Abstract
Conjugated linoleic acids (CLAs) are natural PPARγ ligands, which showed conflicting effects on metabolism in humans. We examined metabolic effects of different isomers of CLA in subjects with PPARγ2 Pro12Ala polymorphisms. A total of 35 men underwent four intervention periods in a crossover study design: subjects with either genotypes received c9, t11 CLA or t10, c12 CLA, a commercially available 1:1 mix of both isomers or reference oil (linoleic acid (LA)). Adipocytokines, insulin, glucose and triglycerides were assessed in the fasting state and after a standardized mixed meal. Across all genotypes, there was a significant (p = 0.025) CLA treatment effect upon postprandial (pp) HOMA-IR values, with c9, t11 CLA and CLA isomer mix improving, but t10, c12 CLA isomer worsening. In Ala12Ala subjects, the t10, c12 isomer caused weight gain (p = 0.03) and tended to increase postprandial insulin levels (p = 0.05). In Pro12Pro subjects, t10, c12 resulted in reduction in waist circumference (p = 0.03). The comparison of the different genotype groups revealed statistically different changes in fasting and postprandial insulin, HOMA-IR and leptin after intervention. c9, t11 CLA and the commercial CLA mix showed beneficial effects on insulin sensitivity compared with LA, while t10, c12 CLA adversely affects body weight and insulin sensitivity in different PPAR genotypes. CLA isomers have different effects on metabolism in Ala and Pro carriers.
Keywords: CLA, PPAR Pro12Ala, Metabolic syndrome, Insulin, Insulin resistance, HOMA, Postprandial, Diabetes, Nutrigenetic, Gene–nutrient interaction, Adiponectin, Adipocytokines, Triglycerides
Introduction
Conjugated linoleic acid (CLA) refers to a group of polyunsaturated fatty acids. The predominant isomer in food is c9t11 CLA (~90% of dietary CLA), followed by minor amounts of t10c12 CLA (~10% of dietary CLA) (Fritsche and Steinhart 1998). CLAs received considerable attention because of their anti-obesity (Park et al. 1997) and anti-diabetic effects (Ryder et al. 2001) in certain animal models. This has led to the promotion of CLA as weight-loss supplements in humans, sold as an equal mix of the two active isomers c9t11 CLA and t10c12 CLA. However, evidence in humans is still inconsistent regarding the significant effect of CLA supplementation on body weight and on insulin sensitivity (Belury 2002; Evans et al. 2002b; Whigham et al. 2007), with some more evidence for body fat loss (Whigham et al. 2007). This could partly be explained by isomer-specific properties of CLA and the different dosages of CLA studied. The lack of consistency between studies may partly be due to the genetic background of individuals, which in turn may result in a unique metabolic response to dietary fat intake (Gomez et al. 2010; Fisher et al. 2009).
PPARγ is a transcription factor, which exerts its action depending on the presence of fatty acids (FA). Differential promoter usage and alternative splicing of the gene generates the PPAR isoforms: PPARγ1 and PPARγ2. PPARγ1 exhibits widespread expression, albeit at low levels, while PPARγ2 is highly expressed in adipose tissue (Fajas et al. 1997). Indeed, the receptor plays a critical role in adipocyte differentiation. Additionally, PPARγ2 regulates insulin sensitivity by transcriptional activation of adipocyte-specific genes involved in insulin signalling, glucose uptake, fatty acid uptake and lipid storage (Armoni et al. 2003; Tan et al. 2006).
A single nucleotide polymorphism (SNP) in the PPARγ2 gene results in a proline to alanin substitution in codon 12 of exon 1 and has been associated in several subgroups (e.g. Caucasians or obese subjects) with reduced risk of type 2 diabetes, a decreased risk of insulin resistance, but paradoxically weight gain (Tonjes et al. 2006). The prevalence of the Ala allele varies from 4% in Asians to 28% in Caucasians (Tonjes et al. 2006). The potential mechanism by which PPARγ2 Ala12Ala carriers have a lower risk of type 2 diabetes despite higher BMI has not been fully elucidated yet. Studies investigating the cellular mechanism of this phenomenon indicate that the Ala variant binds with lower affinity to PPARγ-responsive DNA elements in comparison with the Pro variant. Moreover, reduced transcription of specific PPARγ target genes was reported for cells over-expressing the Ala variant compared with cells over-expressing the wild-type protein (Deeb et al. 1998).
We hypothesized that the SNP in PPARγ2 may influence adipocytokines such as leptin and adiponectin, and we aimed to elucidate which effects a dietary intervention with different CLA isomers has on subjects of different PPARγ2 genotype. For this purpose, fasting and postprandial leptin, adiponectin, insulin, glucose and triglyceride levels were assessed in subjects homozygous for PPARγ2 Pro12Pro and PPARγ2 Ala12Ala after a 4 week dietary intervention period. Subjects received (1) c9t11 CLA, (2) t10c12 CLA, (3) a commercial available mix of both isomers (Tonalin®) and (4) linoleic acid from safflower oil (reference oil) in randomized order. We assessed the effects of CLA isomers within the Pro group and within the Ala group, compared with the effects of reference oil.
Research design and methods
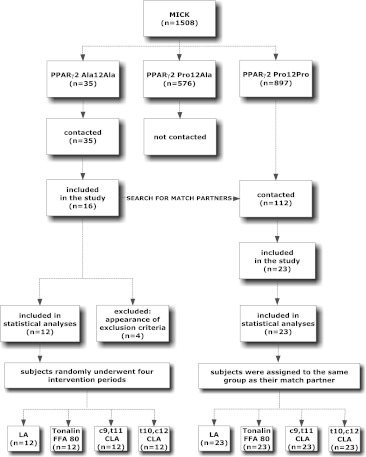
Subjects: 39 healthy middle-aged subjects (45–68 years) were recruited from a population-based cohort (n = 1,508) in Kiel (MICK, Metabolic Intervention Cohort Kiel), which has been previously described (Rubin et al. 2010). A total of 16 male homozygous PPARγ2 Ala12Ala and 23 BMI-matched homozygous control subjects (PPARγ2 Pro12Pro) were included (Fig. 1). Informed consent was obtained from all subjects, and the protocol was approved by the local Ethics Committee of Kiel.
