Abstract
We assessed metabolic changes for darunavir/ritonavir (DRV/r) once daily (qd) versus atazanavir/ritonavir (ATV/r) qd with fixed-dose tenofovir/emtricitabine. This was a phase 4, multicenter, open-label, randomized exploratory study. Treatment-naive, HIV-1-infected adults received DRV/r 800/100 mg qd or ATV/r 300/100 mg qd, both with emtricitabine/tenofovir 200/300 mg qd. Primary end point: change in triglyceride levels from baseline to week 12. Secondary end points: week 12 and week 48 changes in lipid parameters, insulin sensitivity, inflammatory/coagulation/bacterial translocation biomarkers, viral load, CD4+ cell count, and week 48 changes in adipose tissue distribution and subjects' perceptions of body changes. In the DRV/r arm, 32/34 and 29/34 subjects completed weeks 12 and 48, respectively; in the ATV/r arm, 30/31 and 25/31 subjects completed weeks 12 and 48, respectively. Small changes in lipid parameters from baseline to weeks 12 and 48 were observed in both arms. Differences were noted between arms in mean changes in total cholesterol (DRV/r, 20.3 mg/dl; ATV/r, 4.6 mg/dl) and apolipoprotein A1 (DRV/r, 10.7 mg/dl; ATV/r, –0.7 mg/dl) at week 12. At week 48, no clinically relevant differences between arms were noted for changes in any lipid parameter, fasting glucose, or insulin sensitivity. Biomarkers generally decreased and efficacy parameters improved in both arms over 48 weeks. Changes in adipose tissue were small and comparable between arms. Subjects' perceptions of body changes generally improved in both study arms. This first pilot comparison in HIV-1-infected subjects suggests that DRV/r has a metabolic profile similar to ATV/r over 48 weeks of treatment. Further randomized studies are warranted.
Introduction
HIV-infected individuals have a heightened risk of serious non-AIDS conditions, such as cardiovascular disease (CVD), compared with uninfected individuals, due to HIV-induced activation of inflammation and coagulation pathways.1,2 Antiretroviral (ARV) agents, including protease inhibitors (PIs), also contribute to this increased risk as a result of their metabolic complications.3 Specifically, some ritonavir-boosted PIs have been associated with a worsening of lipid parameters and increases in inflammatory markers.4–7 Additionally, some PIs have been associated with insulin resistance in HIV-negative healthy volunteers.8–11 However, this association with insulin resistance seems to be ARV-specific, as opposed to class-specific, as studies evaluating other PIs have failed to demonstrate such an association.12,13 The metabolic complications associated with PI-based therapy can develop within the first week after treatment initiation.6,14–18 Commonly prescribed PIs exert differential effects on lipid, glucose, and insulin parameters, and comprehensive assessment of these metabolic effects will clarify the optimal treatment choices.
Thymidine analogs and, to a lesser degree, PIs and nonnucleoside reverse transcriptase inhibitors have also been associated with lipoatrophy and lipodystrophy.5,19–22 In the HIV-1-infected population, lipodystrophy is an umbrella term that encompasses all changes in fat distribution, including lipohypertrophy, which can include accumulation of fat in the neck, chest, back, breasts, and/or abdomen, and lipoatrophy, which may include fat loss from the limbs, buttocks, and/or face.23–25 HIV-1-associated changes in body shape have the potential to impact a subject's quality of life and well-being, and several studies have linked quality of life to survival of HIV-1-infected subjects.26–28 Interestingly, subjects' perceptions of the changes to their body during ARV therapy are not always in accordance with the objective changes measured in a clinical setting.29 Despite this, few studies have specifically investigated subjects' perceptions of changes in their bodies due to ARV therapy.30,31
Darunavir (DRV; PREZISTA, Janssen Therapeutics, Division of Janssen Products, LP, Titusville, NJ), a PI, combined with low-dose ritonavir (DRV/r), has a favorable lipid profile in healthy and treatment-naive subjects.32,33 Another PI, atazanavir (ATV; REYATAZ, Bristol-Myers Squibb, Princeton, NJ), boosted with low-dose ritonavir (ATV/r), has also demonstrated a favorable metabolic profile in treatment-naive subjects.34 The metabolic effects of DRV/r have been shown to be comparable with those of ATV/r in HIV-negative subjects.33 Furthermore, the metabolic effects of these ARVs have not been directly compared in HIV-1-infected, treatment-naive subjects.
