Highlights
► The metabolism of deoxynivalenol-3-glucoside (D3G) in rats was studied. ► Urine and feces were analyzed by a validated LC–MS/MS biomarker method. ► D3G was readily hydrolyzed to deoxynivalenol (DON) during digestion. ► Most D3G was metabolized by the gut microbiota and recovered in feces. ► D3G is of considerably lower toxicological relevance than DON, at least in rats.
Abbreviations: D3G, deoxynivalenol-3-β-d-glucoside; DON, deoxynivalenol; JECFA, Joint FAO/WHO Expert Committee on Food Additives; DOM-1, de-epoxy deoxynivalenol; DON-GlcA, deoxynivalenol-glucuronide; DOM-1-GlcA, DOM-1-glucuronide; b.w., body weight; SPE, solid phase extraction; MeOH, methanol; ACN, acetonitrile; HPLC, high performance liquid chromatography; MS, mass spectrometry; MS/MS, tandem mass spectrometry; SRM, selected reaction monitoring; DP, declustering potential; CE, collision energy; RA, apparent recovery; SSE, signal suppression/enhancement; RE, recovery of the extraction step; LOD, limit of detection; LOQ, limit of quantification; Z14G, zearalenone-14-β-d-glucoside
Keywords: Deoxynivalenol, Conjugated mycotoxins, ADME, Urine, Feces, Rodent
Abstract
Deoxynivalenol-3-β-d-glucoside (D3G), a plant metabolite of the Fusarium mycotoxin deoxynivalenol (DON), might be hydrolyzed in the digestive tract of mammals, thus contributing to the total dietary DON exposure of individuals. Yet, D3G has not been considered in regulatory limits set for DON for foodstuffs due to the lack of in vivo data. The aim of our study was to evaluate whether D3G is reactivated in vivo by investigation of its metabolism in rats. Six Sprague-Dawley rats received water, DON (2.0 mg/kg body weight (b.w.)) and the equimolar amount of D3G (3.1 mg/kg b.w.) by gavage on day 1, 8 and 15, respectively. Urine and feces were collected for 48 h and analyzed for D3G, DON, deoxynivalenol-glucuronide (DON-GlcA) and de-epoxy deoxynivalenol (DOM-1) by a validated LC–tandem mass spectrometry (MS/MS) based biomarker method. After administration of D3G, only 3.7 ± 0.7% of the given dose were found in urine in the form of analyzed analytes, compared to 14.9 ± 5.0% after administration of DON, and only 0.3 ± 0.1% were detected in the form of urinary D3G. The majority of administered D3G was recovered as DON and DOM-1 in feces. These results suggest that D3G is little bioavailable, hydrolyzed to DON during digestion, and partially converted to DOM-1 and DON-GlcA prior to excretion. Our data indicate that D3G is of considerably lower toxicological relevance than DON, at least in rats.
1. Introduction
The mycotoxin deoxynivalenol (DON), a secondary metabolite of several Fusarium species, is one of the most important mycotoxins in cereal crops worldwide, and the most frequently occurring type B trichothecene in Europe (SCOOP, 2003). DON inhibits protein synthesis and modulates immune responses (reviewed by Pestka, 2010). In animals, toxicity symptoms include feed refusal, vomiting and growth depression (summarized by Pestka, 2007). Furthermore, DON causes inhibition of germination and growth retardation in plants (reviewed by Rocha et al., 2005). However, plants can metabolize DON to a variable extend through enzymatic conjugation to glucose (Berthiller et al., 2009b; Lemmens et al., 2005; Poppenberger et al., 2003). The resulting “masked” mycotoxin deoxynivalenol-3-β-d-glucoside (D3G) affects protein biosynthesis to a far lower extent than DON in vitro and is therefore regarded as a detoxification product of DON in plants (Poppenberger et al., 2003).
D3G was first detected in naturally contaminated wheat and maize in 2005 (Berthiller et al., 2005). Since then, the worldwide occurrence of D3G in different cereal crops has been reported (Berthiller et al., 2009a; De Boevre et al., 2012; Desmarchelier and Seefelder, 2011; Li et al., 2011; Sasanya et al., 2008). The molar percentages of D3G/DON varied strongly in these studies, but reached maximum levels of 46% (Berthiller et al., 2009a). This percentage may increase in the future as a consequence of plant breeding efforts to enhance Fusarium head blight resistance by introgression of resistance loci (Lemmens et al., 2005). Considerable amounts of D3G were found in foodstuffs such as breakfast cereals, snacks and beers (Kostelanska et al., 2009; Malachova et al., 2011). Despite its frequent occurrence, the toxicological relevance of D3G in humans and animals has not yet been evaluated. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) stressed the possibility that D3G is hydrolyzed in the digestive tract of mammals (JECFA, 2011). Although this assumption is not yet supported by in vivo data, a recent study showed that certain intestinal bacteria are capable of cleaving D3G to DON in vitro (Berthiller et al., 2011).
Numerous studies have examined the toxicokinetics of DON in vivo, revealing two major metabolic pathways: de-epoxidation by anaerobic bacteria and conjugation to glucuronic acid. De-epoxy deoxynivalenol (DOM-1), which is at least 50-fold less toxic than DON (Sundstøl Eriksen et al., 2004), is formed by anaerobic ruminal or intestinal microbes (summarized by Zhou et al., 2008). DOM-1 can be excreted via the feces or it can be absorbed and detected in different biological samples of animals, like urine, plasma (reviewed by Rotter et al., 1996), and milk (Seeling et al., 2006). The ability to detoxify DON to DOM-1 in the upper gastrointestinal tract is considered a major cause for the differences regarding the susceptibility to DON among species (Pestka, 2007; Rotter et al., 1996).
The main metabolic pathway of mammals to detoxify resorbed DON is glucuronidation, a phase II reaction which reflects one of the most important mechanisms to inactivate xenobiotics by enhancing their polarity and excretability. Studies in different animal species showed that deoxynivalenol-glucuronide (DON-GlcA) is the major DON metabolite in plasma and urine (summarized by Wu et al., 2007). In humans, the measurement of urinary DON and DON-GlcA levels has been used successfully as a biomarker to assess DON exposure (Turner et al., 2011; Warth et al., 2012a). In addition, the formation of DOM-1-glucuronide (DOM-1-GlcA) in urine of rats has recently been reported (Lattanzio et al., 2011).
