Abstract
Pathogenic Escherichia coli that colonize the small intestine primarily cause gastrointestinal illness in infants and travelers. The main categories of pathogenic E. coli that colonize the epithelial lining of the small intestine are enterotoxigenic E. coli enteropathogenic E. coli and enteroaggregative E. coli. These organisms accomplish their pathogenic process by a complex, coordinated multistage strategy, including non-intimate adherence mediated by various adhesins. These so called “enteroadherent E. coli ” categories subsequently produced toxins or effector proteins that are either secreted to the milieu or injected to the host cell. Finally, destruction of the intestinal microvilli results from the intimate adherence or the toxic effect exerted over the epithelia, resulting in water secretion and diarrhea. In this review, we summarize the current state of knowledge regarding these enteroadherent E. coli strains and the present clinical understanding of how these organisms colonize the human intestine and cause disease.
Keywords: enteropathogenic E. coli, enterotoxigenic E. coli, enteroaggregative E. coli, diarrhea, pathogenic Escherichia coli, virulence, adherence
Introduction
At least six different categories of pathogenic Escherichia coli have been associated with gastrointestinal illness and diarrhea in humans (reviewed in [1]). However, this review focuses on three of these categories of E. coli and their effects exerted on the villous architecture of the small intestine, as well as the impact of their diverse pathogenic properties on diarrheal disease. Enterotoxigenic E. coli cause acute and severe diarrhea as a result of the production of potent toxins altering the biological activity of the intestinal epithelia. Enteropathogenic E. coli employ a type III protein secretion system to orchestrate internal changes in target cells. The intimate contact with the host apical enterocyte membrane results in dysregulation of water and solute transport and the disruption of epithelial barrier structure and function. Finally, enteroaggregative E. coli displays abundant adherence to the intestinal mucosa by the production of different enterotoxins and cytotoxins, resulting in induction of mucosal inflammation (reviewed in [2]). The following sections summarize recent progress in understanding the clinical outcome of these types of E. coli infections with respect to their unique pathogenic properties.
Enterotoxigenic Escherichia coli (ETEC)
ETEC are a very diverse group of pathogenic E. coli that colonize the small intestine and are a major cause of acute secretory diarrhea in developing countries [3]. Every year, ETEC accounts for 280 to 400 million cases in children under 5 years of age and an additional 100 to 400 million cases in children over 5 years of age and in adults [3]. This organism remains a major cause of diarrhea in travelers, including military personnel, and it substantially contributes to both delayed growth and malnutrition due to repeated bouts of infectious diarrhea. Moreover, malnourished children appear to be at a higher risk of acquiring ETEC infections [4, 5].
The pathogen and clinical implications of the disease
As a heterogeneous group of pathogens, ETEC strains have in common the ability to colonize the small intestine with the subsequent production and translocation of the plasmid-encoded heat-labile (LT) and/or heat-stable (ST) enterotoxins [3, 6]. The relative proportion of strains producing LT alone, ST alone or LT/ST, varies from one geographic area to another; but overall, 30-50% of the clinical ETEC isolates seem to produce STs only [7, 8]. In the classic mechanism of ETEC pathogenesis, small intestinal colonization requires plasmid-encoded colonization factors (CFs), thereby enabling the organism to adhere to the mucosa [6]. Until now, more than 25 CFs have been recognized on human ETEC isolates [9]. The types of CF are subdivided by their antigenicity, molecular weight, N-terminal amino acid sequence of the major subunit and structural morphology, either fimbrial, fibrillar, helical or nonfimbrial [9, 1]. In the case of the toxins, the STs can be further classified as STa or STb on the basis of structure and function [10]. STa, which is associated with human disease, binds to guanylyl cyclase receptors on the brush border of the intestine, stimulating their activity and increasing intracellular levels of cyclic GMP and activating the cystic fibrosis transmembrane conductance regulator (CFTR), resulting in impaired absorption of Na+ and H2O efflux into the lumen. STb is mostly associated with porcine ETEC strains but can also been found in human isolates (Figure 1) [10]. In contrast to Sta, the Stb toxin binds to sulphatide, a widely distributed acidic glycosphingolipic. The LT toxin is secreted [11] and it has been associated with lipopolysaccharide on the bacterial surface, acting as an adhesion and facilitating attachment to host cells [12]. LT toxins encompass at least 16 natural polymorphic toxin variants expressed by ETEC human isolates, but only LT1, produced by the reference ETEC H10407 strain, has been intensively studied as a virulence-associated factor and as a mucosal/transcutaneous adjuvant [13].
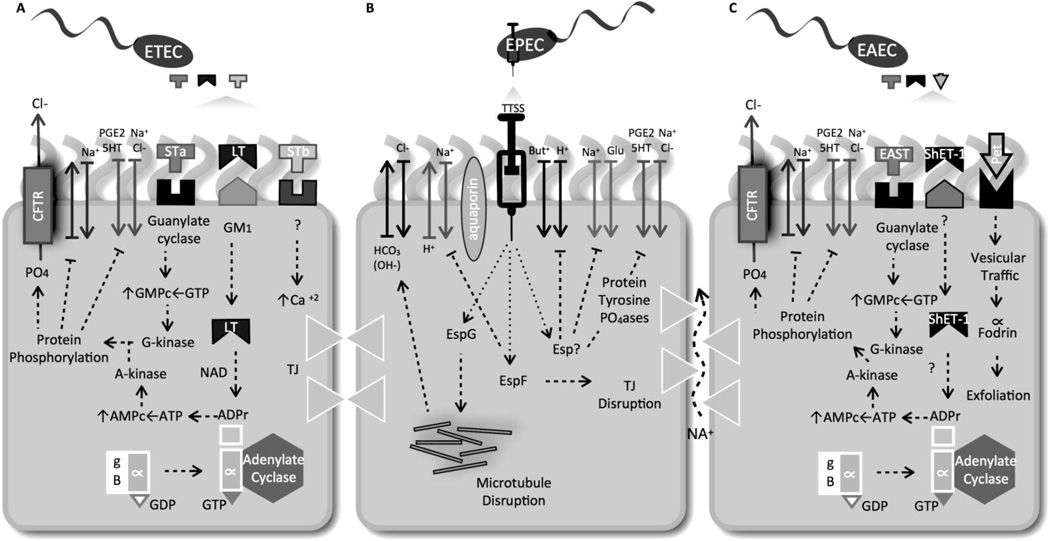
Figure 1. ETEC-, EPEC- and EAEC-induced diarrhea is a multi-factorial event leading to disruption of ion/H2O balance.