Fig. 1.
Recruitment of subjects
Study followed a single-centre, randomized, placebo-controlled, double-blind, crossover design. Thirty-nine men randomly underwent four intervention periods receiving either c-9, t-11CLA or t-10, c-12 CLA, or a commercial available 1:1 mix of both isomers (Tonalin®) or placebo (linoleic acid from safflower oil) as free fatty acids; resulting in daily administration of 4.25 g fatty acids as capsules. These 4.25 g fatty acids were equivalent to 3.4 g active treatment substance per day.
Study participants completed four intervention periods lasting 28 days each. Interventions were separated by a wash-out phase lasting 42 days.
Anthropometry
Body weight was measured with an electronic scale. Height was assessed by using a stadiometer. The body mass index was calculated as weight (kg) divided by height (m) squared. Waist circumference was measured with a constant tape to the nearest 0.1 cm midway between the lower rib margin and the iliac crest, following a standard operating procedure modified after WHO’s guide to physical measurements (WHO). At the end of each intervention period, the oral metabolic tolerance test (OMTT) was made after a minimum of 12-h fasting. OMTT is an industrial manufactured, standardized high-fat liquid test meal (volume, 500 ml) containing the following ingredients: 32.5 g protein (Na-Caseinat; 13.1% energy), 73 g carbohydrates (sucrose, lactose; 29.5% energy), 55 g fat (high oleic safflower oil; 50.4% energy), 10 g alcohol (7% energy), 0.4 g aroma and 600 mg cholesterol; total energy content was 4,259 kJ. Within 10 min after collecting the fasting blood, subjects drank 500 ml of the test meal. Blood withdrawal was repeated 30 min and 1–9 h after ingestion of OMTT. During the test, subjects were allowed to drink water ad libitum.
Blood was collected in dry heparinised or EDTA-containing tubes and centrifuged (3,000 rpm) for 10 min at 4°C. Samples were frozen at −20 and −80°C for later analysis. Serum insulin (0–5 h) was measured by radioimmunoassay (Adaltis Italia S.p.A., Bologna, Italy). Plasma leptin (0–9 h) and adiponectin (0 h and 5 h) were determined by enzyme-linked immunosorbent assay (R&D Systems Inc., Minneapolis, USA) from EDTA + aprotinin plasma. Triglycerides and glucose were assessed enzymatically from fluoride plasma (glucose) and serum (TG), using a clinical laboratory analyser (Konelab, Espoo, Finland) according to the producer’s manual. All samples were measured in duplicate. Insulin and glucose concentrations were used to calculate insulin resistance from the homoeostasis model for insulin resistance (HOMA-IR) model [insulin (μU/ml) × glucose (mg/dl)/405].
Values are expressed as mean ± SEM. The 0–9 h area under the curve (AUC) was calculated by the trapezoidal method. Variables with skewed distributions were logarithmically transformed before parametric analysis. Differences between the two PPAR genotypes were analysed by Mann–Whitney U test. Wilcoxon rank sum test for dependent variables was used for statistical evaluation of postprandial changes within one group. Multiple ANOVA (MANOVA) was used to test the four dietary intervention groups for comparability (treatment and subject as factors). Co-variable was waist circumference. In case of a significant overall test, Duncan’s post hoc test was used to stratify differences. Tests were considered significant at p-value ≤0.05. A p-value ≤0.1 denotes a trend. Statistical analysis was performed with Statgraphics® Plus (Macintosh, version 4.1).
Results
Of 39 men, 38 completed the study. Drop-out reason for one subject was the diagnosis of an aneurysm of the abdominal aorta. Data are based on 35 subjects because three subjects with PPARγ2 Ala12Ala homozygosity were excluded from statistical analysis as they showed increased fasting glucose levels according to the WHO criteria twice during the study. No differences were found between PPAR genotypes in weight, BMI, waist, hip, WHR, fasting and postprandial glucose, insulin, HOMA-IR, triglycerides, adiponectin and leptin at baseline (data not shown).
Anthropometric characteristics, fasting and postprandial values of all subjects after intervention with reference oil, isomer-mix Tonalin®, c9t11 CLA and t10c12 CLA are shown in Table 1. We found a significantly lower postprandial insulin resistance, expressed as HOMA-IR AUC (we are aware that HOMA-IR is only validated for the fasting state) after intervention with isomer mix and c9t11 CLA, compared with t10c12 CLA (p = 0.025), which by trend was shown for postprandial insulin levels as well between intervention periods in overall test (p = 0.054, Table 1). The means of postprandial insulin and HOMA-IR were significantly different between isomer mix and c9t11 CLA, compared with t10c12 CLA (Table 1, marked as unlike letters).
Table 1.