The presence of characteristic metabolites in urine and in feces allows conclusions regarding the absorption and metabolism of mycotoxins (Galtier, 1998). Studies determining the total recovery of orally administered DON in excreta of rats have been performed as early as in the 1980s (Lake et al., 1987; Worrell et al., 1989; Yoshizawa et al., 1983). Depending on whether DON was applied in its pure form or as a radiolabeled compound, observed recoveries ranged from around 15 to 89% of the applied toxin dose, respectively.
D3G has so far not been considered in the regulatory limits for cereal-based food established by the European Commission for DON (European Commission, 2006). Yet, JECFA stated that D3G might be an important contributor to dietary DON exposure and emphasized the need of in vivo data concerning the absorption, distribution, metabolism and excretion (ADME) in order to evaluate the potential health risk of D3G (JECFA, 2011).
The aim of the present study was to determine the fate of orally administered D3G in rats and to compare it with the pattern of DON metabolism. To this end, urine and feces of D3G and DON treated rats were analyzed for D3G, DON, DON-GlcA and DOM-1 by a validated LC–tandem mass spectrometry (MS/MS) based biomarker method. This study provides the first insight into the metabolism and excretion of D3G in vivo, thus contributing to the risk assessment of this masked mycotoxin.
2. Materials and methods
2.1. Chemicals and standard solutions
Methanol (MeOH), acetonitrile (ACN) (both LC grade) and glacial acetic acid (p.a.) were purchased from VWR International GmbH (Vienna, Austria). Water was purified with a with a Purelab Ultra system (ELGA LabWater, Celle, Germany).
DON and DOM-1 standards were obtained from Romer Labs GmbH (Tulln, Austria). D3G was previously purified from DON treated wheat plants (Berthiller et al., 2005) and DON-3-GlcA was chemically synthesized according to the method developed by Fruhmann et al. (2012). For use as analytical standards, solid compounds (DON, D3G, and DON-3-GlcA) were dissolved in ACN. A mixed stock solution, containing 100 μg/mL DON, D3G, DOM-1 and DON-GlcA, was prepared in ACN and stored at −20 °C. Further dilutions for spiking experiments and liquid standards were prepared in MeOH/water (20/80, v/v; feces samples) and ACN/water (10/90, v/v; urine samples).
2.2. Animals and study design
Male Sprague-Dawley rats were obtained from the breeding facility of the Medical University of Vienna (Himberg, Austria) and allowed to acclimatize for one week. The rats (5 months old, 250–280 g body weight (b.w.)) were housed individually in polycarbonate cages (Tecniplast, Hohenpeißenberg, Germany) under controlled conditions (24 ± 1 °C, humidity 50 ± 5%, 12 h light/dark cycle). Pelleted feed (R/M-H, Ssniff, Soest, Germany) and water were provided ad libitum. The rodent diet was analyzed for its concentrations of DON and D3G before the start of the experiment.
Using a repeated measures design, the rats (n = 6) received water, DON (2.0 mg/kg b.w.) and the equimolar amount (6.8 μmol/kg b.w.) of D3G (3.1 mg/kg b.w.) by gavage on days 1, 8 and 15 of the experiment, respectively. Stock solutions of 400 μg/mL DON and 619 μg/mL D3G were prepared by dissolving the solid standards in water. Thereof, volumes of 1.4–1.8 mL were administered to the rats according to their weight. Feed was withdrawn 12 h before the treatment. After administration, the animals were housed individually in polycarbonate metabolic cages (Tecniplast, Hohenpeißenberg, Germany) for 48 h. Urine and feces were collected for the periods 0–24 h and 24–48 h after dosing and volumetrically measured or weighted, respectively. The samples were frozen at −20 °C at the end of the 48 h period.
The study design was approved by both, the Ethics Committee of the Medical University of Vienna and the Austrian Ministry for Science and Research.
2.3. Sample preparation
Urine samples were centrifuged (10 min, 14,000 × g), acidified with 1% of acetic acid and cleaned up by solid phase extraction (SPE) on Strata C18-T cartridges (200 mg, Phenomenex, Aschaffenburg, Germany). After conditioning of the cartridges with 5 mL of MeOH and 5 mL of MeOH/water/acetic acid (5/94/1, v/v/v), 500 μL of urine samples containing 1% of acetic acid were applied. Subsequently, the cartridges were washed with 1 mL of MeOH/water/acetic acid (5/94/1, v/v/v). The analytes were eluted with 2 mL of MeOH/water/acetic acid (70/29/1, v/v/v) and the eluates were evaporated to dryness under compressed air. The residues were reconstituted in 5 mL of ACN/water (20/80, v/v) for LC–MS/MS analysis.
Feces samples were freeze-dried, homogenized and 250 mg aliquots were extracted three times (40/40/20 min) with MeOH/water (50/50, v/v, 3/2/2 mL) on a GFL rotary shaker (Burgwedel, Germany). 500 μL aliquots of the pooled raw extracts were combined with 500 μL cold MeOH. Subsequently, the solutions were vortexed for 15 s and centrifuged at 9000 × g for 10 min. Finally, 300 μL of the supernatants was evaporated to dryness under compressed air and re-dissolved in 300 μL of MeOH/water (20/80, v/v). The samples were vortexed for 30 s and clarified by centrifugation (10 min, 14,000 × g) for LC–MS/MS analysis.
Clean-up procedures for feces and urine as described above resulted in sample dilutions by a factor of 56 and 10, respectively.
