33696 and AI60899 (to J.S.B.).
SUPPORTING INFORMATION AVAILABLE
Interactions between NXL104 and clavulanate with BlaC (Table S1). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.WHO. Global tuberculosis control. 2011;2011 [Google Scholar]
- 2.Netto EM, Dye C, Raviglione MC. Progress in global tuberculosis control 1995–1996, with emphasis on 22 high-incidence countries. Global Monitoring and Surveillance Project. Int J Tuberc Lung Dis. 1999;3:310–320. [PubMed] [Google Scholar]
- 3.WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. 2010. [Google Scholar]
- 4.Goffin C, Ghuysen JM. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol Mol Biol Rev. 1998;62:1079–1093. doi: 10.1128/mmbr.62.4.1079-1093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iland CN. The effect of penicillin on the tubercle bacillus. J Pathol Bacteriol. 1946;58:495–500. doi: 10.1002/path.1700580320. [DOI] [PubMed] [Google Scholar]
- 6.Flores AR, Parsons LM, Pavelka MS., Jr Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to β-lactam antibiotics. Microbiology. 2005;151:521–532. doi: 10.1099/mic.0.27629-0. [DOI] [PubMed] [Google Scholar]
- 7.Hall BG, Barlow M. Revised Ambler classification of β-lactamases. J Antimicrob Chemother. 2005;55:1050–1051. doi: 10.1093/jac/dki130. [DOI] [PubMed] [Google Scholar]
- 8.Hugonnet JE, Blanchard JS. Irreversible inhibition of the Mycobacterium tuberculosis β-lactamase by clavulanate. Biochemistry. 2007;46:11998–12004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay LW, Xu H, Blanchard JS. Structures of the Michaelis complex (1.2 A) and the covalent acyl intermediate (2.0 A) of cefamandole bound in the active sites of the Mycobacterium tuberculosis β-lactamase K73A and E166A mutants. Biochemistry. 2010;49:9685–9687. doi: 10.1021/bi1015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tremblay LW, Hugonnet JE, Blanchard JS. Structure of the covalent adduct formed between Mycobacterium tuberculosis β-lactamase and clavulanate. Biochemistry. 2008;47:5312–5316. doi: 10.1021/bi8001055. [DOI] [PubMed] [Google Scholar]
- 11.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, 3rd, Blanchard JS. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnefoy A, Dupuis-Hamelin C, Steier V, Delachaume C, Seys C, Stachyra T, Fairley M, Guitton M, Lampilas M. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor. J Antimicrob Chemother. 2004;54:410–417. doi: 10.1093/jac/dkh358. [DOI] [PubMed] [Google Scholar]
- 13.Livermore DM, Mushtaq S, Warner M, Miossec C, Woodford N. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum β-lactamases and carbapenemases. J Antimicrob Chemother. 2008;62:1053–1056. doi: 10.1093/jac/dkn320. [DOI] [PubMed] [Google Scholar]
- 14.Stachyra T, Pechereau MC, Bruneau JM, Claudon M, Frere JM, Miossec C, Coleman K, Black MT. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam β-lactamase inhibitor. Antimicrob Agents Chemother. 2010;54:5132–5138. doi: 10.1128/AAC.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktas Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with beta-lactams against Gram-negative bacteria, including OXA-48 beta-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2012;39:86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Endimiani A, Choudhary Y, Bonomo RA. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother. 2009;53:3599–3601. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother. 2009;64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolle LE. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16:349–360. doi: 10.1155/2005/385768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay LW, Fan F, Blanchard JS. Biochemical and structural characterization of Mycobacterium tuberculosis β-lactamase with the carbapenems ertapenem and doripenem. Biochemistry. 2010;49:3766–3773. doi: 10.1021/bi100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 21.Potterton E, Briggs P, Turkenburg M, Dodson E. A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr. 2003;59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 22.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 23.Pannu NS, Murshudov GN, Dodson EJ, Read RJ. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr D Biol Crystallogr. 1998;54:1285–1294. doi: 10.1107/s0907444998004119. [DOI] [PubMed] [Google Scholar]
- 24.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.