Anthropometric characteristics, fasting and postprandial values of all subjects at the end of each intervention period
| All subjects (n = 35) | |||||
|---|---|---|---|---|---|
| Preparation | Linoleic acid from safflower oil | Tonalin® (50:50 mix) | Cis-9, trans-11 CLA | Trans-10, cis-12 CLA | p-Value within group |
| Anthropometry | |||||
| Weight | 84.2 (±2.0) | 84.2 (±2.0) | 84.1 (±2.0) | 84.0 (±2.0) | 0.851 |
| BMI (kg/m2) | 26.1 (±0.5) | 26.1 (±0.4) | 26.0 (±0.4) | 26.0 (±0.5) | 0.900 |
| Waist (cm) | 102.1 (±1.5) | 102.1 (±1.6) | 102.3 (±1.5) | 101.3 (±1.5) | 0.172 |
| Hip (cm) | 104.8 (±1.0) | 104.8 (±1.0) | 104.8 (±1.0) | 104.0 (±1.0) | 0.824 |
| Waist/hip ratio | 0.97 (±0.01) | 0.97 (±0.01) | 0.98 (±0.01) | 0.97 (±0.01) | 0.645 |
| Fasting values | |||||
| Plasma glucose (mg/dl) | 96.5 (±1.4) | 98.2 (±1.5) | 97.5 (±1.5) | 96.5 (±1.4) | 0.487 |
| Serum insulin (μU/ml) | 12.5 (±1.0) | 12.9 (±1.0) | 12.5 (±1.1) | 12.2 (±1.0) | 0.898 |
| HOMA-IR (μU/ml*mg/dl/405 | 3.0 (±0.3) | 3.2 (±0.2) | 3.1 (±0.3) | 2.9 (±0.3) | 0.828 |
| Triacylglycerols (mg/dl) | 127.0 (±10.1) | 124.4 (±10.8) | 119.1 (±8.9) | 124.7 (±10.6) | 0.787 |
| Adiponectin (ng/ml) | 7,334 (±467) | 7,452 (±497) | 7,681 (±493) | 7,172 (±479) | 0.152 |
| Plasma leptin (pg/ml) | 5,054 (±532)a | 4,732 (±482)a,b | 5,027 (±559)a | 4,425 (±528)b | 0.054 |
| Postprandial values | |||||
| Glucose AUC (mg/dl) | 483.0 (±8.9) | 484.5 (±8.3) | 472.3 (±7.9) | 447.6 (±23.4) | 0.111 |
| Insulin AUC (μU/ml) | 155.6 (±11.3)b | 137.5 (±10.2)a | 143.6 (±10.8)a | 157.2 (±13.0)b | 0.054 |
| HOMA-IR AUC | 189.0 (±14.8)a,b | 167.3 (±13.4)a | 169.2 (±13.7)a | 195.4 (±19.5)b | 0.025* |
| Triacylglycerols (mg/dl) | 1,661 (±130) | 1,621 (±126) | 1,536 (±111) | 1,666 (±135) | 0.445 |
| Adiponectin 5 h (ng/ml) | 7,364 (±471) | 7,401 (±467) | 7,540 (±484) | 7,067 (±470) | 0.161 |
| Leptin AUC (pg/ml) | 39,092 (±4,060) | 38,220 (±3,934) | 39,502 (±4,386) | 36,467 (±4,523) | 0.320 |
All values are expressed as mean ± SEM
CLA conjugated linoleic acid, BMI body mass index
* Significant differences between intervention periods in overall test (p ≤ 0.05; MANOVA). Duncan′s post hoc test was used to describe where the differences between intervention groups appeared. Means that are significantly different from others are marked as unlike letters. p-Value ≤ 0.1 was seen as a trend. To demonstrate where differences occurred in analyses, means were marked as unlike letters, too. For details of diets, see research design and methods
The mean concentrations for postprandial leptin were lower after t10c12 intervention compared with c9t11 and reference oil (Table 1).
Homozygous subjects for the Ala variant had significantly higher body weight and BMI after intervention with t10c12 CLA when compared with reference oil, isomer mix and c9t11 (p = 0.027, p = 0.034, respectively, Table 2).
Table 2.
Anthropometric characteristics, fasting and postprandial values of subjects homozygote for PPARγ2 Ala12Ala at the end of each intervention period
| PPARγ2 Ala12Ala homozygous (n = 12) | |||||
|---|---|---|---|---|---|
| Preparation | Linoleic acid from safflower oil | Tonalin® (50:50 mix) | Cis-9, trans-11 CLA | Trans-10, cis-12 CLA | p-Value within group |
| Anthropometry | |||||
| Weight | 81.9 (±3.9)a | 82.1 (±3.9)a | 82.4 (±3.8)ab | 82.9 (±3.9)b | 0.027* |
| BMI (kg/m2) | 25.8 (±0.8)a | 25.8 (±0.8)a | 25.9 (±0.8)ab | 26.1 (±0.8)b | 0.034* |
| Waist (cm) | 102.0 (±2.8) | 102.3 (±2.9) | 102.4 (±3.0) | 102.3 (±3.0) | 0.971 |
| Hip (cm) | 103.1 (±2.0) | 102.9 (±1.9) | 103.5 (±2.0) | 102.9 (±1.9) | 0.779 |
| Waist/hip ratio | 0.99 (±0.01) | 0.99 (±0.01) | 0.99 (±0.01) | 0.99 (±0.02) | 0.678 |
| Fasting values | |||||
| Plasma glucose (mg/dl) | 98.4 (±2.4) | 98.7 (±1.8) | 97.8 (±2.9) | 97.2 (±2.1) | 0.845 |
| Serum insulin (μU/ml) | 12.6 (±1.4) | 12.1 (±1.0) | 12.8 (±1.0) | 12.5 (±1.0) | 0.951 |
| HOMA-IR | 3.1 (±0.4) | 3.0 (±0.3) | 3.1 (±0.3) | 3.0 (±0.3) | 0.981 |
| Triacylglycerols (mg/dl) | 136.5 (±17.4) | 121.7 (±17.7) | 123.5 (±16.2) | 123.3 (±12.2) | 0.668 |
| Adiponectin (ng/ml) | 7,319 (±679) | 7,260 (±630) | 7,657 (±783) | 7,093 (±744) | 0.855 |
| Plasma leptin (pg/ml) | 5,295 (±868) | 5,437 (±1,009) | 5,686 (±1,064) | 5,210 (±1,089) | 0.550 |
| Postprandial values | |||||
| Glucose AUC (mg/dl) | 482.7 (±15.0) | 490 (±18.1) | 476 (±15.5) | 491.2 (±14.2) | 0.541 |
| Insulin AUC (μU/ml) | 165.2 (±20.4)a | 130.7 (±17.3)b | 144.4 (±10.5)a,b | 176.0 (±19.4)a | 0.054 |
| HOMA-IR AUC | 200.3 (±26.5)a,b | 167.2 (±16.3)a | 172.1 (±17.1)a | 219.3 (±31.4)b | 0.070 |
