Abstract
Recent studies have shown that the microtubule-stabilizing drug, paclitaxel, which is commonly used for the treatment of prostate cancer inhibits signaling from the androgen receptor (AR) by inhibiting its nuclear accumulation downstream of microtubule stabilization. This mechanism is independent of paclitaxel-induced mitotic arrest and could provide an alternative mechanism of drug action that can explain its clinical activity. In this review, we highlight the importance of signaling and trafficking pathways that depend on intact and dynamic microtubules and as such they represent downstream targets of microtubule inhibitors. We showcase prostate cancer, which is driven by the activity of the androgen receptor (AR), as recent reports have revealed a connection between the microtubule-dependent trafficking of AR and the clinical efficacy of taxanes. Identification and further elucidation of microtubule-dependent tumor-specific pathways will help us better understand the molecular basis of clinical taxane resistance as well as identify individual patients more likely to respond to treatment.
1. Introduction
In 2012, prostate cancer will be diagnosed in over 240,000 men with approximately 28,000 deaths attributable to prostate cancer (1). Prostate cancer is a heterogeneous disease, driven primarily by androgen receptor (AR) signaling, and has been traditionally treated with androgen deprivation therapy (ADT). Although our understanding of the molecular basis of prostate cancer has significantly increased over the past decade, ADT for men who develop metastases is still basically the same as first proposed 60 years ago – interfere with androgen signaling (2). The goal of ADT is to block active AR signaling, either by eliminating the ligand or affecting the receptor directly. While most prostate cancer patients are initially sensitive to androgen withdrawal, loss of sensitivity to ADT occurs, leading to the development of castrate-resistant prostate cancer (CRPC) (3). Similarly, even with the introduction of new and more effective therapies that target the androgen axis -such as the CYP17A1 inhibitor, abiraterone, which targets the central synthesis of testosterone and the AR-antagonist MDV3100- ADT is not curative (3). The molecular disturbances that contribute to prostate cancer progression in the setting of castrate levels of circulating androgen, have been reviewed elsewhere (4–7), but almost universally allow for the continued function of the AR as a transcription factor resulting in androgen-driven prostate cancer growth. Consequently, targeting the androgen axis has remained a key concept in the development of novel therapeutic strategies.
In 2004, the combination of docetaxel plus prednisone was established as the standard of care for first-line treatment of patients with CRPC, making taxanes the first class of chemotherapy drugs shown to improve survival in CRPC (8, 9). At the cellular level the taxanes (paclitaxel, docetaxel and cabazitaxel) bind β-tubulin and stabilize microtubules, resulting in mitotic arrest and cell death (10, 11). Microtubules are dynamic cytoskeletal polymers critically important for several cellular functions including structural support and the formation of the mitotic apparatus. During cell division, microtubule dynamics increase significantly (4–100 fold) to enable fast “search and chromosome capture” functions required for mitosis (10). Therefore, drugs that stabilize microtubules, like the taxanes, interfere with mitotic cell progression by suppressing microtubule dynamics. This key observation, supported by numerous in vitro studies, has led to the common belief that the clinical activity of taxanes stems from their antimitotic activity (12). However, this mechanism of action has not helped us understand the molecular basis of clinical response and resistance to taxane chemotherapy, as this model applies primarily to rapidly dividing cells and tissues. It is important to emphasize here, that patients’ tumors have significantly lower rates of cell division than cancer cells growing in vitro. For example, prostate cancer doubles every 33–577 days (13, 14), in contrast to the rapidly dividing prostate cancer cells grown in tissue culture with doubling times between 30–48 h (15, 16); therefore, mitotic arrest alone cannot account for the therapeutic benefit of taxane-based chemotherapy (17, 18). Thus, the effects of taxanes on interphase microtubules and the cellular pathways that depend directly on intact microtubules could provide an alternate mechanism of action for this class of drugs. While this notion challenges the existing paradigm of taxanes exerting their clinical activity exclusively through inhibition of mitosis, by shifting the focus to interphase microtubules, it provides a unique opportunity to dissect how these drugs work and why they are not effective in all tumor types and all patients. This then raises the question of what are the functions of interphase microtubules that are critical for the growth and survival of the tumor?