(A) In an ETEC infection, the LT toxin (A1 subunit) catalyzes the ADP-ribosylation of the α-subunit of Gs-protein, resulting in activation of adenylate cyclase and increased levels of intracellular cAMP. The activation of the cAMP-dependent A kinase results in phosphorylation of apical membrane transporters (i.e., the cystic fibrosis transmembrane conductance regulator, CFTR), resulting in secretion of Cl− and HCO3− and decrease absorption of Na+ and Cl−. STa acts by binding to the GC-C membrane receptor. Activation of GC-C results in increased levels of intracellular cGMP, elevated Cl− secretion and decreased Na absorption, due to activation of cGMP-dependent kinase (G-kinase) and/or the cAMP dependent kinase (A-kinase). (B) EPEC infection decreases epithelial ion absorption resulting in diarrhea as a result of the bacterial protein effectors secreted by the type III secretion system (TTSS) having an effect on Cl− and Na+ ion activity. Some TTSS secreted proteins Esps) affecting ion balances are: EspF, inhibiting function of Na+/H+ exchange isoform 3 (NH3); EspG, disrupting microtubules and leading to decrease in apical Cl−/HCO3− exchange activity. EPEC, via unknown effector molecules decrease butyrate (But) absorption and induced activation of protein tyrosine phosphatases (PTPases). This results in decreasing function of serotonin transporter (SERT) and increasing prostaglandin E -2 (PGE-2) and 5-hydroxytryptamine 5-HT) availability, further affecting ion absorption and motility, resulting in diarrhea. (C) EAEC produces two plasmid-encoded toxins, the E. coli heat stable enterotoxin (EAST) and the plasmid encoded-toxin (Pet). Further, the chromosomally-encoded protein involved in colonization (Pic) (not shown), the Shigella enterotoxin 1 (ShET1) and hemolysin E (HlyE) are also produced. Although several toxins are secreted by EAEC, the mechanism of action associated with the EAEC-mediated diarrheal disease has not been fully established but involves G-kinase and A-kinase-dependent mechanism.
The diarrhea produced by ETEC is secretory. The disease begins with a sudden onset of watery stool (without blood or inflammatory cells) (Table 1). Other signs and symptoms include headache, fever, nausea and vomiting, leading to dehydration (reviewed in [7]). The LT and ST enterotoxins are responsible for the electrolyte and fluid lost into the intestinal lumen and the onset of diarrhea after a short incubation period of 1–2 days. The patients are afebrile and usually the self-limited diarrhea lasts only 3 to 4 days; however, some patients have prolonged diarrheal illness lasting a week or more [14]. In travelers, the diarrhea in the majority of the cases resolves without the need for medical care, and might include other symptoms like cramping, nausea, and low grade fever [15]. With adequate treatment, the mortality is very low (<1%) [10].
Table 1.
Characteristics of enteroadherent E. coli affecting the human small intestine.*
| Category | Clinical manifestations | Susceptible population |
Virulence factors |
Diagnostic | Treatment |
|---|---|---|---|---|---|
| ETEC | watery stool (without blood or inflammatory cells) leading to dehydration, headache, fever, nausea and vomiting |
Children 0–5 years of age and adults traveling to developing countries |
ST, LT, CFs |
Culture, detection of ST (STa, STb) and LT, CFAs using ELISAa and PCR- based methodsa |
Self-limited, responsive to oral rehydration therapy (low response in children < 2 years). Antimicrobial therapy on individual cases |
| EPEC | Secretory and persistent diarrhea, anorexia, low fever, and rapid wasting |
Children 0–2 years of age, occasionally adults |
pEAF, BFP, LEE and Nle effectors |
Culture, adherence patterns (LA LAL, etc), serotyping, PCR-based methodsa |
Self-limited, responsive to oral rehydration therapy. Antimicrobial therapy on individually cases |
| EAEC | Persistent and acute diarrhea, mucoid stools, abdominal pain, nausea, vomiting, occasionally fever |
People of all ages in developing and industrialized countries, HIV- infected adults |
EAST Pet Pic ShET-1 Aap AAF/II |
Culture, adherence pattern (stacked-brick pattern), pAA DNA probe, multiplex and real-time PCR assaysa |
Self-limited, responsive to oral rehydration therapy. Antimicrobial therapy on individual cases |
ST, heat-stable toxin
LT, heat-labile toxin
CFA, Colonization factors
LEE, Locus of enterocyte effacement
HIV, Human immunodeficiency virus
pEAF, Plasmid enteroadherente factor of EPEC
BFP, Bundle forming pilus
EAST, Enteroaggregative heat-stable toxin
Pet, Plasmid encoded-toxin
Pic, Protein involved in colonization
ShET-1, Shigella enterotoxin-1
Aap, dispersin
AAF/II, Aggregative adherence factor II
Commercial kits available for clinical use
Current progress in prevention and treatment
Natural immunity to ETEC infection has been described in people repeatedly challenged with this bacterium [16]. However, ETEC remains the most common cause of diarrhea; therefore, there is intense work trying to obtain an ETEC vaccine. The design of such a vaccine is based on the knowledge of the mechanisms of immune protection during ETEC infections, and, as such, studies suggest that immune responses against CFs may protect against ETEC [17, 8]. Strains expressing CFs within specific isolates have been shown to induce substantial immune responses not only against homologous CFs, but also against other CFs [7]. Based on the identification of CFs as key protective ETEC antigens, the approach to develop a vaccine included preparing killed ETEC that express the most important immunogenic CFs [8]. Thus, CFs on ETEC inactivated by mild formalin-treatment have been shown to be more stable than purified CFs in the gastrointestinal milieu, as well as to retain immunogenicity, fimbrial structure and capacity to bind to eukaryotic cells. The vaccine, which is not clinically available, yet, it is recommended to be delivered by the oral or gastrointestinal route to induce optimal local intestinal immune responses [8].