| Triacylglycerols (mg/dl) | 1,595 (±116) | 1,461 (±101.2) | 1,569 (±125) | 1,644 (±118) | 0.480 |
| Adiponectin 5 h (ng/ml) | 7,486 (±249)a | 7,254 (±248)a | 7,377 (±249)a | 6,613 (±249)b | 0.091 |
| Leptin AUC (pg/ml) | 40,801 (±7241) | 43,816 (±8,090) | 43,088 (±8232) | 44,390 (±9,294) | 0.905 |
All values are expressed as mean ± SEM
CLA conjugated linoleic acid, BMI body mass index
* Significant differences between intervention periods in overall test (p ≤ 0.05; MANOVA). Duncan′s post hoc test was used to describe where the differences between intervention groups appeared. Means that are significantly different from others are marked as unlike letters. p-Value ≤0.1 was seen as a trend. To demonstrate where differences occurred in analyses, means were marked as unlike letters, too. For details of diets, see research design and methods
Ala allele carriers had lower insulin AUC levels after intervention with the 50:50 isomer mix compared with reference oil and t10c12 CLA (p = 0.054), and tended to have a lower HOMA-IR AUC after both Tonalin® and c9t11 intervention; however, the p-value within group did not reach statistical significance (p = 0.07, Table 2). Postprandial adiponectin mean values after t10c12 intervention were lower in this subgroup compared with control and Tonalin® (Table 2).
Pro12Pro subjects’ waist circumference was significantly lower after intervention with t10c12 CLA, when compared with reference oil, isomer mix and c9t11 CLA (p = 0.033, Table 3). The mean values of postprandial leptin were significantly lower after t10c12 intervention compared with reference oil and c9t11 intervention in Pro12 homozygous (Table 3).
Table 3.
Anthropometric characteristics, fasting and postprandial values of subjects homozygote for PPARγ2 Pro12Pro at the end of each intervention period
| Preparation | Linoleic acid from safflower oil | Tonalin® (50:50 mix) | Cis-9, trans-11 CLA | Trans-10, cis-12 CLA | p-Value within group |
|---|---|---|---|---|---|
| Anthropometry | |||||
| Weight | 85.3 (±2.3) | 85.3 (±2.3) | 85.0 (±2.3) | 84.5 (±2.3) | 0.690 |
| BMI (kg/m2) | 26.2 (±0.6) | 26.2 (±0.5) | 26.1 (±0.5) | 26.0 (±0.5) | 0.591 |
| Waist (cm) | 102.1 (±1.5)a | 102.1 (±1.6)a | 102.3 (±1.5)a | 101.3 (±1.5)b | 0.033* |
| Hip (cm) | 105.8 (±1.1) | 105.8 (±1.1) | 105.5 (±1.1) | 104.5 (±1.1) | 0.772 |
| Waist/hip ratio | 0.96 (±0.01) | 0.96 (±0.01) | 0.97 (±0.01) | 0.96 (±0.01) | 0.686 |
| Fasting values | |||||
| Glucose (mg/dl) | 95.6 (±1.7) | 97.9 (±2.0) | 97.4 (±1.8) | 96.2 (±1.8) | 0.440 |
| Insulin (μU/ml) | 12.4 (±1.4) | 13.2 (±1.5) | 12.4 (±1.6) | 12.0 (±1.4) | 0.789 |
| HOMA-IR | 3.0 (±0.4) | 3.3 (±0.4) | 3.0 (±0.4) | 2.9 (±0.3) | 0.707 |
| Triacylglycerols (mg/dl) | 122.0 (±12.6) | 125.8 (±13.8) | 117.0 (±10.8) | 125.4 (±14.9) | 0.930 |
| Adiponectin (ng/ml) | 7,342 (±678) | 7,552 (±689) | 7,695 (±644) | 7,215 (±628) | 0.110 |
| Leptin (pg/ml) | 4,929 (±682)a | 4,365 (±513)a,b | 4,683 (±650)a,b | 4,015 (±570)b | 0.083 |
| Postprandial values | |||||
| Glucose AUC (mg/dl) | 483.2 (±11.3) | 481.7 (±8.6) | 470.7 (±9.2) | 483.1 (±13.4) | 0.221 |
| Insulin AUC (μU/ml) | 150.5 (±13.8) | 141.0 (±12.8) | 143.2 (±15.7) | 147.4 (±17.0) | 0.688 |
| HOMA-IR AUC | 183.1 (±18.2) | 170.2 (±16.2) | 167.6 (±19.1) | 181.4 (±24.8) | 0.446 |
| Triacylglycerols (mg/dl) | 1,695 (±190) | 1,704 (±184) | 1,520 (±154) | 1,679 (±199) | 0.254 |
| Adiponectin 5 h (ng/ml) | 7,296 (±626) | 7,479 (±645) | 7,456 (±623) | 7,304 (±617) | 0.799 |
| Leptin AUC (pg/ml) | 38,201 (±4,997)a | 35,301 (±4,266)a,b | 37,630 (±5,206)a | 32,483 (±4,804)b | 0.063 |
All values are expressed as mean ± SEM
CLA conjugated linoleic acid, BMI body mass index
* Significant differences between intervention periods in overall test (p ≤ 0.05; MANOVA). Duncan’s post hoc test was used to describe where the differences between intervention groups appeared. Means that are significantly different from others are marked as unlike letters. p-Value ≤0.1 was seen as a trend. To demonstrate where differences occurred in analyses, means were marked as unlike letters, too
The genotype groups showed statistically different changes in fasting and postprandial insulin, HOMA-IR and leptin after interventions: a greater increase in insulin and HOMA-IR (p-value between genotype groups: p = 0.001, p = 0.002, respectively) and postprandial insulin and postprandial HOMA-IR (p = 0.001, p ≤ 0.001, respectively) in Ala12 homozygous; and a lower decrease in fasting and postprandial leptin (p = 0.004, p ≤ 0.001, respectively, Table 4) in these rare allele homozygous.