2.4. HPLC–MS/MS parameters
Analysis was performed on an 1100 series high performance liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) coupled to a QTrap 4000 LC–MS/MS System (AB Sciex, Foster City, CA) equipped with a Turbo V electrospray ionization (ESI) source. Chromatographic separation was achieved on an Atlantis® T3 column (3.0 mm × 150 mm, 3 μm, Waters, Vienna, Austria) equipped with a 4 mm × 3 mm C18 security guard cartridge (Phenomenex, Torrance, CA, USA). Eluent A was composed of water/acetic acid (99.9/0.1, v/v) and eluent B of ACN/acetic acid (99.9/0.1, v/v). After an initial period of 2 min at 5% B, the proportion of B was increased linearly to 25% (at 3 min), 90% (at 14.8 min) and 96% (at 15 min). After a hold-time of 2 min at 96% B, the column was re-equilibrated for 2 min at 5% B. The temperature of the column oven was 35 °C, while the flow rate was set to 600 μL/min. The injection volume was 5 μL.
Mass spectrometric analysis was performed in the selected reaction monitoring (SRM) mode after negative electrospray ionization. The following settings were used: source temperature 550 °C, curtain gas 20 psi, nebulizer gas (GS1) 50 psi, auxiliary gas (GS2) 50 psi, ion spray voltage −4000 V, collision gas high, SRM dwell time 50 ms. Mass transitions used for the analysis as well as optimized analyte-dependent parameters are given in Table 1.
Table 1.
Mass transitions and optimized SRM parameters of the target analytes. Values are given in the order quantifier ion, qualifier ion.
| Analyte | tRa (min) | Precursor ion (m/z) | Product ion (m/z) | DPb (V) | CEc (V) |
|---|---|---|---|---|---|
| DON | 6.29 | 355.3 [M+Ac]− | 59.1 | −40 | −30 |
| 355.3 [M+Ac]− | 265.2 | −40 | −12 | ||
| D3G | 6.13 | 517.3 [M+Ac]− | 427.3 | −60 | −28 |
| 517.3 [M+Ac]− | 457.3 | −60 | −18 | ||
| DON-GlcA | 6.18 | 471.3 [M−H]− | 112.9 | −95 | −44 |
| 471.3 [M−H]− | 265.2 | −60 | −18 | ||
| DOM-1 | 6.68 | 339.3 [M+Ac]− | 59.1 | −45 | −40 |
| 339.3 [M+Ac]− | 249.1 | −40 | −18 |
Retention time.
Declustering potential.
Collision energy.
2.5. Method validation
Validation of the method included determination of the apparent recovery (RA), the signal suppression/enhancement (SSE), the recovery of the extraction step (RE), the repeatability (RSD) as well as the limits of detection and quantification (LODs and LOQs). Feces and urine samples of the control group were spiked in triplicate with appropriate amounts of standard mixtures prior to and after extraction. Method validation for feces was performed for DON, DOM-1 and D3G at 8 different spiking levels, corresponding to a working range of 1–300 ng/mL in the measurement solutions. For urine, method performance characteristics were determined for DON, DOM-1, D3G and DON-GlcA in an extended working range of 1–500 ng/mL in the measurement solutions (9 spiking levels).
2.6. Data evaluation
All samples were analyzed using Analyst software version 1.5.2 (AB Sciex, Foster City, CA). By plotting the peak area versus the analyte concentration in MS Excel (2007), linear regressions curves were obtained for each analyte and sample type. Thereof, the performance characteristics RA and SSE were calculated according to Sulyok et al. (2006). The RE was calculated by dividing the obtained mean values for the RA by the determined mean values for the SSE. The repeatability of the method, expressed as relative standard deviation, was calculated from the triplicate analysis of the different spiking levels. The LODs and LOQs were calculated from the spiking levels closest to a signal-to-noise ratio (S/N) of 3:1 and 10:1, respectively, and assessed for both, liquid standards and spiked samples.
Urine and feces samples from treated rats were extracted and analyzed in duplicate. Sample concentrations were determined on the basis of peak areas using external calibration (Analyst). If samples showed signal-to-noise ratios lower than 3:1 and 10:1, respectively, half of the LOD and half of the LOQ values were used for further calculations. Obtained mean values were corrected for the RA. The total amounts of excreted DON, D3G and their metabolites were calculated considering the volumes of the urine and the weights of the feces samples, respectively, which were collected from the individual rats per day.
3. Results and discussion
3.1. Sample preparation and method validation
An LC–MS/MS based method was developed for the direct quantification of DON, DON-GlcA, DOM-1 and D3G in urine and feces of rats. Results of the method validation are listed in Table 2.
Table 2.
Method performance characteristics for the matrices urine (n = 27) and feces (n = 24).
| Matrix | Analyte | RAa ± RSD (%) | SSEb ± RSD (%) | REc ± RSD (%) | LODd (ng/mL) | LOQe (ng/mL) |
|---|---|---|---|---|---|---|
| Urine | DON | 34 ± 1 | 39 ± 5 | 88 ± 5 | 2.7 | 6.9 |
| D3G | 45 ± 3 | 56 ± 10 | 81 ± 13 | 0.6 | 2.1 | |
| DON-GlcA | 89 ± 4 | 97 ± 3 | 92 ± 6 | 3.0 | 13.7 | |
| DOM-1 | 24 ± 3 | 27 ± 9 | 89 ± 11 | 5.1 | 17.0 | |
| Feces | DON | 77 ± 6 | 77 ± 2 | 100 ± 6 | 1.6 | 3.6 |
| D3G | 56 ± 15 | 66 ± 5 | 86 ± 18 | 1.7 | 8.6 | |
| DOM-1 | 69 ± 2 | 63 ± 2 | 108 ± 2 | 2.7 | 8.5 | |
Apparent recovery.
Signal suppression/enhancement.
Extraction recovery.
Limits of detection in spiked measurement solutions.
Limits of quantification in spiked measurement solutions.
Urine was cleaned-up by SPE and diluted before injection. Initially we tried a dilute-and-shoot approach, as successfully performed by Warth et al. (2011). However, this procedure did not sufficiently reduce matrix interferences in our samples. Therefore, SPE was employed for sample clean-up. Acidification of the solutions used in SPE increased the extraction recoveries. Still, signal suppression could not be eliminated for all analytes determined in urine, especially for DOM-1 (27%) and DON (39%). Consequently, apparent recoveries ranged from 24% (DOM-1) to 89% (DON-GlcA) (see Table 2). In future methods, the use of [13C]-labeled internal standards could compensate for this limitation of the method. Still the repeatability of the method was excellent, with RSDs for all analytes ≤4%.