2. Interphase microtubules as targets for taxane chemotherapy
In interphase cells, microtubules cover the entire area of the cell’s cytoplasm, originating from the microtubule organizing center (MTOC), right outside the nucleus, and extending all the way to the plasma membrane, providing ample surface for protein-protein interactions. In epithelial cells, microtubules display an inherent polarity having their slow-growing minus-end embedded in the MTOC and their fast-growing plus-end oriented towards the plasma membrane. This polarity is utilized by microtubule-based motor proteins, moving cargos either towards the nucleus (dynein) or towards the plasma membrane (kinesins), thereby, allowing for the directional flow of signal information within the cell, which ultimately dictates cell function (19–21). All of these qualities make microtubules centralized nodes of dynamic signaling pathways (22), which remain largely unexplored and their therapeutic potential unexploited.
We have focused our efforts into identifying pathways that depend on intact and dynamic microtubules and whose disruption by taxane treatment would be fatal for tumor cell survival. We have shown that the activity of certain transcription factors depends on the chemomechanics of the microtubule cytoskeleton, and therefore represent indirect targets of taxane activity. These factors, include the tumor suppressor p53, whose nuclear accumulation and trafficking requires intact microtubules and the dynein minus-end directed motor protein (23, 24), and the hypoxia-inducible factor 1 (HIF-1α) whose activity is tightly regulated by microtubule dynamics through microtubule-dependent mRNA trafficking to sites of active protein translation (25, 26). Interestingly, this mechanism does not apply to renal cell carcinoma (RCC) where HIF-1α regulation is independent of microtubules (27). These results can potentially explain the lack of taxane clinical activity in RCC, while identification of the cellular factors that link HIF-1α to microtubules and are missing or are deregulated in RCC can provide a new therapeutic strategy for the treatment of this disease.
Additional proteins whose translocation is microtubule-mediated are the retinoblastoma protein (Rb) (28), the glucocorticoid receptor (GR) (29) and the parathyroid hormone receptor protein (PTHrP) (30). Komlodi-Pasztor et al provide a detailed list of other proteins that traffic on or associate with microtubules (14).
In prostate cancer, specifically, we and others have recently shown that taxane chemotherapy impairs AR signaling activity, not through mitosis, but by impairing AR nuclear translocation and inhibiting subsequent transcriptional activation of ARE-containing target genes (31, 32). Other recent studies showed that paclitaxel-induced inhibition of AR activity is mediated by FOXO1, an AR-suppressive nuclear transcription factor (33) and that docetaxel treatment can down-regulate the expression of AR and prostate specific antigen (PSA) in prostate cancer cell lines (34).
Prompted by the established clinical activity of taxanes in CRPC together with the fact that AR continues to drive disease progression despite prior anti-androgen therapies (35), we set out to investigate the role of tubulin and the impact of microtubule-targeting drugs on AR trafficking and signaling.
The results presented in our recent study (31) provide a mechanistic insight for the clinical activity of taxanes in CRPC by revealing an unconventional link between a nuclear transcription factor and the chemomechanics of the microtubule cytoskeleton. As illustrated in Figure 1, AR associates with microtubules and is trafficked towards the nucleus with the aid of the minus-end-directed motor protein dynein. It is well established that upon ligand binding AR dimerizes and the ligand-receptor complex translocates to the nucleus (36). However, the mechanism enabling this translocation was previously unknown. The recent studies (31, 32) identify microtubules as the “highway tracks” that enable the rapid and targeted nuclear “delivery” of AR, which is required for its transcriptional activity. What remains to be solved however, is whether AR binds microtubules directly, remaining tethered in the cytoplasm and associating with dynein only after ligand binding; or whether AR associates with dynein in the cytoplasm and following ligand binding the receptor-ligand complex gets recruited to the microtubule for trafficking. Recent data from our laboratory support the first model as we show that AR association with microtubules is diminished in the presence of ligand (Figure 2) and that the co-precipitation of AR with dynein is enhanced following ligand stimulation (30). These results suggest that unliganded AR, at steady state, is tethered to the microtubule cytoskeleton and that upon ligand binding the complex associates with dynein and is released from the microtubule. In vitro studies using recombinant AR protein and purified microtubules are required to further investigate AR binding affinity to microtubules. Regardless of direct or indirect binding of AR to the microtubule, dynein’s function is critical for the trafficking not only of AR but also other proteins for which nuclear localization is critical to their respective physiological roles, such as p53 (23), Rb (28) and PTHrP (30). With the implication of dynein in this mechanism, it has also become important to decipher the role of the dynein accessory proteins that mediate cargo recognition specific for AR. This information will enable the development of dynein small molecule inhibitors, which in combination with taxane chemotherapy, could prove significantly beneficial to CRPC patients.