It has long been demonstrated that second-time travelers develop anti-LT antibodies and have lower rates of LT-ETEC travelers’ diarrhea compared with newly arrived visitors [18]. In such cases, antibodies against ETEC LT and major CFs co-operated synergistically for protection against LT-producing ETEC expressing homologous CFs [19]. Other studies in humans have revealed that repeated oral antigen administration was optimal in inducing intestinal immune responses and, therefore, oral inactivated vaccines, which consist of toxin antigen and whole cells, i.e. the licensed recombinant cholera B subunit (rCTB)-WC cholera vaccine Dukoral, have been developed. Other oral ETEC vaccine consisting of rCTB and formalin-inactivated E. coli bacteria expressing major CFs has been shown to be safe and immunogenic in adults and children in different countries. The status of this vaccines remains at clinical trials [19].
However, phase I safety and immunogenicity study in healthy adult volunteers was completed as a three-strain combination, live attenuated vaccine known as ACE527. This vaccine proved to be well tolerated and immunogenic at dose levels of 1010 and 1011 total CFU [20*]. Strong immune responses to LTB and to CFs expressed on all three constituent strains were induced, with at least 50% of subjects in the high-dose group responding to LTB, CFA/I, CS3, and CS6. This is the first study showing that an oral cellular ETEC vaccine induces both mucosal and serum responses [20*].
Epidemiological studies indicate that immune responses to uncharacterized, chromosomally encoded antigens could contribute to protection resulting from repeated ETEC infections. As such, studies of immune responses to ETEC infection had identified a class of surface-expressed molecules known as autotransporters (AT), which have been fully identified with the completion of the genome sequence of the prototype ETEC strain H10407 [21*]. Two chromosomally encoded ATs identified in ETEC were found immunogenic and protective in an animal model, possibly meaning that conserved ATs might contribute to the protective immune responses that follow natural ETEC infection and therefore, offering new potential targets for vaccine development [22]. In addition, other ETEC vaccines include one more candidate which is active against cholera toxin and confers cross protection against ETEC [23]. Finally, a recombinant, transdermal LT vaccine has also been recently evaluated [24]; however, this and other vaccines are available for clinical trials but are not commercially available.
In the case of antibiotic treatment, prophylaxis can be considered for individuals in whom a single episode of travelers’ diarrhea would have costly adverse consequences, for example, during critically important trips, or when travelers’ diarrhea might adversely affect an underlying illness. Several antimicrobials have demonstrated efficacy in the treatment of ETEC-associated diarrhea, including rifaximin, fluoroquinolones and azithromycin (reviewed in [25]). Rifaximin, a rifamycin derivative that is generally well tolerated, has received significant attention for its use in prophylaxis [26*]. Rifaximin has been approved only for the treatment of travelers’ diarrhea caused by non-invasive types of E. coli as it is not effective in treating invasive enteric pathogens [26*]. Therefore, rifaximin remains an excellent choice for treatment of travelers’ diarrhea due to its favorable pharmacokinetics, in vitro susceptibility profile, and efficacy and safety data from clinical trials [27]. Further, rifaximin is currently approved in the United States by the FDA for the treatment of travelers' diarrhea caused by noninvasive diarrheagenic E. coli and is approved in more than 30 other countries for a variety of gastrointestinal disorders [26].
Enteropathogenic Escherichia coli
Enteropathogenic Escherichia coli (EPEC) isolates are a significant cause of infantile diarrhea worldwide and particularly in developing countries. The populations more susceptible to EPEC gastroenteritis are infants less than 2 years of age, with significant mortality rates (10–40%) [28, 29].
The pathogen and clinical implications of the disease
EPEC is characterized for the production of a characteristic histopathological lesion on the apical surface of the enterocytes known as Attaching and Effacing (A/E). EPEC strains are known to possess specific determinants of virulence, i.e., an EPEC adherence factor plasmid (pEAF) and the chromosomal-encoded pathogenicity island known as the Locus of Enterocyte Effacement (LEE) [30]. Adherence, as the first step in the colonization process of EPEC, is mediated by several surface factors that promote the localized adherence phenotype (LA) on intestinal epithelial cells [31]. The bundle forming pilus (BFP), encoded in pEAF, is responsible for the formation of microcolonies in association with the LA phenotype (LA), followed by a type III secretion (LEE encoded)-mediated injection of effector proteins, effacement of microvilli and, finally, intimate adherence on intestinal epithelial cells (reviewed in [1, 31, 32]).
EPEC is divided into two groups, typical EPEC (tEPEC) and atypical EPEC (aEPEC). tEPEC is characterized by the presence of the pEAF, while this plasmid is absent in aEPEC [33]. aEPEC strains are generally defined as E. coli strains that may or may not belong to the classical EPEC O-serogroups that can produce A/E lesions, do not express BFP, and lack Shiga-toxin-encoding genes. Because more than 200 O-serogroups have been identified among aEPEC strains and many are non-typeable [30, 34, 35*], investigation regarding clinical significance of this group of pathogens has been complicated.
The LEE pathogenicity island, as a key virulence determinant, encodes the type III secretion system involved in translocation of bacterial effector proteins to the host cell, and which are either encoded within the LEE or elsewhere in the EPEC genome (non-LEE or Nle). The functions of the large repertoire of EPEC effectors has been grouped depending on the cellular pathway affected as cytoskeletal re-arrangers, immune modulators, or those causing electrolyte disturbances and destruction of microvilli (Figure 1) [36, 37].
Upon colonization and initiation of intestinal disturbances, several manifestations of the EPEC-associated disease in humans are evident, including diarrhea, anorexia, rapid wasting and sometimes death within several days (Table 1). EPEC diarrhea is a self-limiting disease due to a direct relation with the status of host immune response, and EPEC infection has been found to induce activation of innate immune cells, leading to both stimulation of a protective antibody response and deleterious inflammation [38*]. However, EPEC infections remain one of the main causes of persistent secretory diarrhea. “Secretory” diarrhea results from increased chloride secretion and decreased sodium absorption, resulting in increased mucosal permeability. Persistent diarrhea is defined as the passage of loose stools for more than 2 weeks, with progression to chronic diarrhea at the 4-week mark [39, 40]. Overall, the damage caused by EPEC to the mucosa of the small intestine can be summarized as the deregulation of water and solute transport and the disruption of the epithelial barrier structure and function [41, 28, 39, 40].