Feces samples were freeze-dried, extracted, diluted and injected. During method development it became obvious that one-step extraction of feces samples resulted in low and non-repeatable extraction recoveries. By performing three subsequent extractions, the RE was increased to ≥86% for all analytes. Besides protein precipitation with pure MeOH, dilution of the samples was needed in order to further decrease matrix effects. Finally, apparent recoveries ranging from 56% (D3G) to 77% (DON) were achieved.
LODs and LOQs corresponded to S/N ratios of 3/1 and 10/1, respectively. In standard solutions, LODs ranged from 0.1 to 1.8 ng/mL, while LOQs were between 0.4 and 5.9 ng/mL. LODs and LOQs obtained in urine and feces were, however higher due to the dilution of the samples by a factor of 10 and 56, respectively. In urine, LODs for DON, D3G, DON-GlcA and DOM-1 were 27, 5.7, 30 and 51 ng/mL, respectively. Corresponding LOQs of 69, 21, 137 and 170 ng/mL were determined. In freeze-dried feces, LODs and LOQs for DON, D3G and DOM-1 were 90, 95 and 151 ng/g and 202, 482 and 476 ng/g, respectively. The obtained LODs and LOQs were sufficiently low for the measurements of the target analytes relevant in our study.
Altogether, an extensive validation of the employed method was performed, which ensured accurate quantification of the mycotoxins biomarkers in urine and feces samples.
3.2. DON, D3G and their metabolites in urine of treated rats
Concentrations of DON, D3G, DON-GlcA and DOM-1 in the analyzed urine samples were in the range of 97–2200 ng/mL, 143–239 ng/mL, 265–8750 ng/mL and 285–388 ng/mL, respectively. Daily volumes of urine varied between 11 and 33 mL per rat. Table 3 presents the total amounts of DON, D3G and their metabolites excreted in urine in the time periods 0–24 h and 24–28 h after oral application of water, DON and D3G, respectively. For better comparability of the results, data are expressed as molar amounts.
Table 3.
Total amounts of recovered DON, D3G, DON-GlcA and DOM-1 in urine. Mean values ± SD (n = 6) are given for the indicated time periods after oral administration of water, 2.0 mg/kg b.w. DON and 3.1 mg/kg b.w. D3G, respectively <!-- no-mfc -->(6.8 μmol toxin/kg b.w.).<!-- /no-mfc -->.
| Treatment | Time period (h) | DON ± SD (nmol) | D3G ± SD (nmol) | DON-GlcA (nmol) | DOM ± SD (nmol) |
|---|---|---|---|---|---|
| Water | 0–24 | 1.3 ± 0.7 | n.d. | 1.2 ± 0.8 | n.d. |
| 24–48 | 1.9 ± 1.6 | n.d. | 1.1 ± 0.7 | n.d. | |
| DON | 0–24 | 79 ± 28 | n.d. | 180 ± 72 | 14 ± 6.0 |
| 24–48 | 10 ± 2.0 | n.d. | 16 ± 5.9 | 4.3 ± 1.6 | |
| D3G | 0–24 | 26 ± 4.9 | 6.9 ± 2.2 | 24 ± 5.0 | 15 ± 7.3 |
| 24–48 | 2.4 ± 1.7 | 0.2 ± 0.1 | 1.9 ± 1.1 | 4.7 ± 1.7 |
n.d., not detected (analyte concentration in all samples <LOD).
Following oral application of water, we detected small amounts of DON and DON-GlcA in urine of rats. Several rodent diets were analyzed for DON and D3G already before the start of the experiment. One commercial rodent diet showed reasonably low DON and D3G concentrations (160 μg/kg DON and <30 μg/kg D3G) and therefore was considered suitable for our study. Since concentrations of DON and DON-GlcA were smaller than the respective limit of quantification in the majority of the measured samples, dietary DON intake was not of major relevance for the outcome to the experiment.
In the urine samples of DON exposed rats, DON, DON-GlcA and DOM-1 were determined. Based on the molar amounts excreted on both days, 88.2 ± 6.8% of the total urinary metabolites were eliminated within 24 h after administration. This is in accordance with previous kinetic studies in rats, where urinary DON excretion decreased after 24 h (Lake et al., 1987; Meky et al., 2003). High variations in the amounts of daily excreted analytes were observed. This effect is probably due to the low absorption of DON in one of the six rats. In detail, urinary DON excretion of rat number 2 was 26.8 nmol within 24 h after dosing, whereas values between 76.5 and 111 nmol were found with the other rats. Thus, besides parameters like species specificity, the route of administration (both reviewed by Rotter et al., 1996) and the dose (Goyarts and Dänicke, 2006) also variations between individuals and the status of their digestion can influence DON metabolism.
DOM-1 has been identified as a DON metabolite in rat urine already in 1983 (Yoshizawa et al., 1983). Since then, data concerning the presence and the amount of urinary DOM-1 excretion in rats have been inconsistent (Lattanzio et al., 2011; Meky et al., 2003). In the current experiment, we found elevated DOM-1 concentrations in urine from 5 out of 6 animals. However, considerably lower amounts of DOM-1 were detected in comparison to DON and DON-GlcA. Thus, elimination of DON in form of DOM-1 in urine seems to be of minor relevance, at least in our experiment.