Table 4.
Changes from baseline in anthropometric, fasting and postprandial parameters at the end of PUFA supplementation in two different PPAR genotypes
| Preparation | PPARγ2 Pro12Pro homozygous (n = 23) | PPARγ 2 Ala12Ala homozygous (n = 12) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linoleic acid from safflower oil | Tonalin FFA (50:50 mix) | Cis-9, trans-11 CLA | Trans-10, cis-12 CLA | Linoleic acid from safflower oil | Tonalin FFA 80 (50:50 mix) | Cis-9, trans-11 CLA | Trans-10, cis-12 CLA | p-Value between genotypes | p-Value preparation | |
| Anthropometry | ||||||||||
| Δ Weight (kg) | 0.7 (±0.4) | 0.7 (±0.4) | 0.4 (±0.5) | −0.1 (±0.5) | −0.2 (±0.4) | −0.1 (±0.4) | 0.2 (±0.6) | 0.7 (±0.2) | ns | ns |
| Δ BMI (kg/m2) | 0.2 (±0.1) | 0.2 (±0.1) | 0.1 (±0.2) | 0.0 (±0.2) | −0.1 (±0.1) | 0.0 (±0.1) | 0.1 (±0.2) | 0.1 (±0.2) | ns | ns |
| Δ Waist (cm) | 1.3 (±0.4) | 1.2 (±0.4) | 1.4 (±0.7) | 0.0 (±0.6) | 0.9 (±0.8) | 1.3 (±0.5) | 1.3 (±0.7) | 1.3 (±0.7) | ns | ns |
| Δ Hip (cm) | −0.3 (±0.5) | −0.3 (±0.5) | −0.5 (±0.6) | −1.0 (±0.5) | −0.4 (±0.6) | −0.5 (±0.7) | 0.0 (±0.5) | −0.5 (±0.6) | ns | ns |
| Δ Waist/hip ratio | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | ns | ns |
| Fasting | ||||||||||
| Δ Plasma glucose (mg/dl) | −2.6 (±1.5) | −0.3 (±1.4) | −0.8 (±1.2) | −2.0 (±1.5) | −0.4 (±1.6) | −0.1 (±1.8) | −1.1 (±2.1) | −1.6 (±0.9) | ns | ns |
| Δ Serum insulin (μU/ml) | 1.2 (±0.8)a | 2.1 (±0.7)a | 1.2 (±0.7)a | 0.9 (±0.7)a | 3.8 (±1.2)b | 3.3 (±1.4)b | 4.0 (±1.0)b | 3.7 (±0.9)b | p ≤ 0.001* | ns |
| Δ HOMA−IR | 0.3 (±0.2)a | 0.5 (±0.2)a | 0.3 (±0.2)a | 0.2 (±0.2)a | 0.9 (±0.3)b | 0.8 (±0.3)b | 0.9 (±0.3)b | 0.8 (±0.2)b | p ≤ 0.002* | ns |
| Δ Triacylglycerols (mg/dl) | 3.9 (±6.2) | 7.7 (±7.9) | −1.1 (±7.8) | 7.3 (±6.3) | 12.0 (±6.2) | −2.8 (±19.5) | −5.7 (±11.6) | −1.2 (±11.4) | ns | ns |
| Δ Adiponectin (ng/ml) | −245 (±120) | −35 (±126) | 109 (±189) | −372 (±186) | 103 (±233) | 44 (±329) | 91 (±223) | −123 (±470) | ns | ns |
| Δ Plasma leptin (pg/ml) | −1,116.8 (±580)a | −1,680.5 (±610)a | −1,363 (±499)a | −2,031 (±506)a | −82 (±509)b | 58 (±650)b | 310 (±419)b | −166 (±412)b | p ≤ 0.000* | ns |
| Postprandial | ||||||||||
| Δ Glucose AUC (mg/dl) | 7.7 (±8.7) | 6.3 (±8.4) | −4.8 (±9.2) | 7.7 (±10.2) | 0.3 (±8.1) | 7.3 (±13.7) | −6.8 (±11.2) | 8.8 (±10.0) | ns | ns |
| Δ Insulin AUC (μU/ml) | 9.4 (±9.8)a | −0.2 (±6.1)a | 2.0 (±9.9)a | 6.2 (±10.4)a | 55.1 (±17.7)b | 20.6 (±15.8)b | 34.3 (±11.9)b | 65.9 (±20.0)b | p ≤ 0.000* | ns |
| Δ HOMA−IR AUC | 11.7 (±13.8)a | −1.1 (±10.2)a | −3.7 (±14.1)a | 10.1 (±16.5)a | 67.7 (±22.1)b | 29.1 (±22.3)b | 39.5 (±16.3) | 86.7 (±29.4)b | p ≤ 0.001* | ns |
| Δ Triacylglycerols (mg/dl) | 178 (±118.0) | 188 (±134.2) | 4.0 (±98.1) | 162.7 (±93.7) | 73.0 (±152.3) | −61.1 (±119.6) | 8.0 (±148.2) | 121.2 (±158.4) | ns | ns |
| Δ Adiponectin 5 h (ng/ml) | −267 (±185) | −83 (±212.6) | −106 (±205) | −258 (±215) | 332 (±320) | 90 (±392) | 211 (±231) | −550 (±292) | ns | ns |
| Δ Leptin AUC (pg/ml) | −11,718 (±4,526)a | −14,619 (±4,573)a | −12,289 (±4,287)a | −17,436 (±4,297)a | −5,654 (±4,427)b | −2,639 (±4,944)b | −3,367 (±2,992)b | −2,065 (±3,255)b | p ≤ 0.004* | ns |
All values are expressed as Δ from baseline value (mean ± SEM)
CLA conjugated linoleic acid, BMI body mass index
* Significant differences between genotype groups (p ≤ 0.05; MANOVA). Duncan’s post hoc test was used to describe where the differences between intervention groups appeared. Means that are significantly different from others are marked as unlike letters
Discussion
The most important finding of our study is an inverse reaction of insulin resistance to CLA interventions with c9t11 and t10c12 isomers with a beneficial effect of c9t11 isomer on subjects with equal distribution of PPARγ2 Ala and Pro homozygous. Therefore, this study shows the clear need to differentiate the isomers in CLA intervention studies and could explain the conflicting results in studies using a CLA mix with both isomers like Tonalin® or the c10t12 isomer. In these studies, a positive effect of CLA on body weight was shown, but a negative effect on insulin sensitivity (Riserus et al. 2002), and the authors concluded that CLA generally are more harmful than beneficial. Our results demonstrate that a supplementation with c9t11 and isomer mix in a group of subjects with equal parts of PPARγ Pro12 and 12Ala genotype has beneficial effects on postprandial insulin sensitivity compared with the control oil.