Current progress in prevention and treatment
The estimated prevalence of EPEC as a cause of diarrheal disease is 8.8% in community-based cohort studies, and 9.1% and 15.6%, respectively, in the in the outpatient and inpatient settings, making EPEC the second most common cause of inpatient diarrhea after rotavirus (25.4%) [41, 35*]. Interestingly, the prevalence of EPEC infections, mainly caused by tEPEC, has decreased in the last several decades, possibly due to breast-feeding promotion, improvement of sanitation, or overestimated diagnosis based on serological tests. Further, several studies have found EPEC at similar frequencies among diarrheal and control samples [41, 35*, 42]. In contrast, aEPEC has become important as the cause of endemic diarrhea in children in developing countries, as well as the causative agent of diarrheal outbreaks. Current data indicate that aEPEC is more prevalent and produces a longer duration of diarrhea than does tEPEC in both developing and developed countries [35*, 34, 42]. Although an animal reservoir for aEPEC is unknown, aEPEC isolates have been detected in domestic animals [42, 35*].
Oral rehydration remains the best therapy for EPEC infectious, leading to decreased morbidity and mortality [43]. However, children with an acute EPEC infection are more likely to fail to respond to oral rehydration therapy, while those developing persistent diarrhea, require hospitalization and may suffer from cow's milk intolerance [43, 44]. Therefore, antimicrobials are recommended for EPEC persistent infections, in which the choice of effective treatment may be crucial for patient recovery and even survival [34]. An association was recently reported between classical EPEC serotypes and antimicrobial resistance, with the identification of a conjugative and multi-resistant plasmid in classical serogroups of EPEC [34]. The most common antibiotic resistances found in classical EPEC are ampicillin, tetracycline, streptomycin and sulfonamides; a high percentage of tEPEC strains are multi-resistant. aEPEC has shown resistance only to trimethoprim. Therefore, the antibiotics recommended are ceftazidime, ceftriaxone, imipenem or piperacillin-tazobactam, for which no resistance has been reported [34, 42]. The use of probiotics has been proposed as an alternative therapy for treatment of A/E pathogens, including EPEC, because they promote tight junction formation and integrity of intestinal barrier function in vitro and in animal models of infection [45, 46].
Current progress in diagnostics
Serotyping (O:H) and adherence to tissue cultured cells have been historically used to diagnose patients with EPEC-associated diarrhea,. However, fast, easy and inexpensive diagnostic methods are required to define optimal treatment and prevention for children in endemic areas [35*]. In addition, aEPEC strains include diverse serotypes, coming mainly from non-classical, non-typeable EPEC serogroups, many of which are non-motile [33]. Thus, some efforts have been directed toward improving and optimizing phenotypic and genotypic assays used for EPEC identification, and these include determining rapid adherence patterns on HEp-2/HeLa cells and immunological detection of BFP, which allows investigators to distinguishing tEPEC from aEPEC [33, 35*, 39].
A large number of multiplex PCRs and real-time PCRs assays have been designed to detect EPEC genes from stool cultures, such as eae and bfpA, encoding intimin and BfpA (major Bfp subunit), respectively [47, 48]. The advantage of these methods is that they are more sensitive and specific; however, further phenotypic characterization is required to define an aEPEC strain and, therefore, this type of methodology is still limited to research settings and is used in case of an outbreak, episodes of severe diarrhea and epidemiologic studies [33, 47, 48]. Two recent studies have shown significant promise for developing rapid reliable diagnostic tests to detect tEPEC and aEPEC isolates. The first reported a comparative analysis of multiplex PCR (mPCR) with serogrouping and PCR-RFLP of the fliC gene [49]. This assay allows differentiation of tEPEC and aEPEC strains from the overall EPEC isolates and can be adapted, when required to allow screening of a large number of isolates [49]. The second study investigated whether Shiga toxin-producing E. coli (STEC) can be distinguished from EPEC (tEPEC and aEPEC) strains, by using mPCR methodology with specific biomarkers associated with each strain’s respective virulence genotype. The study demonstrated consistent amplification of genes specific to the prototype STEC O157:H7 and EPEC O127:H6 E2348/69 strains [50*]. Further, the method was used to screen with high sensitivity and specificity those clinical samples from hemolytic uremic syndrome and diarrheal patients [50*]. As a result of these advances, mPCR approaches are currently the leading diagnostic methodology for the differentiation of pathogenic E. coli strains.
Enteroaggregative Escherichia coli
The EAEC category is heterogeneous, and associated with cases of acute or persistent diarrhea in children and adults worldwide (reviewed in [51, 52]). EAEC has received increasing attention during the past decade as a cause of watery diarrhea, which in a significant proportion of patients becomes persistent. Further, sporadic cases and outbreaks of EAEC-caused diarrhea have been also described (reviewed in [53]).
The pathogen and clinical implications of the disease
The classical definition of EAEC pathogenesis indicates that the micro-organism has the ability to adhere to epithelial cells in a very characteristic ‘stacked-brick’ pattern and is capable of forming biofilms [53]. Although many studies searching for specific virulence determinants of EAEC have been published, the process by which this pathogen causes persistent diarrhea is still unknown (Table 1). However, in vitro organ cultures studies have confirmed that EAEC adheres to the intestinal mucosa and creates a mucoid biofilm on the small bowel surface [54]. Therefore, it has been proposed that host cellular changes during EAEC infection result in digestive-absorptive abnormalities due to these biofilm-forming bacteria, prolonging the diarrhea (Figure 1) [54]. Further, it is well known that EAEC requires a variety of virulence factors, including the so-called aggregative adherence fimbriae and enterotoxins, to cause damage, but since EAEC strains are usually recovered from healthy as well as diseased subjects, the overall mechanisms by which EAEC exerts its pathogenic process remains poorly characterized (reviewed in [51, 52]). Several EAEC strains carry the aggR regulon, that comprises virulence genes defining typical, pathogenic EAEC from aggR-negative, atypical strains, which are considered non-pathogenic [55, 51]. Although several studies demonstrated that EAEC strains are able to invade cultured epithelial cells, the contribution of this invasion to the disease remains unknown [56, 57]. Further, in a recent study which attempted to link the distribution of genes encoding putative invasins with the ability of aggR-positive and aggR-negative EAEC isolates to enter intestinal epithelial cells, there was no correlation between the invasion efficiency of these strains and the presence of any particular gene involved in invasion [58].