Presented here are the week 12 primary end point analysis and the week 48 results of METABOLIK (Metabolic Evaluation in Treatment-naïves Assessing the impact of two BOosted protease inhibitors on LIpids and other marKers), an exploratory study evaluating metabolic outcomes of DRV/r-based therapy compared with those of ATV/r-based therapy in treatment-naive, HIV-1-infected adult subjects. Additionally, we report changes in adipose tissue distribution and subjects' perceptions of body changes over the course of the trial.
Materials and Methods
Study design and treatment
METABOLIK was a 48-week, phase 4, multicenter, open-label, randomized study that assessed changes in fasting lipids, glucose, insulin, insulin sensitivity, biomarkers, and the safety and efficacy of DRV/r-based versus ATV/r-based therapy in HIV-1-infected, treatment-naive adults. Adult subjects were randomized in a 1:1 ratio, stratified by sex, to receive DRV/r 800/100 mg once daily (qd) or ATV/r 300/100 mg qd, both with a fixed-dose combination of emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) 200/300 mg qd. Both DRV and ATV, with ritonavir, were administered within 30 min of a meal to maximize drug exposure.
Subject population
Eligible subjects were at least 18 years old and naive to ARV therapy (≤10 days' previous ARV therapy at any point) with HIV-1 RNA 1000 copies/ml or higher; there were no CD4+ count restrictions. Subjects were required to have demonstrated sensitivity to DRV, ATV, TDF, and FTC by resistance testing (DRV, ATV, and TDF susceptibility determined by Antivirogram, Virco Lab, Inc., Raritan, NJ; FTC susceptibility determined by virco® TYPE HIV-1, Virco Lab, Inc., Raritan, NJ). Exclusion criteria included body mass index greater than 30 kg/m2; fasting glucose greater than 110 mg/dl; low-density lipoprotein (LDL) greater than 130 mg/dl; triglycerides greater than 200 mg/dl; alanine aminotransferase greater than 2.5 times the upper limit of normal; creatinine clearance 50 ml/min/m2 or lower; evidence of significantly decreased hepatic function or decompensation; presence of any Centers for Disease Control and Prevention active AIDS-defining illness (Category C conditions), except stable cutaneous Kaposi's sarcoma or wasting syndrome; acute or chronic hepatitis A, B, or C; grade 3 or 4 laboratory abnormalities; history of significant cardiac, vascular, pulmonary, gastrointestinal, endocrine, neurologic, hematologic, rheumatologic, psychiatric, or metabolic disturbances; use of any non-ARV investigational agents within 90 days of screening; receipt of anabolic steroids, atypical antipsychotics, or growth hormones; use of disallowed concomitant therapy; and pregnancy or breastfeeding. Use of lipid-lowering medications, either prescription (e.g., statins or fibrates) or over-the-counter (e.g., fish oil), was prohibited from 28 days before baseline through week 12 of the trial. The use of lipid-lowering medications was allowed after week 12. All subjects provided written informed consent.
Study evaluations
The primary end point was the change in triglyceride levels from baseline to week 12. Secondary end points included week 12 and week 48 changes in other lipid parameters that included total cholesterol (TC), high-density lipoprotein (HDL), LDL, and apolipoproteins (apo) A1 and B. Lipid parameters were tested for normality. Additional secondary end points assessed at week 12 and week 48 included changes in glucose and insulin levels, insulin sensitivity (as measured by the homeostasis model assessment of insulin resistance [HOMA-IR] method),35 inflammatory biomarkers (interleukin [IL]-1 beta, IL-6, tumor necrosis factor receptor II [TNF RII], high sensitivity C-reactive protein [hs-CRP]), coagulation biomarkers (fibrinogen, d-dimer), and the microbial translocation biomarker lipopolysaccharide (LPS). In addition, polychromatic flow cytometry was used to assess changes in the percentage of CD3+/CD4+ and CD3+/CD8+ T cells, along with their markers of immune activation (CD38+/HLADR+); senescence (CD28−/CD57+); proliferation (Ki67+); and naive/memory subsets (naive, CCR7+/CD45RA+; central memory, CCR7+/CD45RA−; effector memory, CCR7−/CD45RA−; terminal effector, CCR7−/CD45RA+).