The main urinary metabolite was found to be DON-GlcA, representing 63.4 ± 6.4% of the total analytes excreted in urine. Meky et al. (2003) implicated DON-GlcA as the major urinary metabolite on the basis of indirect quantification after hydrolysis of urine samples. In the study by Lattanzio et al. (2011), the presence of two DON-GlcA isomers in rat urine (without further details concerning their molecular structures) was postulated. Also Warth et al. (2012a) recently showed the occurrence of two DON-GlcA isomers in human urine after DON exposure, identifying both DON-3-GlcA and DON-15-GlcA. In contrast, in vitro synthesis of DON-GlcA by rat liver microsomes seemingly resulted only in formation of DON-3-GlcA (Wu et al., 2007). In our experiment, identical retention times and quantifier/qualifier-ratios were observed for DON-3-GlcA in standard solutions and for DON-GlcA in urine samples. Additionally, a minor second DON-GlcA peak at later retention time than DON-3-GlcA was found in the urine of rat number 2 and at even lower concentrations also in the urine of all other rats. Furthermore, urine samples were analyzed by the LC–MS/MS method developed by Warth et al. (2012a) which allows the separation and the quantification of DON-3-GlcA and DON-15-GlcA. The analysis confirmed the presence of DON-3-GlcA in rat urine, while DON-15-GlcA was not detected in any sample. The minor peak was investigated by MS/MS experiments and enzymatic hydrolysis with β-glucuronidase (according to Warth et al., 2012a) and assumed to be another DON-GlcA isomer. Based on these findings, we conclude that DON is mainly metabolized to DON-3-GlcA in the used rat strain. Conjugation to a – yet unidentified – other DON-GlcA (which was not quantified in our experiments) occurred only to minor extent.
Recently, the occurrence of another DON-metabolite in rat urine, DOM-1-GlcA, was reported (Lattanzio et al., 2011). After enzymatic hydrolysis of urine samples, we observed an increase in the DOM-1 concentration of 2.0- to 3.2-fold, indicating the presence of DOM-1-GlcA. Yet, direct quantification of DOM-1-GlcA was not possible due to the lack of a suitable standard.
Following oral application of D3G, we detected D3G as well as DON, DON-GlcA and DOM-1 in rat urine. In principle, after oral administration an effective gastrointestinal absorption leads to high urinary excretion of a toxin or its metabolites, whereas fecal elimination indicates lack of absorption (Galtier, 1998). D3G was determined in all urine samples collected 0–24 h after administration, proving that this masked mycotoxin is bioavailable in rats. Yet, amounts of urinary excreted D3G/day did not exceed 9.9 nmol Furthermore, only traces of D3G were found after 24 h. Thus, the absorption of D3G seems to be very limited. Currently, only one previous study evaluated the fate of mycotoxin glucosides in vivo. In a feeding experiment with zearalenone-14-β-d-glucoside (Z14G), Gareis et al. (1990) did not detect Z14G in urine of swine. Seemingly, bioavailability of Z14G and D3G differs, as was to be expected.
In recent years concerns have been raised that cleavage of D3G could increase total DON intake of individuals. In the urine of the exposed rats, D3G was mainly eliminated in form of DON and DON-GlcA (67.7 ± 7.0%). Therefore, our findings demonstrate that DON is liberated from D3G in vivo, absorbed and subsequently metabolized to DON-GlcA. Yet, considerably lower amounts of DON and DON-GlcA were determined in the urine of D3G treated rats in comparison to DON treatment. Thus, DON exposure due to the ingestion of D3G seems to be marginal, at least in rats.
3.3. DON, D3G and their metabolites in feces of treated rats
Concentrations of DON and DOM-1 in the analyzed feces samples were between 217–17,700 ng/mL and 819–7740 ng/mL, respectively. The daily amounts of freeze-dried feces/animal ranged from 3 to 9 g per animal. The total amounts of excreted DON, DOM-1 and D3G in feces are given in Table 4.
Table 4.
Total amounts of recovered DON, D3G, DON-GlcA and DOM-1 in feces. Mean values ± SD (n = 6) are given for the indicated time periods after oral administration of water, 2.0 mg/kg b.w. DON and 3.1 mg/kg b.w. D3G, respectively <!-- no-mfc -->(6.8 μmol toxin/kg b.w.).<!-- /no-mfc -->.
| Treatment | Time period (h) | DON ± SD (nmol) | D3G ± SD (nmol) | DON-GlcA (nmol) | DOM ± SD (nmol) |
|---|---|---|---|---|---|
| Water | 0–24 | 1.9 ± 0.9 | n.d. | n.d. | n.d. |
| 24–48 | 1.8 ± 0.4 | n.d. | n.d. | n.d. | |
| DON | 0–24 | 77 ± 57 | n.d. | n.d. | 120 ± 29 |
| 24–48 | 5.6 ± 2.3 | n.d. | n.d. | 53 ± 15 | |
| D3G | 0–24 | 200 ± 130 | 1.9 ± 1.5 | n.d. | 120 ± 33 |
| 24–48 | 5.5 ± 3.9 | n.d. | n.d. | 46 ± 25 |
n.d., not detected (analyte concentration in all samples <LOD).
Independent of the treatment, DON-GlcA was not detected in any of the samples, as expected. Even if one presumes a significant enterohepatic recycling (biliary excretion of DON-GlcA to intestines) complete hydrolysis of the conjugate DON-GlcA by bacterial glucuronidase would occur before fecal excretion and before freezing of the fecal samples.
Similar to the results obtained from the analysis of urine, traces of DON were observed in rat feces after administration of water due to the dietary DON intake. DOM-1 was not detected in the feces samples of this group, which could be explained by the higher method's LODs and LOQs compared to DON and by only partial conversion.
Following DON application, DON and DOM-1 were found in rat feces. The de-epoxidation of DON by rat gut microbes was demonstrated by Worrell et al. (1989). Furthermore, DOM-1 was determined as the major DON-metabolite in feces (Lake et al., 1987; Worrell et al., 1989; Yoshizawa et al., 1983). In accordance, we observed DOM-1 amounts in feces exceeding those of DON in 5 out of 6 animals. Considerable amounts of DOM-1 (up to 78.1 nmol) were excreted even 24–48 after dosing.
In the feces of rats dosed with D3G, the vast majority of the metabolites (99.5 ± 0.4%) was excreted in form of DON and DOM-1. Only traces of D3G were detected in three out of six samples 0–24 h after treatment. These findings prove that the non-absorbed proportion of D3G is almost completely cleaved to DON and subsequently metabolized to DOM-1 in the gut. Our results are in line with in vitro data from Berthiller et al. (2011), who showed that several intestinal bacteria have the capability to hydrolyze D3G to DON. Similarly, Gareis et al. (1990) demonstrated that Z14G is completely cleaved during digestion, indicating that mycotoxin glucosides in general can be deconjugated in the digestive tract of mammals.