Upon adhesion, EAEC produce several toxins, including a heat-stable enterotoxin (EAST-1). Several studies have suggested a role of EAST1 in diarrhea; however, the correlation between diarrhea and the presence of an astA gene (encoding for EAST-1) remains inconclusive [59]. Another toxin characterized in EAEC strains is the plasmid-encoded toxin (Pet) [60] a type V serine protease autotransporter that has been shown to induce increased mucus release, exfoliation of cells, and development of crypt abscesses. The eukaryotic target of Pet is the actin-binding protein alpha-fodrin, and cleavage of this protein results in disruption of the actin cytoskeleton organization [60]. Further, it has been reported that Pet also cleaves spectrin, but recent evidence suggests that mechanisms other than host spectrin redistribution occur during Pet intoxication [61]. Interestingly, a case-control study of moderate-to-severe acute diarrhea among children, 0–59 months of age, was used to identify potential virulence factors among the EAEC strains. The data showed that only a subset of EAEC strains were pathogenic and identified the gene encoding the autotransporter SepA protease as being strongly associated with diarrhea among the EAEC strains tested [62*]. Further investigation is required to define the contribution of SepA to the pathogenesis of EAEC.
Current progress in prevention and treatment
One of the hallmarks during EAEC infection of the gastrointestinal tract is the release of IL-8, resulting in neutrophil recruitment and gastroenteritis [51, 52]. However, an incomplete understanding of the effect of EAEC adherence to intestinal epithelial cells (IECs) and the subsequent innate responses elicited has resulted in an inadequate development of effective treatments. Recent studies have characterized and identified the EAEC processes that might be contributing to the mechanisms underlying EAEC-induced mucosal inflammatory responses. One study found that EAEC adherence contributes to IECs antimicrobial innate immunity, inducing a strong expression of IL-8 and CCL20, consistent with its greater in vivo propensity to induce inflammatory diarrhea [63*]. A subsequent study found that polymorphonuclear neutrophils (PMN) transepithelial migration promoted an enhanced attachment of EAEC to intestinal epithelial cells, and raised the possibility that EAEC-induced PMN infiltration may favor colonization and thus pathogenesis of EAEC in the host [64*].
EAEC infections are often persistent and significantly inflammatory. Moreover, they have resulted in a high worldwide incidence of antibiotic-resistant EAEC isolates [65]. Because of these factors, the antibiotic of choice to treat EAEC-induced diarrhea is ciprofloxacin, an antibiotic with bactericidal activity against a wide spectrum of bacteria. Interestingly, it has been shown that sub-minimum inhibitory concentrations of ciprofloxacin inhibited EAEC adhesion to glass- and tissue-cultured cells [66]. However, if the EAEC surface charges changed, e.g., in a mutant lacking the surface protein dispersin, then the sensitivity to ciprofloxacin was significantly reduced when compared with the same activity in a wild-type strain. Therefore, maintenance of the EAEC surface’s hydrophobicity is required to maintain the effectiveness of ciprofloxacin treatment [66]. Unfortunately, recent reports indicate an increased resistance to nearly all of the antibiotics tested against EAEC, including ciprofloxacin [67], implying that a continuous surveillance of EAEC susceptibility patterns worldwide and development of novel antimicrobial therapies are required to combat EAEC infections.
Conclusion
Significant progress has occurred in recent years relative to understanding the pathogenic processes of these enteroadherent E. coli categories affecting the small intestine, and undoubtedly this knowledge will continue evolving. However, a larger emphasis on the effectiveness of novel treatments is required to ultimately reduce the number of cases and outbreaks still affecting endemic regions. Because of the wide nature of clinical symptoms observed with these enteroadherent E. coli strains and the decreased diagnostic sensitivity of these pathogens, future assays that target stable traits might improve diagnostic sensitivity. To facilitate diagnosis and patient management, future methods would also ideally allow for an assessment of the organism's potential to cause severe disease. Discovery of pathogen-specific virulence factors in those so-called “atypical” strains or from outbreak-producing isolates is required to increase our repertoire of factors that can be incorporated into effective vaccines. Further, as nucleotide sequences of more E. coli strains become available, comparative genomic studies might identify targets that can be used to improve detection, virulence profiling, and vaccine development for these pathogenic E. coli categories.
Finally, a new category of E. coli linked to inflammatory bowel disease has emerged as a potential enteroadherent pathogen. The so-called Adherent Invasive E. coli (AIEC) category represents a group of isolates found in the ileal mucosa of patients with Crohn’s disease (CD), and which do not possess the classical virulence factors found in other E. coli pathotypes. Unlike non-pathogenic commensal E. coli the AIEC isolates have been shown to efficiently adhere to and invade epithelial cells and macrophages, survive and replicate within macrophages, without triggering host cell death, and to induce the release of large amounts of TNF-α from infected macrophages [68, 69, 70, 71]. The ability of AIEC to further stimulate production of pro-inflammatory cytokines, such as IFN and TNF-α, suggest that these bacteria might contribute to the persistence and the chronic inflammatory response observed in CD patients. However, since further work is required to establish the direct link between development of CD and the pathogenesis of AIEC associated with this human disease, this new E. coli category was not further discussed in this review.
Acknowledgements
The work in AGT’s laboratory was supported by NIH/NIAID grants AI079154 and AI09956001. The laboratories of YM-L and MA-H were supported by institutional funds from the VIEP-BUAP MALI-NAT12-I and VIEP-BUAP ARHM-NAT12-I.