For measuring insulin sensitivity, HOMA-IR was calculated using the following formula: {[fasting insulin (μU/ml)]×[fasting glucose (mmol/liter)]}/22.5.36 The lower limits of quantification (laboratory variability; normal reference range) for the biomarker assays are as follows: IL-1 beta, 0.125 pg/ml (6.7%; <0.201 pg/ml); IL-6, 0.2 pg/ml (4.3%; <11.83 pg/ml); TNF RII, 1 pg/ml (2.6%–4.8%; 1003–3170 pg/ml); hs-CRP, 0.15 mg/liter (<2%; <5.00 mg/liter); fibrinogen, 42 mg/dl (<4%; 211–372 mg/dl); and d-dimer, 109 ng/ml (<4%; <201 ng/ml; ICON Central Laboratories Inc., Farmingdale, NY). For the determination of LPS concentration, the QCL-1000 assay (Lonza Biologics Inc., Walkersville, MD), with a sensitivity range of 0.1–1.0 endotoxin units/ml (1 endotoxin unit=100 pg), and low intraassay variability, was used. Change in log10 HIV-1 RNA from baseline, proportion of subjects with virologic response (HIV-1 RNA<50 copies/ml), change in CD4+ cell count through week 48, and safety were also assessed.
For analysis of fat redistribution, computed tomography (CT) scans were performed at the L4–L5 level and mid-thigh at baseline and week 48, and centrally analyzed for total (TAT), subcutaneous (SAT), visceral (VAT), and peripheral (PAT) adipose tissue by BioClinica Imaging Technologies, Inc. (Newtown, PA). The 27-item, self-reported Assessment of Body Change and Distress (ABCD) questionnaire37 was administered at baseline and at weeks 12 and 48 and was composed of three parts. The categorical portion of the questionnaire asked subjects to assign one of five answers, ranging from ”all of the time” to ”none of the time,“ to questions encompassing a wide range of disease-related issues. The yes/no portion of the questionnaire asked subjects to indicate whether they had noticed changes in aspects of their physical appearance. Finally, the questionnaire asked subjects to rate their overall satisfaction with their body. The questionnaire had a recall period of 4 weeks and measured subjects' perceived body changes, physical and emotional distress, social concerns, and health behavioral changes. Responses to the ABCD questionnaire were not considered or reported as adverse events (AEs).
Fasting (at least 8 h) blood samples for laboratory assessments, lipids, and efficacy parameters were taken at weeks 0, 4, 8, 12, 24, 36, and 48, and for biomarker tests at weeks 0, 4, 12, 24, and 48. Lipid parameters, glucose, and insulin assessments were analyzed by ICON Central Laboratories (Farmingdale, NY). Biomarkers were analyzed by Rush University Medical Center (Chicago, IL) and ICON Central Laboratories. The incidence and type of all AEs and serious AEs (SAEs) were recorded from the signing of the informed consent form through the completion of the last study-related procedure. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Statistical analysis
The primary end point for this study was change in triglyceride levels from baseline to week 12. Assuming a standard deviation (SD) of 75 mg/dl for the primary end point and a two-sided 95% confidence interval (CI) with a precision of 42 mg/dl on each side of the estimated difference, it would be required that at least 50 subjects complete the study (25 subjects per treatment arm). To allow for dropouts, an overall sample size of 60 subjects was planned. Descriptive statistics for the preplanned analyses of the primary and secondary end points at week 12 and week 48 are reported. The lipid-evaluable population used for the week 12 and week 48 lipid end points includes subjects completing week 12 who had a baseline value and at least one postdose fasting lipid value and no relevant protocol deviations or violations; lipid analyses were completed using observed values. All other end points use observed values for the intent-to-treat (ITT) population. The lipid and biomarker end points were estimated using mean values with 95% CIs. Response rates were derived using the confirmed virologic response (CVR; HIV-1 RNA<50 copies/ml, confirmed by two consecutive assessments at least 14 days apart) algorithm for the ITT population.