We previously postulated that D3G is hydrolyzed to DON in distal parts of the intestine, since the toxin was found to be resistant to acidic conditions and several digestive enzymes (Berthiller et al., 2011). In total, we observed higher amounts of DON in rat feces after D3G treatment compared to DON treatment. As DON is mainly absorbed in the small intestine (Dänicke et al., 2004), our data lead to the assumption that D3G is hydrolyzed distal therefrom. However, detected amounts of DON in feces varied over a wide range (82–427 nmol), which impedes firm conclusions. Thus, further experiments with more specific study designs are necessary to verify this hypothesis.
3.4. Total amounts recovered
It should be emphasized here that the toxins were applied to the animals by gavage to ensure complete administration. These conditions are artificial, compared to the regular uptake of the compounds with feed. Further studies (e.g. with other animal species) shall take this into account, preferably delivering the compounds mixed into the diet.
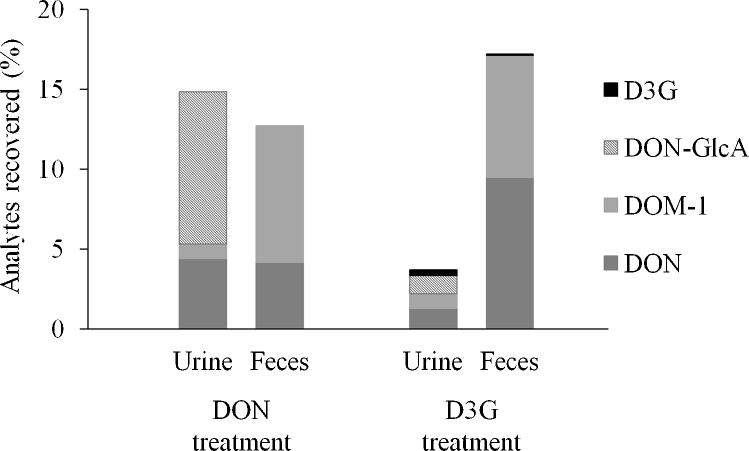
After DON application, the overall amount of the recovered analytes was 554 ± 64 nmol, representing 27.6 ± 3.6% of the administered dose. In urine, 14.9 ± 5.0% of the applied dose were recovered, whereas detected analytes in feces accounted for 12.7 ± 3.4% (Fig. 1). The major metabolite was DON-GlcA (9.5%). Yoshizawa et al. (1983) recovered around 15% of the applied toxin dose after oral administration of 6 mg/kg DON. These data correlate well with our own findings, especially if taken into account, that analysis of DON-GlcA was not implemented in that study. In contrast, significantly higher recoveries of around 89% were observed after administration of 10 mg/kg [14C]-DON in rats (Lake et al., 1987; Worrell et al., 1989). Lake et al. (1987) found 25% and 64% of the administered dose in urine and feces, respectively, while Meky et al. (2003) recovered 37% of the applied dose in urine. Hence, although we also obtained a lower recovery in urine, the differences regarding the detected amounts of analytes in feces are more striking. Several reasons may account for this phenomenon.
Fig. 1.
Excretion of DON, D3G and their metabolites in urine and feces of rats (n = 6) treated with DON or D3G. Values are expressed as equivalent percentages of the administered dose.
First, DON elimination via feces is not completed within 48 h after toxin application, as indicated by our own data and demonstrated by Lake et al. (1987). Therefore, the lower amounts recovered in feces can be explained to a certain degree by the short sampling period. Furthermore, the experimental setup, leading to freezing of feces samples with a delay of up to 48 h, might have an influence. Although the analytes included in our analysis are known to be stable under different cooling conditions (Warth et al., 2012b), microbial degradation of the analytes before freezing, resulting in the formation of unknown metabolites, cannot be excluded.
Above all, excretion was determined on the basis of radioactivity in the studies using [14C]-labeled DON. As a consequence, the obtained total recoveries could also include yet unidentified DON metabolites. The formation of such unknown metabolites, most possibly in the distal end of the intestine, has been suggested before (Sundstøl Eriksen et al., 2003) and would explain the lower recoveries of our experiment. Therefore, an important task in the future will be the evaluation of such metabolites and their subsequent characterization on a high resolution mass spectrometer.
Nevertheless, by using a repeated measures study design we clearly focused on the metabolism of D3G in comparison to that of DON. The total recovery of administered D3G was 20.9 ± 6.6%, with feces being the main excretory route (17.2 ± 6.6%; Fig. 1). Only 3.7 ± 0.7% of the applied dose were recovered in urine, with D3G representing 0.3 ± 0.1%. Thus, our data show that D3G and its metabolites are considerably less absorbed than DON in rats and therefore most likely less bioavailable. A lower absorption of glycosylated plant metabolites in comparison to their parent aglycones has been described in the literature before, for instance for isoflavones (reviewed by Mortensen et al., 2009). DON and DON-GlcA found in the urine accounted for 1.3 ± 0.3% and 1.2 ± 0.3% of the administered dose, respectively (2.5 ± 0.1% in total). The other urinary metabolites D3G and DOM-1 are assumed to possess a reduced protein synthesis inhibition or a lower cytotoxicity compared to DON (Poppenberger et al., 2003; Sundstøl Eriksen et al., 2004). In the DON treatment group, urinary DON and DON-GlcA represented 4.4 ± 1.4% and 9.5 ± 3.6%, which sum up to 13.9 ± 4.7% of the administered dose. Therefore, D3G seems to be of reduced toxicological relevance compared to DON, at least in rats.
In conclusion, this study demonstrates that D3G is partly bioavailable in rats. However, the majority of administered D3G was cleaved during digestion and subsequently excreted in feces. Thus, D3G present in food and feed seems to have a significantly lower toxic equivalency compared to DON. Due to the differences regarding the anatomy and gut microbiota, the bioavailability and metabolization may be species dependent and should be experimentally determined in the future. In such follow-up studies, also the bioavailability of D3G should be monitored, by application of the substance both orally and into the bloodstream by injection, followed by the determination of its concentration. Currently, the limited availability of pure D3G precludes testing of larger animals such as swine.