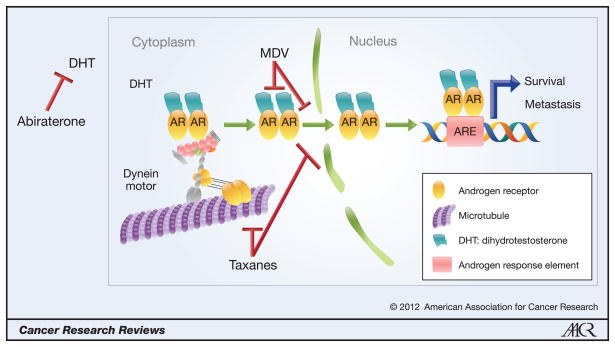
Figure 1. Proposed model of taxane mechanism of action in prostate cancer.
This model represents a novel mechanism of action for taxanes in prostate cancer, which implicate this class of drugs in critical interphase cellular functions such as AR intracellular transport and signaling. In the model, AR associates with microtubules and translocates to the nucleus via the motor protein dynein. This transport is made possible due to the inherent polarity of microtubules, which is recognized by the minus-end-directed motor protein dynein, to transport cargoes towards the nucleus. Taxanes, which bind to and hyperstabilize microtubules, inhibit this trafficking and subsequently prevent AR from reaching the nucleus and activating target genes. This mechanism of action predicts that the combination of a taxane with an inhibitor of AR ligand synthesis (i.e. abiraterone) or with inhibitors of AR ligand interaction (i.e. MDV3100) would be synergistic in the clinical setting as there will be inhibition of AR signaling axis by two different but converging pathways. Additionally, the model predicts that a small molecule inhibitor of dynein, would similarly impair AR nuclear accumulation, and would also be synergistic in combination with a taxane. Finally, the model suggests that a putative small molecule inhibitor targeting the interaction between AR and microtubules or dynein could be used therapeutically for CRPC treatment.
Figure 2. Ligand treatment decreases the association of AR with microtubules.
A microtubule co-sedimentation assay using whole cell lysate from PC3:mCh-tub cells transfected with GFP-AR(wt) was carried out in the presence or absence of the synthetic DHT analog (R1881 10 nM). Briefly, a total of 1 mg of precleared cell extract (HSS) from the transfected cells was incubated for 30 minutes with 10 μM exogenous purified bovine brain tubulin and subjected to a cycle of polymerization with 20 μM paclitaxel at 37°C. The samples were centrifuged at 100,000 g to separate the microtubule polymers (WP) from the soluble tubulin dimers (WS), resolved by SDS-PAGE and immunoblotted for the presence of AR and tubulin. Note that R1881 treatment decreases the amount of AR protein that co-sediments with the microtubule polymer, as can be seen by the shift from 85% to 44% of AR in the WP (%P = 100*WP/(WP+WS). Protein quantification was performed using ImageJ (National Institutes of Health) software. Tubulin was detected as microtubule polymers in the WP fraction in both conditions.
3. Using the microtubule-AR axis to understand clinical taxane resistance in prostate cancer
The model presented in Figure 1 provides a basis for understanding clinical taxane resistance in prostate cancer. Darshan et al. showed that perturbation of the microtubule-AR axis is an important determinant of taxane activity, independent of mitosis, as AR cytoplasmic sequestration in circulating tumor cells isolated from CRPC patients significantly correlated with clinical response to taxane chemotherapy.
Taxanes bind β-tubulin, suppress microtubule dynamics and hyper-stabilize the microtubule cytoskeleton by inducing the formation of microtubule bundles; a hallmark of effective drug-target engagement. Microtubule bundling is the first cellular insult that leads to disruption of downstream pathways. This mechanism implies that taxane chemotherapy should be most effective against tumor types in which microtubule-dependent pathways drive tumor progression, like the AR pathway in prostate cancer.