Footnotes
Disclosure
Dr. M. Arenas-Hernandez’s and Dr. Y Martinez-Laguna’s institution has received grant support from VIEP. Dr. A Torres’ institution has received grant support from the NIH.
References
- 1.Torres AG, Zhou X, Kaper JB. Adherence of Diarrheagenic Escherichia coli Strains to Epithelial cells. Infect Immun. 2005;73:18–29. doi: 10.1128/IAI.73.1.18-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Future Directions for Research on Enterotoxigenic Escherichia coli Vaccines for Developing Countries. Wkly Epidemiol Rec. 2006;81:97–104. [PubMed] [Google Scholar]
- 4.Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea. J Travel Med. 2008;15:110–118. doi: 10.1111/j.1708-8305.2008.00189.x. [DOI] [PubMed] [Google Scholar]
- 5.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun. 2007;75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svennerholm AM, Tobias J. Vaccines against enterotoxigenic Escherichia coli. Expert Rev Vaccines. 2008;7:795–804. doi: 10.1586/14760584.7.6.795. [DOI] [PubMed] [Google Scholar]
- 9.Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 10.Turner SM, Scott-Tucker A, Cooper LM, Henderson IR. Weapons of mass destruction: virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 2006;263:10–20. doi: 10.1111/j.1574-6968.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 11.Dorsey FC, Fischer JF, Fleckenstein JM. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell Microbiol. 2006;8:1516–1527. doi: 10.1111/j.1462-5822.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson AM, Kaushik RS, Francis DH, Fleckenstein JM, Hardwidge PR. Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells. J Bacteriol. 2009;191:178–186. doi: 10.1128/JB.00822-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasaro MA, Mathias-Santos C, Rodrigues JF, Ferreira LC. Functional and immunological characterization of a natural polymorphic variant of a heat-labile toxin (LT-I) produced by enterotoxigenic Escherichia coli (ETEC) FEMS Immunol Med Microbiol. 2009;55:93–99. doi: 10.1111/j.1574-695X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoder JS, Cesario S, Plotkin V, Ma X, Kelly-Shannon K, Dworkin MS. Outbreak of enterotoxigenic Escherichia coli infection with an unusually long duration of illness. Clin Infect Dis. 2006;42:1513–1517. doi: 10.1086/503842. [DOI] [PubMed] [Google Scholar]
- 15.Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000;62:585–589. doi: 10.4269/ajtmh.2000.62.585. [DOI] [PubMed] [Google Scholar]
- 16.Steffen R, Castelli F, Dieter Nothdurft H, Rombo L, Jane Zuckerman N. Vaccination against enterotoxigenic Escherichia coli a cause of travellers’ diarrhea. J Travel Med. 2005;12:102–107. doi: 10.2310/7060.2005.12207. [DOI] [PubMed] [Google Scholar]
- 17.Sack DA, Shimko J, Torres O, Bourgeois AL, Francia DS, Gustafsson B, et al. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhea of travellers to Guatemala and Mexico. Vaccine. 2007;25:4392–4400. doi: 10.1016/j.vaccine.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 18.DuPont HL, Haynes GA, Pickering LK, Tjoa W, Sullivan P, Olarte J. Diarrhea of travelers to Mexico. Relative susceptibility of United States and Latin American students attending a Mexican University. Am J Epidemiol. 1977;105:37–41. doi: 10.1093/oxfordjournals.aje.a112353. [DOI] [PubMed] [Google Scholar]
- 19.Svennerholm AM. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res. 2011;133:188–194. [PMC free article] [PubMed] [Google Scholar]
- 20. Harro C, Sack D, Bourgeois AL, Walker R, DeNearing B, Feller A, et al. Combination Vaccine Consisting of Three Live Attenuated Enterotoxigenic Escherichia coli Strains Expressing a Range of Colonization Factors and Heat-Labile Toxin Subunit B Is Well Tolerated and Immunogenic in a Placebo-Controlled Double-Blind Phase I Trial in Healthy Adults. Clin Vaccine Immunol. 2011;18:2118–2127. doi: 10.1128/CVI.05342-11.. This report describes the safety and immunogenicity results from the first-in-human dose-escalation trial of ACE527, a three live attenuated ETEC vaccine strains which collectively express several CSs and LTB.
- 21. Crossman LC, Chaudhuri RR, Beatson SA, Wells TJ, Desvaux M, Cunningham AF, et al. A commensal gone bad: complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407. J Bacteriol. 2010;192:5822–5831. doi: 10.1128/JB.00710-10.. This paper describes for the first time the complete genomic sequence of E. coli H10407, a prototypical strain of enterotoxigenic E. coli.
- 22.Harris JA, Roy K, Woo-Rasberry V, Hamilton DJ, Kansal R, Qadri F, et al. Directed Evaluation of Enterotoxigenic Escherichia coli Autotransporter Proteins as Putative Vaccine Candidates. PLoS Negl Trop Dis. 2011;5:e1428. doi: 10.1371/journal.pntd.0001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner A, Wiedermann U. Travellers’ diarrhoea – pros and cons of different prophylactic measures. Wien Klin Wochenschr. 2009;121:13–18. doi: 10.1007/s00508-009-1228-1. [DOI] [PubMed] [Google Scholar]
- 24.Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371:2019–2025. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 25.Flores J, Okhuysen PC. Enterotoxigenic Escherichia coli. In: Torres AG, editor. Pathogenic Escherichia coli in Latin America. Bentham Sciencie Publishers Ltd; 2010. pp. 84–94. [Google Scholar]
- 26. Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17–25. doi: 10.1097/MOG.0b013e328333dc8d. This review describes the most recent advances in rifaximin treatment and prevention of travelers’ diarrhea.