Conflict of interest statement
The authors declare to have no conflict of interests.
Acknowledgements
The authors thank the Federal Ministry of Economy, Family and Youth, the National Foundation for Research, Technology and Development, BIOMIN Holding GmbH and Nestec Ltd. for funding the Christian Doppler Laboratory for Mycotoxin Metabolism. The financial support by the Austrian Science Fund (FWF projects L475, F3706 and F3708) is greatly acknowledged. Furthermore, we express our gratitude to Alfred Dutter for the care of the animals and the administration of the toxins to the animals by gavage. We also thank Benedikt Warth for the additional MS/MS measurements of urine samples. Finally, we thank Oliver Greitbauer and Veronika Slavik for their help during sample preparation.
References
- Berthiller F., Dall’Asta C., Schuhmacher R., Lemmens M., Adam G., Krska R. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography–tandem mass spectrometry. Journal of Agricultural and Food Chemistry. 2005;53:3421–3425. doi: 10.1021/jf047798g. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Dall’Asta C., Corradini R., Marchelli R., Sulyok M., Krska R., Adam G., Schuhmacher R. Occurrence of deoxynivalenol and its 3-β-d-glucoside in wheat and maize. Food Additives and Contaminants. 2009;26:507–511. doi: 10.1080/02652030802555668. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Schuhmacher R., Adam G., Krska R. Formation, determination and significance of masked and other conjugated mycotoxins. Analytical and Bioanalytical Chemistry. 2009;395:1243–1252. doi: 10.1007/s00216-009-2874-x. [DOI] [PubMed] [Google Scholar]
- Berthiller F., Krska R., Domig K.J., Kneifel W., Juge N., Schuhmacher R., Adam G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicology Letters. 2011;206:264–267. doi: 10.1016/j.toxlet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dänicke S., Valenta H., Döll S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Archives of Animal Nutrition. 2004;58:169–180. doi: 10.1080/00039420410001667548. [DOI] [PubMed] [Google Scholar]
- De Boevre M., Di Mavungu J.D., Maene P., Audenaert K., Deforce D., Haesaert G., Eeckhout M., Callebaut A., Berthiller F., Van Peteghem C., De Saeger S. Development and validation of an LC–MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Additives and Contaminants. 2012;29:819–835. doi: 10.1080/19440049.2012.656707. [DOI] [PubMed] [Google Scholar]
- Desmarchelier A., Seefelder W. Survey of deoxynivalenol and deoxynivalenol-3-glucoside in cereal-based products by liquid chromatography electrospray ionization tandem mass spectrometry. World Mycotoxin Journal. 2011;4:29–35. [Google Scholar]
- European Commission, 2006. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R1881:20120401:EN:PDF (retrieved 06.06.12).
- Fruhmann P., Warth B., Hametner C., Berthiller F., Horkel E., Adam G., Sulyok M., Krska R., Fröhlich J. Synthesis of deoxynivalenol-3-β-d-O-glucuronide for its use as biomarker for dietary deoxynivalenol exposure. World Mycotoxin Journal. 2012;5:127–132. [Google Scholar]
- Galtier P. Biological fate of mycotoxins in animals. Revue de Medecine Veterinaire. 1998;149:549–554. [Google Scholar]
- Gareis M., Bauer J., Thiem J., Plank G., Grabley S., Gedek B. Cleavage of zearalenone-glycoside, a masked mycotoxin, during digestion in swine. Journal of Veterinary Medicine Series B. 1990;37:236–240. doi: 10.1111/j.1439-0450.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Goyarts T., Dänicke S. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pig. Toxicology Letters. 2006;163:171–182. doi: 10.1016/j.toxlet.2005.10.007. [DOI] [PubMed] [Google Scholar]
- JECFA, 2011. Evaluation of certain contaminants in food: seventy-second report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 959. http://whqlibdoc.who.int/trs/WHO_TRS_959_eng.pdf (retrieved 06.06.12).
- Kostelanska M., Hajslova J., Zachariasova M., Malachova A., Kalachova K., Poustka J., Fiala J., Scott P.M., Berthiller F., Krska R. Occurrence of deoxynivalenol and its major conjugate, deoxynivalenol-3-glucoside, in beer and some brewing intermediates. Journal of Agricultural and Food Chemistry. 2009;57:3187–3194. doi: 10.1021/jf803749u. [DOI] [PubMed] [Google Scholar]
- Lake B.G., Phillips J.C., Walters D.G., Bayley D.L., Cook M.W., Thomas L.V., Gilbert J., Startin J.R., Baldwin N.C.P., Bycroft B.W., Dewick P.M. Studies on the metabolism of deoxynivalenol in the rat. Food and Chemical Toxicology. 1987;25:589–592. doi: 10.1016/0278-6915(87)90019-6. [DOI] [PubMed] [Google Scholar]
- Lattanzio V.M.T., Solfrizzo M., De Girolamo A., Chulze S.N., Torres A.M., Visconti A. LC–MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. Journal of Chromatography B. 2011;879:707–715. doi: 10.1016/j.jchromb.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Lemmens M., Scholz U., Berthiller F., Dall’Asta C., Koutnik A., Schuhmacher R., Adam G., Buerstmayr H., Mesterházy Á., Krska R. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Molecular Plant–Microbe Interactions. 2005;18:1318–1324. doi: 10.1094/MPMI-18-1318. [DOI] [PubMed] [Google Scholar]
- Li F.Q., Yu C.C., Shao B., Wang W., Yu H.X. Natural occurrence of masked deoxynivalenol and multi-mycotoxins in cereals from China harvested in 2007 and 2008. Chinese Journal of Preventive Medicine. 2011;45:57–63. [PubMed] [Google Scholar]