Despite the success of taxanes in CRPC treatment, their efficacy varies from patient to patient while it remains unclear why individual patients respond to paclitaxel but not docetaxel and vice versa, even though these drugs share the same mechanism of action and a common binding site on β-tubulin. In 2010, the docetaxel-analog cabazitaxel was approved by the FDA for the treatment of CRPC patients who have previously failed docetaxel-based therapy (37). This highlights, once again, the activity of this class of drugs in CRPC while raising the question of what is the molecular basis of clinical taxane resistance. According to the model presented here, clinical taxane resistance could arise as a result of: 1) impaired drug uptake, potentially due to the presence of P-glycoprotein (P-gp) or other drug transporters; 2) impaired binding to β-tubulin, possibly due to the presence of tubulin mutations at the drug binding site or overexpression of βIII tubulin isotype; 3) presence of AR mutations or splice variants that do not require microtubule-based transport; and 4) dysregulation of dynein-cargo interaction. Regarding the first possibility, limited studies have not suggested a significant correlation between P-gp expression and response to taxane treatment in prostate cancer patients (38). Similarly, tubulin alterations such as β-tubulin mutations or altered isotype expression, have not been associated with response to taxane-based therapy in CRPC either (39). Conversely, alterations of AR have been extensively studied in CRPC albeit not in the context of taxane resistance. Recent studies have demonstrated the presence of alternatively spliced AR variants, such as ARv567 and AR-V7 that arise following castration (40–43). These variants lack the ligand-binding domain, are insensitive to ADT and are constitutively active in the nucleus, which allows for continuous AR transcriptional activity. The ARv567 variant was shown to be present in 59% of CRPC patients and to arise in response to ADT or to the newer AR-targeted therapies, such as abiraterone (44). The frequency of this molecular alteration in CRPC makes it imperative to determine whether these variants are under microtubule-control, similar to wild-type AR, and whether they would respond to taxane treatment. Our model predicts that any AR variant lacking the microtubule- or dynein-binding domain would be insensitive to taxane treatment and thereby, has the potential to serve as a predictive biomarker of clinical taxane activity. Finally, regarding the dysregulation of dynein-cargo interaction, our model predicts that any aberration/mutation in the dynein motor protein that impairs cargo (AR) recognition and/or transport should lead to taxane resistance.
4. Therapeutic implications and perspectives
The model presented herein, suggests that simultaneous targeting of different pathways that inhibit AR signaling, may result in greater or more durable anti-tumor effects. Specifically, combination of a taxane, which interferes with AR nuclear translocation, with an inhibitor of androgen synthesis (e.g. abiraterone) or an inhibitor of AR-ligand interaction such as MDV3100, which also inhibits AR nuclear accumulation (by yet an undefined mechanism) (45), could yield enhanced therapeutic efficacy. To this end, a Phase I trial is currently testing the combination of docetaxel and abiraterone in patients with CRPC. More importantly, understanding the precise mechanisms by which prostate cancer cells circumvent AR signaling inhibition will allow development of novel, more targeted therapies. For instance, the development of a small molecule inhibitor of dynein, or of dynein-AR interaction could be added to the space of CRPC therapy. Similarly, the recent identification of an N-terminal-targeted AR inhibitor which has the potential to target both AR wild-type and variants, as the N-terminal domain is conserved (46, 47) should be synergistic in combination with a taxane. A deeper understanding of the mechanisms used by a prostate cancer cell to bypass AR inhibition as well as the cellular factors that regulate AR signaling in CRPC is required in order to develop approaches that will allow men to live with metastatic prostate cancer beyond the 1–2 years typically associated with responses to chemotherapy after ADT.
Summary
In summary, our group’s work highlights the importance of microtubule-dynein dependent trafficking for transcription factors, such as AR, that require rapid and targeted nuclear translocation upon specific stimuli. In addition, this work challenges the existing paradigm whereby the clinical activity of taxanes in prostate cancer is attributed solely to the drugs’ antimitotic effects and highlights the therapeutic importance of the signaling events that are impaired downstream of drug-induced microtubule disruption.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (R01 CA137020-01 and U54 CA143876) (PG), the NIH Physical Sciences Oncology Center at Cornell (PG and DN), the Weill Cornell Clinical and Translational Science Center (DN and PG) a Creativity Award from the Prostate Cancer Foundation (PG and DN) and support from the Genitourinary Oncology Research Fund (DN).