- 27.Koo HL, DuPont HL, Huang DB. The role of rifaximin in the treatment and chemoprophylaxis of travelers’ diarrhea. Ther Clin Risk Manag. 2009;5:841–848. doi: 10.2147/tcrm.s4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapointe TK, O’Connor PM, Buret AG. The role of epithelial malfunction in the pathogenesis of enteropathogenic E. coli-induced diarrea. Lab Invest. 2009;89:964–970. doi: 10.1038/labinvest.2009.69. [DOI] [PubMed] [Google Scholar]
- 29.Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Molecular Microbiology. 2011;80:1420–1438. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- 30.Bardiau M, Szalo M, Mainil JG. Initial adherence of EPEC, EHEC and VTEC to host cells. Vet Res. 2010;41:57. doi: 10.1051/vetres/2010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphries RM, Armstrong GD. Sticky situation: localized adherence of enteropathogenic Escherichia coli to the small intestine epithelium. Future Microbiol. 2010;5:1645–1661. doi: 10.2217/fmb.10.124. [DOI] [PubMed] [Google Scholar]
- 32.Gomes TAT, Gonzalez-Pedrajo B. Enteropathogenic Escherichia coli (EPEC) In: Torres AG, editor. Pathogenic Escherichia coli in Latin America. Betham Science Publishers Ltd; 2010. pp. 25–47. [Google Scholar]
- 33.Hernandes RT, Elias WP, Vieira MAM, Gomes TAT. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett. 2009;297:137–149. doi: 10.1111/j.1574-6968.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 34.Scaletsky ICA, Souza TB, Aranda KRS, Okeke IN. Genetic elements associated with antimicrobial resistance in enteropathogenic Escherichia coli (EPEC) from Brazil. BMC Microbiol. 2010;10:25. doi: 10.1186/1471-2180-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ochoa TJ, Contreras CA. Enteropathogenic Escherichia coli infection in children. Curr Opin Infect Dis. 2011;24:478–483. doi: 10.1097/QCO.0b013e32834a8b8b.. This review discussed the current knowledge about atypical EPEC as a human pathogen.
- 36.Bugarel M, Martin A, Fach P, Beutin L. Virulence gene profiling of enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli strains: a basis for molecular risk assessment of typical and atypical EPEC strains. BMC Microbiol. 2011;11:142. doi: 10.1186/1471-2180-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 38. Calderon Toledo C, Arvidsson I, Karpman D. Cross-reactive protection against enterohemorrhagic Escherichia coli infection by enteropathogenic E. coli in a mouse model. Infect Immun. 2011;79:2224–2233. doi: 10.1128/IAI.01024-10.. Using a murine model of infection, this study showed that enteropathogenic E. coli infections may protect against enterohemorrhagic E. coli infections.
- 39.Pawlowski SW, Warren CA, Guerrant R. Diagnosis and Treatment of Acute or Persistent Diarrhea. Gastroenterology. 2009;136:1874–1886. doi: 10.1053/j.gastro.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodges K, Gill R. Infectious diarrhea. Cellular and molecular mechanisms. Gut Microb. 2010;1:4–21. doi: 10.4161/gmic.1.1.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abba K, Sinfield R, Hart CA, Garner P. Pathogens associated with persistent diarrhea in children in low and middle income countries: systematic review. BMC Infect Dis. 2009;9:88. doi: 10.1186/1471-2334-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García PG, Silva VL, Dinis CG. Ocurrence and antimicrobial Drug Susceptibility Patterns of commensal and diarrheagenic Escherichia coli in fecal microbiota from children with and without acute diarrhea. J Microbiol. 2011;49:46–52. doi: 10.1007/s12275-011-0172-8. [DOI] [PubMed] [Google Scholar]
- 43.Ochoa TJ, Barletta F, Contreras C, Mercado E. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg. 2008;102:852–856. doi: 10.1016/j.trstmh.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fagundes-Neto U, Scaletsky IC. The gut at war: the consequences of enteropathogenic Escherichia coli infection as a factor of diarrhea and malnutrition. Sao Paulo Med J. 2000;118:21–29. doi: 10.1590/S1516-31802000000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu ZH, Shen TY, Zhang P, Ma YL, Moyer MP, Qin HL. Protective effects of Lactobacillus plantarum against epithelial barrier dysfunction of human colon cell line NCM460. World J Gastroenterol. 2010;16:5759–5765. doi: 10.3748/wjg.v16.i45.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Britton RA, Versalovic J. Probiotics and Gastrointestinal Infections. Interdiscip Perspect Infect Dis. 2008;2008:290769. doi: 10.1155/2008/290769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardegen C, Messler S, Henrich B, Pfeffer K, Würthner J, MacKenzie CR. A set of novel multiplex Taqman real-time PCRs for the detection of diarrhoeagenic Escherichia coli and its use in determining the prevalence of EPEC and EAEC in a university hospital. Ann Clin Microbiol Antimicrob. 2010;9:5. doi: 10.1186/1476-0711-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mainil JG, Bardiau M, Ooka T, Ogura Y, Murase K, Etoh Y, et al. Typing of O26 enterohaemorrhagic and enteropathogenic Escherichia coli isolated from humans and cattle with IS621 multiplex PCR-based fingerprinting. J Appl Microbiol. 2011;111:773–786. doi: 10.1111/j.1365-2672.2011.05089.x. [DOI] [PubMed] [Google Scholar]
- 49.Bouzari S, Aslani MM, Oloomi M, Jafari A, Dashti A. Comparison of multiplex PCR with serogrouping and PCR-RFLP of fliC gene for the detection of enteropathogenic Escherichia coli (EPEC) Braz J Infect Dis. 2011;15:365–369. doi: 10.1016/s1413-8670(11)70206-9. [DOI] [PubMed] [Google Scholar]
- 50. Botkin DJ, Galli L, Sankarapani V, Soler M, Rivas M, Torres AG. Development of a Multiplex PCR Assay for detection of Shiga toxin-producing Escherichia coli, enterohemorrhagic E. coli, and enteropathogenic E. coli strains. Front Microbiol. 2012;2:1. doi: 10.3389/fcimb.2012.00008.. This new mPCR methodology permitted the differentiation of enteropathogenic E. coli, Shiga toxin-producing E. coli and enterohemorrhagic E. coli strains from other pathogenic E. coli.