In support of our own results, Riserus et al. (2002) reported t10c12 CLA isomer-specific side effects on insulin sensitivity. In abdominally obese men, glucose and insulin levels were increased and insulin sensitivity was decreased significantly in the t10c12 CLA group, compared with a non-specified control, probably olive oil, but not with the CLA mix group (Riserus et al. 2002). Interestingly, Riserus et al. found an association between t10c12 CLA and body weight, maybe because subjects were not stratified by genotype. Our results suggest that subjects homozygous for the rare Ala12Ala polymorphism in PPARγ2 had a higher BMI after intervention with the t10c12 CLA -isomer, in contrast to the common allele homozygous Pro12Pro, which showed the opposite reaction to t10c12 intervention with a lower waist circumference. The waist-reducing effect is in accordance with many recent studies that showed a positive effect on body weight, taking into account that 85% of the population are Pro-allele carriers.
Only few human data are available about the effect of individual CLA isomers on metabolic parameters. In most human studies, an equal mix of the t10c12 CLA isomer and the c9t11 CLA isomer was provided because this is the commercial product. Compared to the few individual isomer studies, we pre-treated the single isomer preparations in identical manner as is usually done with the commercial CLA product (=addition of mixed tocopherols) in order to avoid any bias that might occur by inadequate storage conditions either in the lab or at the volunteers’ home, taking into account that CLA are unsaturated fatty acids. In addition, our study provides information about CLA effects on postprandial metabolism. Furthermore, this is the first study in humans that focuses on gene-nutrient interactions with respect to the PPARγ2 locus and CLA isomers.
Adiponectin plays also an important role in regulation of insulin sensitivity. A functional PPAR response element was identified in the adiponectin promoter, suggesting that PPARγ regulates adiponectin expression (Iwaki et al. 2003).
Reports of a difference in plasma adiponectin concentration between Ala carriers and Pro12Pro homozygous are conflicting. An association of the Ala12Ala allele with low adiponectin concentrations was found in healthy Japanese (Yamamoto et al. 2002; Takata et al. 2004), but a lack of association in healthy Caucasians has also been reported (Thamer et al. 2003; Tan et al.2006; Helwig et al.2007). We found no significant differences in fasting or postprandial adiponectin between different PPARγ2 genotypes at baseline and after interventions; therefore, our results are consistent with previous findings in Caucasian populations. However, 12Ala homozygous showed a significantly reduced postprandial adiponectin after t10c12 intervention compared with the other intervention groups.
Leptin is another hormone secreted directly by adipocytes and is suggested to be modulated by PPARγ (Kallen and Lazar 1996; Reseland et al.2001). In our study, we found no significant difference between BMI-matched genotypes in fasting and postprandial leptin levels at baseline, but leptin levels were higher in the Ala12Ala group at the end of each FA intervention. The latter results are in agreement with the findings of Cole et al. (2000), Evans et al. (2002a, b) and Simon et al. (2002) who reported higher fasting leptin levels in humans carrying the Ala12Ala allele of PPARγ2, which is assumed to exert lower activity (Cole et al.2000; Evans et al.2002a; Simon et al.2002).
The functional impact of Pro12Ala polymorphism on postprandial leptin levels is sparsely elucidated in humans. In line with fasting values, leptin AUC did not differ between PPAR genotypes at baseline but was higher in the Ala group compared with the Pro group after dietary supplementation, suggesting an impact of CLA on leptin values in the Pro group. Carriers of the common Pro-allele had significantly lower waist circumference after intervention with t10c12 CLA isomer, when compared with reference oil, c9t11 CLA and CLA isomer mix. Fasting leptin and postprandial leptin AUC levels, when compared with reference oil, were lower in carriers of the Pro variant after t10c12 CLA treatment, although this association failed statistical significance. Circulating leptin levels increase exponentially with increasing fat mass (Lonnqvist et al.1995; Considine et al.1996). Interventions that reduce fat mass also lower adipocytokine levels. It is possible that the changes in plasma leptin values that we observed may reflect a reduction in waist circumference as an indicator for intra abdominal fat mass.