Footnotes
Disclosure of potential conflicts of interest: D.M. Nanus and P. Giannakakou are paid consultants for Sanofi-Aventis. The other author disclosed no potential conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C. The Treatment of Cancer of the Prostate : (The 1943 Address in Surgery before the Royal College of Physicians and Surgeons of Canada) Can Med Assoc J. 1944;50:301–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17:3876–83. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 4.Attard G, Richards J, de Bono JS. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin Cancer Res. 2011;17:1649–57. doi: 10.1158/1078-0432.CCR-10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryce A, Ryan CJ. Development and clinical utility of abiraterone acetate as an androgen synthesis inhibitor. Clin Pharmacol Ther. 2012;91:101–8. doi: 10.1038/clpt.2011.275. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–8. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 7.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 9.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 11.Torres K, Horwitz SB. Mechanisms of Taxol-induced cell death are concentration dependent. Cancer Res. 1998;58:3620–6. [PubMed] [Google Scholar]
- 12.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–17. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 13.Berges RR, Vukanovic J, Epstein JI, CarMichel M, Cisek L, Johnson DE, et al. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–80. [PMC free article] [PubMed] [Google Scholar]
- 14.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011 doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh AK, Steele R, Ray RB. Knockdown of MBP-1 in human prostate cancer cells delays cell cycle progression. J Biol Chem. 2006;281:23652–7. doi: 10.1074/jbc.M602930200. [DOI] [PubMed] [Google Scholar]
- 16.Butterworth KT, McCarthy HO, Devlin A, Ming L, Robson T, McKeown SR, et al. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- and phenotype. Int J Cancer. 2008;123:760–8. doi: 10.1002/ijc.23418. [DOI] [PubMed] [Google Scholar]
- 17.Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors Targeting Mitosis: Tales of How Great Drugs against a Promising Target Were Brought Down by a Flawed Rationale. Clin Cancer Res. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]
- 18.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–22. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 20.Asrih M, Pellieux C, Papageorgiou I, Lerch R, Montessuit C. Role of ERK1/2 activation in microtubule stabilization and glucose transport in cardiomyocytes. Am J Physiol Endocrinol Metab. 2011;301:E836–43. doi: 10.1152/ajpendo.00160.2011. [DOI] [PubMed] [Google Scholar]
- 21.Babich A, Burkhardt JK. Lymphocyte signaling converges on microtubules. Immunity. 2011;34:825–7. doi: 10.1016/j.immuni.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Etienne-Manneville S. From signaling pathways to microtubule dynamics: the key players. Curr Opin Cell Biol. 2010;22:104–11. doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2:709–17. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- 24.Giannakakou P, Nakano M, Nicolaou KC, O’Brate A, Yu J, Blagosklonny MV, et al. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc Natl Acad Sci U S A. 2002;99:10855–60. doi: 10.1073/pnas.132275599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 26.Carbonaro M, O’Brate A, Giannakakou P. Microtubule disruption targets HIF-1alpha mRNA to cytoplasmic P-bodies for translational repression. J Cell Biol. 2011;192:83–99. doi: 10.1083/jcb.201004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbonaro M, Escuin D, O’Brate A, Thadani-Mulero M, Giannakakou P. Microtubules Regulate Hypoxia-inducible Factor-1alpha Protein Trafficking and Activity: IMPLICATIONS FOR TAXANE THERAPY. J Biol Chem. 2012;287:11859–69. doi: 10.1074/jbc.M112.345587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth DM, Moseley GW, Glover D, Pouton CW, Jans DA. A microtubule-facilitated nuclear import pathway for cancer regulatory proteins. Traffic. 2007;8:673–86. doi: 10.1111/j.1600-0854.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 29.Harrell JM, Murphy PJ, Morishima Y, Chen H, Mansfield JF, Galigniana MD, et al. Evidence for glucocorticoid receptor transport on microtubules by dynein. J Biol Chem. 2004;279:54647–54. doi: 10.1074/jbc.M406863200. [DOI] [PubMed] [Google Scholar]
- 30.Lam MH, Thomas RJ, Loveland KL, Schilders S, Gu M, Martin TJ, et al. Nuclear transport of parathyroid hormone (PTH)-related protein is dependent on microtubules. Mol Endocrinol. 2002;16:390–401. doi: 10.1210/mend.16.2.0775. [DOI] [PubMed] [Google Scholar]
- 31.Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan L, Chen S, Wang Y, Watahiki A, Bohrer L, Sun Z, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 2009;69:8386–94. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: implications for PSA surrogacy. Prostate. 2009;69:1579–85. doi: 10.1002/pros.21004. [DOI] [PubMed] [Google Scholar]
- 35.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 36.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 37.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 38.Hamidovic A, Hahn K, Kolesar J. Clinical significance of ABCB1 genotyping in oncology. J Oncol Pharm Pract. 2010;16:39–44. doi: 10.1177/1078155209104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 40.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Inaugural Article: Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Sadar MD. Small molecule inhibitors targeting the “achilles’ heel” of androgen receptor activity. Cancer Res. 2011;71:1208–13. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]