- 51.Flores J, Okhuysen PC. Enteroaggregative Escherichia coli infection. Curr Opin Gastroenterol. 2009;25:8–11. doi: 10.1097/MOG.0b013e32831dac5e. [DOI] [PubMed] [Google Scholar]
- 52.Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12–18. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 53.Weintraub A. Enteroaggregative Escherichia coli: epidemiology, virulence and detection. J Med Microbiol. 2007;56:4–8. doi: 10.1099/jmm.0.46930-0. [DOI] [PubMed] [Google Scholar]
- 54.Andrade JA, Freymüller E, Fagundes-Neto U. Adherence of enteroaggregative Escherichia coli to the ileal and colonic mucosa: an in vitro study utilizing the scanning electron microscopy. Arq Gastroenterol. 2011;48:199–204. doi: 10.1590/s0004-28032011000300009. [DOI] [PubMed] [Google Scholar]
- 55.Dudley EG, Abe C, Ghigo JM, Latour-Lambert P, Hormazabul JC, Nataro JP. An IncI1 plasmid contributes to the adherence of the atypical enteroaggregative Escherichia coli strain C1096 to cultured cells and abiotic surfaces. Infect Immun. 2006;74:2102–2114. doi: 10.1128/IAI.74.4.2102-2114.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abe CM, Knutton S, Pedroso MZ, Freymüller E, Gomes TA. An enteroaggregative Escherichia coli strain of serotype O111:H12 damages and invades cultured T84 cells and human colonic mucosa. FEMS Microbiol Lett. 2001;203:199–205. doi: 10.1111/j.1574-6968.2001.tb10841.x. [DOI] [PubMed] [Google Scholar]
- 57.Pereira AC, Britto-Filho JD, de Carvalho JJ, das Gracas de Luna M, Rosa AC. Enteroaggregative Escherichia coli (EAEC) strains enter and survive within cultured epithelial cells. Microb Pathog. 2008;45:310–314. doi: 10.1016/j.micpath.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Sobieszczañska B, Duda AK, Turniak M, Duda-Madej A, Franiczek R, Kasprzykowska U. Characterization of genes associated with internalization of enteroaggregative Escherichia coli. Microb Pathog. 2011;50:141–147. doi: 10.1016/j.micpath.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Konno T, Yatsuyanagi J, Saito S. Virulence gene profiling of enteroaggregative Escherichia coli heat-stable enterotoxin 1-harboring E. coli (EAST1EC) derived from sporadic diarrheal patients. FEMS Immunol Med Microbiol. 2011 doi: 10.1111/j.1574-695X.2011.00913.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Navarro-Garcia F. Enteroaggregative Escherichia coli plasmid-encoded toxin. Future Microbiol. 2010;5:1005–1013. doi: 10.2217/fmb.10.69. [DOI] [PubMed] [Google Scholar]
- 61.Cappello RE, Estrada-Gutierrez G, Irles C, Giono-Cerezo S, Bloch RJ, Nataro JP. Effects of the plasmid-encoded toxin of enteroaggregative Escherichia coli on focal adhesion complexes. FEMS Immunol Med Microbiol. 2011;61:301–314. doi: 10.1111/j.1574-695X.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boisen N, Scheutz F, Rasko DA, Redman JC, Persson S, Simon J, et al. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J Infect Dis. 2012;205:431–444. doi: 10.1093/infdis/jir757.. In this paper, an mPCR and comparative genome hybridization analysis of a collection of enteroaggregative E. coli (EAEC) strains was performed and a subset of EAEC strains which are pathogenic was defined, including the identification of a marker for the most virulent isolates.
- 63. Edwards LA, Bajaj-Elliott M, Klein NJ, Murch SH, Phillips AD. Bacterial-epithelial contact is a key determinant of host innate immune responses to enteropathogenic and enteroaggregative Escherichia coli. PLoS One. 2011;6:e27030. doi: 10.1371/journal.pone.0027030. This paper characterized which bacterial motifs contribute to the innate epithelial response to enteropathogenic E. coli and enteroaggregative E. coli, using a range of E. coli isogenic mutant strains.
- 64. Boll EJ, Struve C, Sander A, Demma Z, Krogfelt KA, McCormick BA. Enteroaggregative Escherichia coli promotes transepithelial migration of neutrophils through a conserved 12-lipoxygenase pathway. Cell Microbiol. 2012;14:120–132. doi: 10.1111/j.1462-5822.2011.01706.x.. This paper found that polymorphonuclear neutrophils (PMN) transepithelial migration promoted enhanced attachment of enteroaggregative E. coli (EAEC) to T84 cells and suggested that EAEC-induced PMN infiltration may favor colonization and thus pathogenesis of EAEC.
- 65.Aslani MM, Alikhani MY, Zavari A, Yousefi R, Zamani AR. Characterization of enteroaggregative Escherichia coli (EAEC) clinical isolates and their antibiotic resistance pattern. Int J Infect Dis. 2011;15:e136–e139. doi: 10.1016/j.ijid.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Mortensen NP, Fowlkes JD, Maggart M, Doktycz MJ, Nataro JP, Drusano G, et al. Effects of sub-minimum inhibitory concentrations of ciprofloxacin on enteroaggregative Escherichia coli and the role of the surface protein dispersin. Int J Antimicrob Agents. 2011;38:27–34. doi: 10.1016/j.ijantimicag.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Ouyang-Latimer J, Jafri S, VanTassel A, Jiang ZD, Gurleen K, Rodriguez S, et al. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008. Antimicrob Agents Chemother. 2011;55:874–878. doi: 10.1128/AAC.00739-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darfeuille-Michaud A. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn's disease. Int J Med Microbiol. 2002;292:185–193. doi: 10.1078/1438-4221-00201. [DOI] [PubMed] [Google Scholar]
- 69.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 70.Eaves-Pyles T, Allen CA, Taormina J, Swidsinski A, Tutt CB, Jezek GE, et al. Escherichia coli isolated from a Crohn's disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int J Med Microbiol. 2008;298:397–409. doi: 10.1016/j.ijmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Nash JH, Villegas A, Kropinski AM, Aguilar-Valenzuela R, Konczy P, Mascarenhas M, et al. Genome sequence of adherent-invasive Escherichia coli and comparative genomic analysis with other E. coli pathotypes. BMC Genomics. 2010;11:667. doi: 10.1186/1471-2164-11-667. [DOI] [PMC free article] [PubMed] [Google Scholar]