Findings of Belury et al. (2003) indicated that indeed dietary CLA can modulate leptin levels. They reported that CLA reduced leptin in zucker diabetes fatty rats (Belury et al. 1999) and humans with type 2 diabetes (Belury et al.2003). Diabetic subjects were supplemented with a dietary CLA mix (6 g/d) or safflower oil placebo for 8 weeks. Plasma level of CLA was inversely correlated with the change in body weight and serum leptin. Interestingly, this association was only significant for the t10c12 CLA, but not for cis-9, trans-11 isomer. This suggests that lower body weight and serum leptin values are attributed to the t10c12 isomer in the plasma. However, supplementation with CLA in non-obese humans (3 g/d) had a modest and transient effect on leptin, as observed by Medina et al. (2000) (Medina et al.2000). Our findings support the view of Belury et al. (2003), because results suggest that individual CLA isomers reduced leptin levels in humans, especially in those carrying the common Pro12Pro allele of PPARγ2. The mechanism underlying this phenomenon remains to be clarified.
Furthermore, in our study, intervention with t10c12 CLA tended to result in lower levels of the insulin-sensitizing adiponectin, compared with reference oil and commercial CLA mix. Since adiponectin has been reported to correlate inversely with body weight (Arita et al.1999), lower adiponectin levels might be the result of weight gain after intervention with t10c12 CLA in the rare genotype group. This in turn might be responsible for the tendency to increased postprandial insulin resistance observed here.
Reduced levels of adiponectin after treatment with CLA or CLA isomers have also been reported in experimental mouse models, and these changes were accompanied by an increase in plasma insulin levels (Poirier et al.2006); the observation has also been reported from human adipocytes (Kennedy et al.2008).
Kennedy et al. (2008) demonstrated that t10c12 CLA, but not c9t11 CLA, antagonizes ligand-stimulated activation of PPARγ, possibly via PPARγ phosphorylation, resulting in its degradation. The authors conclude that c9t11 CLA and t10c12 CLA might have opposite effects on PPARγ and its target gene adiponectin. Our results support these findings. In the Ala group, levels of adiponectin tended to be lower after intervention with t10c12 CLA, but were equal after intervention with c9t11 CLA and isomer mix.
Several investigators suggest an interaction between genotypes and fatty acids including CLA (Roche 2005), but to our knowledge, this is the first study with a focus on the isomer-specific CLA effects on PPARγ2 Ala12Ala SNP carriers compared with the Pro12Pro wild-type carriers. We could show for the first time in vivo gene nutrition interaction of CLA on PPARγ2 SNP.
In summary, individual CLA isomers have different effects on metabolism in Ala- and Pro carriers. CLA isomer c9t11 and the commercial CLA mix seem to have beneficial effects on insulin sensitivity compared with LA, while t10c12 CLA adversely affects body weight and insulin sensitivity in the rare PPAR genotype. In order to clearly demonstrate the effects of CLA on adiponectin in humans, specifically designed studies would be required. Furthermore, future studies investigating the potentially broader use of CLA for weight management purposes might take the genetic make-up of individuals into account.
Acknowledgments
Federal Ministry of Education and Research (BMBF) Germany project grant AZ 20 0312823A/B; Cognis GmbH Monheim, Germany.
Conflict of interest
D.B. has been employee of Cognis GmbH while the study was designed and conducted. C.L., J.S. received research support from Cognis GmbH for this and several other studies, and J.S. gave a talk and statements on CLA paid for by Cognis GmbH. The other authors declare no conflict of interest.
References
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Armoni M, Kritz N, Harel C, Bar-Yoseph F, Chen H, Quon MJ, Karnieli E (2003) Peroxisome proliferator-activated receptor-{gamma} represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J Biol Chem 278:30614–30623. doi:10.1074/jbc.M304654200 [DOI] [PubMed]
- Belury MA, Vanden Heuvel JP (1999) Modulation of diabetes by conjugated linoleic acid. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ (eds) Advances in conjugated linoleic acid research, vol 1. AOCS Press Champaign, IL, pp 404–411
- Belury MA. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–2998. doi: 10.1093/jn/131.10.2995. [DOI] [PubMed] [Google Scholar]
- Belury MA, Mahon A, Banni S. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J Nutr. 2003;133:257S–260S. doi: 10.1093/jn/133.1.257S. [DOI] [PubMed] [Google Scholar]
- Cole SA, Mitchell BD, Hsueh WC, Pineda P, Beamer BA, Shuldiner AR, Comuzzie AG, Blangero J, Hixson JE. The Pro12Ala variant of peroxisome proliferator-activated receptor-gamma2 (PPAR-gamma2) is associated with measures of obesity in Mexican Americans. Int J Obes Relat Metab Disord. 2000;24:522–524. doi: 10.1038/sj.ijo.0801210. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Deeb SS, Fajas L, Nemoto M, Pihlajamaki J, Mykkanen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- Evans M, Brown J, McIntosh M. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J Nutr Biochem. 2002;13:508. doi: 10.1016/S0955-2863(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Evans ME, Brown JM, McIntosh MK. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J Nutr Biochem. 2002;13:508–516. doi: 10.1016/S0955-2863(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre A-M, Saladin R, Najib J, Laville M, Fruchart J-C, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Fisher E, Boeing H, Fritsche A, Doering F, Joost HG, Schulze MB. Whole-grain consumption and transcription factor-7-like 2 (TCF7L2) rs7903146: gene-diet interaction in modulating type 2 diabetes risk. Br J Nutr. 2009;101:478–481. doi: 10.1017/S0007114508020369. [DOI] [PubMed] [Google Scholar]