- Malachova A., Dzuman Z., Veprikova Z., Vaclavikova M., Zachariasova M., Hajslova J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: the major mycotoxins found in cereal-based products on the Czech market. Journal of Agricultural and Food Chemistry. 2011;59:12990–12997. doi: 10.1021/jf203391x. [DOI] [PubMed] [Google Scholar]
- Meky F.A., Turner P.C., Ashcroft A.E., Miller J.D., Qiao Y.-L., Roth M.J., Wild C.P. Development of a urinary biomarker of human exposure to deoxynivalenol. Food and Chemical Toxicology. 2003;41:265–273. doi: 10.1016/s0278-6915(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Mortensen A., Kulling S.E., Schwartz H., Rowland I., Ruefer C.E., Rimbach G., Cassidy A., Magee P., Millar J., Hall W.L., Birkved F.K., Sorensen I.K., Sontag G. Analytical and compositional aspects of isoflavones in food and their biological effects. Molecular Nutrition and Food Research. 2009;53:266–309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- Pestka J.J. Deoxynivalenol, toxicity, mechanisms and animal health risks. Animal Feed Science and Technology. 2007;137:283–298. [Google Scholar]
- Pestka J.J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Archives of Toxicology. 2010;84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- Poppenberger B., Berthiller F., Lucyshyn D., Sieberer T., Schuhmacher R., Krska R., Kuchler K., Glössl J., Luschnig C., Adam G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry. 2003;278:47905–47914. doi: 10.1074/jbc.M307552200. [DOI] [PubMed] [Google Scholar]
- Rocha O., Ansari K., Doohan F.M. Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Additives and Contaminants. 2005;22:369–378. doi: 10.1080/02652030500058403. [DOI] [PubMed] [Google Scholar]
- Rotter B.A., Prelusky D.B., Pestka J.J. Toxicology of deoxynivalenol (vomitoxin) Journal of Toxicology and Environment Health. 1996;48:1–34. doi: 10.1080/009841096161447. [DOI] [PubMed] [Google Scholar]
- Sasanya J.J., Hall C., Wolf-Hall C. Analysis of deoxynivalenol, masked deoxynivalenol, and Fusarium graminearum pigment in wheat samples, using liquid chromatography–UV–mass spectrometry. Journal of Food Protection. 2008;71:1205–1213. doi: 10.4315/0362-028x-71.6.1205. [DOI] [PubMed] [Google Scholar]
- Scientic Cooperation on Questions Relating to Food (SCOOP), 2003. Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states. SCOOP Task 3.2.10 Final Report. http://ec.europa.eu/food/fs/scoop/task3210.pdf (retrieved 06.06.12).
- Seeling K., Dänicke S., Valenta H., Van Egmond H.P., Schothorst R.C., Jekel A.A., Lebzien P., Schollenberger M., Razzazi-Fazeli E., Flachowsky G. Effects of Fusarium toxin-contaminated wheat and feed intake level on the biotransformation and carry-over of deoxynivalenol in dairy cows. Food Additives and Contaminants. 2006;23:1008–1020. doi: 10.1080/02652030600723245. [DOI] [PubMed] [Google Scholar]
- Sulyok M., Berthiller F., Krska R., Schuhmacher R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Communications in Mass Spectrometry. 2006;20:2649–2659. doi: 10.1002/rcm.2640. [DOI] [PubMed] [Google Scholar]
- Sundstøl Eriksen G., Pettersson H., Lindberg J.E. Absorption, metabolism and excretion of 3-acetyl don in pigs. Archives of Animal Nutrition. 2003;57:335–345. doi: 10.1080/00039420310001607699. [DOI] [PubMed] [Google Scholar]
- Sundstøl Eriksen G., Pettersson H., Lundh T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food and Chemical Toxicology. 2004;42:619–624. doi: 10.1016/j.fct.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Turner P.C., Ji B.T., Shu X.O., Zheng W., Chow W.H., Gao Y.T., Hardie L.J. A biomarker survey of urinary deoxynivalenol in China. The Shanghai women's health study. Food Additives and Contaminants. 2011;28:1220–1223. doi: 10.1080/19440049.2011.584070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth B., Sulyok M., Berthiller F., Schuhmacher R., Fruhmann P., Hametner C., Adam G., Fröhlich J., Krska R. Direct quantification of deoxynivalenol glucuronide in human urine as biomarker of exposure to the Fusarium mycotoxin deoxynivalenol. Analytical and Bioanalytical Chemistry. 2011;401:195–200. doi: 10.1007/s00216-011-5095-z. [DOI] [PubMed] [Google Scholar]
- Warth B., Sulyok M., Fruhmann P., Berthiller F., Schuhmacher R., Hametner C., Adam G., Fröhlich J., Krska R. Assessment of human deoxynivalenol exposure using an LC–MS/MS based biomarker method. Toxicology Letters. 2012;211:85–90. doi: 10.1016/j.toxlet.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Warth B., Sulyok M., Fruhmann P., Mikula H., Berthiller F., Schuhmacher R., Hametner C., Angie Abia W., Adam G., Fröhlich J., Krska R. Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Communications in Mass Spectrometry. 2012;26:1533–1540. doi: 10.1002/rcm.6255. [DOI] [PubMed] [Google Scholar]
- Worrell N.R., Mallett A.K., Cook W.M., Baldwin N.C.P., Shepherd M.J. The role of gut micro-organisms in the metabolism of deoxynivalenol administered to rats. Xenobiotica. 1989;19:25–32. doi: 10.3109/00498258909034673. [DOI] [PubMed] [Google Scholar]
- Wu X., Murphy P., Cunnick J., Hendrich S. Synthesis and characterization of deoxynivalenol glucuronide: its comparative immunotoxicity with deoxynivalenol. Food and Chemical Toxicology. 2007;45:1846–1855. doi: 10.1016/j.fct.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Takeda H., Ohi T. Structure of a novel metabolite from deoxynivalenol, a trichothecene mycotoxin, in animals. Agricultural and Biological Chemistry. 1983;47:2133–2135. [Google Scholar]
- Zhou T., He J., Gong J. Microbial transformation of trichothecene mycotoxins. World Mycotoxin Journal. 2008;1:23–30. [Google Scholar]