- Fritsche J, Steinhart H. Amounts of conjugated linoleic acid (CLA) in German foods and evaluation of daily intake. Zeitschrift für Lebensmitteluntersuchung und-Forschung A. 1998;206:77–82. doi: 10.1007/s002170050218. [DOI] [Google Scholar]
- Gomez P, Perez-Martinez P, Marin C, Camargo A, Yubero-Serrano EM, Garcia-Rios A, Rodriguez F, Delgado-Lista J, Perez-Jimenez F, Lopez-Miranda J. APOA1 and APOA4 gene polymorphisms influence the effects of dietary fat on LDL particle size and oxidation in healthy young adults. J Nutr. 2010;140:773–778. doi: 10.3945/jn.109.115964. [DOI] [PubMed] [Google Scholar]
- Helwig U, Rubin D, Kiosz J, Schreiber S, Folsch UR, Nothnagel M, Doring F, Schrezenmeir J. The minor allele of the PPARgamma2 pro12Ala polymorphism is associated with lower postprandial TAG and insulin levels in non-obese healthy men. Br J Nutr. 2007;97:847–854. doi: 10.1017/S0007114507665179. [DOI] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1996;93:5793–5796. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A, Overman A, LaPoint K, Hopkins R, West T, Chuang C-C, Martinez K, Bell D, McIntosh MK (2008) Conjugated linoleic acid-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol. J Lipid Res 50(2):225–232 [DOI] [PMC free article] [PubMed]
- Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, Nelson GJ, Erickson KL. Conjugated linoleic acid supplementation in humans: effects on circulating leptin concentrations and appetite. Lipids. 2000;35:783–788. doi: 10.1007/s11745-000-0586-y. [DOI] [PubMed] [Google Scholar]
- Park Y, Albright KJ, Liu W. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- Poirier H, Shapiro JS, Kim RJ, Lazar MA (2006) Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes 55:1634–1641. doi:10.2337/db06-0036 [DOI] [PubMed]
- Reseland JE, Haugen F, Hollung K, Solvoll K, Halvorsen B, Brude IR, Nenseter MS, Christiansen EN, Drevon CA. Reduction of leptin gene expression by dietary polyunsaturated fatty acids. J Lipid Res. 2001;42:743–750. [PubMed] [Google Scholar]
- Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002;25:1516–1521. doi: 10.2337/diacare.25.9.1516. [DOI] [PubMed] [Google Scholar]
- Roche HM. Fatty acids and the metabolic syndrome. Proc Nutr Soc. 2005;64:23–29. doi: 10.1079/PNS2004405. [DOI] [PubMed] [Google Scholar]
- Rubin D, Helwig U, Nothnagel M, Folsch UR, Schreiber S, Schrezenmeir J (2010) Association of postprandial and fasting triglycerides with traits of the metabolic syndrome in the metabolic intervention cohort kiel. Eur J Endocrinol 162:719–727 [DOI] [PubMed]
- Ryder JW, Portocarrero CP, Song XM, Cui L, Yu M, Combatsiaris T, Galuska D, Bauman DE, Barbano DM, Charron MJ, Zierath JR, Houseknecht KL. Isomer-specific antidiabetic properties of conjugated linoleic acid: improved glucose tolerance, skeletal muscle insulin action, and UCP-2 gene expression. Diabetes. 2001;50:1149–1157. doi: 10.2337/diabetes.50.5.1149. [DOI] [PubMed] [Google Scholar]
- Simon I, Vendrell J, Gutierrez C, Fernandez-Real JM, Vendrell I, Gallart L, Fontova R, Richart C. Pro12Ala substitution in the peroxisome proliferator-activated receptor-gamma is associated with increased leptin levels in women with type-2 diabetes mellitus. Horm Res. 2002;58:143–149. doi: 10.1159/000064770. [DOI] [PubMed] [Google Scholar]
- Takata N, Awata T, Inukai K, Watanabe M, Ohkubo T, Kurihara S, Inaba M, Katayama S. Pro12Ala substitution in peroxisome proliferator-activated receptor[gamma]2 is associated with low adiponectin concentrations in young Japanese men. Metabolism. 2004;53:1548. doi: 10.1016/j.metabol.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Tan GD, Neville MJ, Liverani E, Humphreys SM, Currie JM, Dennis L, Fielding BA, Karpe F. The in vivo effects of the Pro12Ala PPARgamma2 polymorphism on adipose tissue NEFA metabolism: the first use of the Oxford Biobank. Diabetologia. 2006;49:158–168. doi: 10.1007/s00125-005-0044-z. [DOI] [PubMed] [Google Scholar]
- Thamer C, Machicao F, Fritsche A, Stumvoll M, Häring H. No influence of the PPAR[gamma]2 Pro12Ala genotype on serum adiponectin concentrations in healthy Europeans. Metabolism. 2003;52:798. doi: 10.1016/S0026-0495(03)00080-5. [DOI] [PubMed] [Google Scholar]
- Tonjes A, Scholz M, Loeffler M, Stumvoll M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with Pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care. 2006;29:2489–2497. doi: 10.2337/dc06-0513. [DOI] [PubMed] [Google Scholar]
- Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hirose H, Miyashita K, Nishikai K, Saito I, Taniyama M, Tomita M, Saruta T. PPAR[gamma]2 gene Pro12Ala polymorphism may influence serum level of an adipocyte-derived protein, adiponectin, in the Japanese population. Metabolism. 2002;51:1407. doi: 10.1053/meta.2002.35586. [DOI] [PubMed] [Google Scholar]