Results
Subject population and baseline characteristics
In total, 34 subjects (29 men) received DRV/r-based regimens and 31 (27 men) received ATV/r-based regimens. In the DRV/r arm, 32 subjects completed week 12 and 29 subjects completed week 48; in the ATV/r arm, 30 and 25 subjects completed week 12 and week 48, respectively. The lipid-evaluable populations included 28 and 27 subjects in the DRV/r and ATV/r arms, respectively. Of five (14.7%) subjects in the DRV/r arm who discontinued prior to week 48, two withdrew consent, one was noncompliant, one was lost to follow-up, and one relocated. Of six (19.4%) subjects in the ATV/r arm who discontinued early, two discontinued due to AEs (one with grade 3 leukocytoclastic vasculitis and one with grade 1 increased blood creatinine), one discontinued due to pregnancy, one discontinued because of investigational product dispensing error, one was lost to follow-up, and one withdrew consent. At baseline, DRV/r subjects had higher mean log10 baseline viral loads, lower median CD4+ counts, and lower TC and LDL levels compared with ATV/r subjects (Tables 1 and 2).
Table 1.
Baseline Demographics and Disease Characteristics
| Parameter | DRV/r n=34 | ATV/r n=31 |
|---|---|---|
| Male, n (%) | 29 (85.3) | 27 (87.1) |
| Age, median (range), years | 36.5 (19.0–58.0) | 35.0 (20.0–65.0) |
| Race, n (%) | ||
| Asian | 0 | 2 (6.5) |
| Black | 13 (38.2) | 17 (54.8) |
| White | 21 (61.8) | 12 (38.7) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 7 (20.6) | 7 (22.6) |
| BMI, mean (SD) | 23.8 (3.1) | 24.5 (3.6) |
| Worst clinical stage of HIV infection, n (%) | ||
| A | 30 (88.2) | 26 (83.9) |
| B | 4 (11.8) | 4 (12.9) |
| C | 0 | 1 (3.2) |
| CD4+ count, median (range), cells/mm3 | 267 (10–532) | 316 (39–813) |
| Viral load, mean (SD), log10 copies/ml | 5.0 (0.8) | 4.6 (0.7) |
| Viral load, median (range), copies/ml | 137,000 (642–2,450,000) | 46,100 (397–637,000) |
DRV/r, darunavir/ritonavir; ATV/r, atazanavir/ritonavir; BMI, body mass index; SD, standard deviation.
Table 2.
Changes in Primary and Secondary Lipid End Points, Glucose, Insulin, Insulin Sensitivity, and Inflammatory/Coagulation Biomarkers over 12 and 48 Weeks (Observed Values)
| |
DRV/r n=28 |
ATV/r n=27 |
|
||||
|---|---|---|---|---|---|---|---|
| |
BL |
Change from BL at week 12 |
BL |
Change from BL at week 12 |
Difference in week 12 mean change between arms (95% CI) |
||
|
Primary end point,amean (SD) | |||||||
| TG, mg/dl |
113.7 (57.4) |
22.0 (62.7) |
114.2 (84.1) |
8.1 (81.2) |
13.8 (−25.8, 53.4) |
||
| BL | Change from BL at week 12 | Change from BL at week 48 | BL | Change from BL at week 12 | Change from BL at week 48 | Difference in week 48 mean change between arms (95% CI) | |
| Secondary lipid end points,amean (SD) | |||||||
| TG, mg/dl | 113.7 (57.4) | 22.0 (62.7) | 26.1 (69.0) | 114.2 (84.1) | 8.1 (81.2) | 9.6 (73.7) | 16.5 (−25.0, 58.0) |
| TC, mg/dl | 141.8 (28.3) | 20.3 (30.5) | 22.3 (30.7) | 165.1 (30.0) | 4.6 (26.7) | 11.8 (31.9) | 10.5 (−7.7, 28.8) |
| LDL, mg/dl | 84.6 (21.9) | 13.6 (25.1) | 14.7 (25.9) | 100.2 (23.9) | 9.6 (20.8) | 13.9 (27.1) | 0.8 (−14.6, 16.3) |
| HDL, mg/dl | 37.9 (13.4) | 6.6 (11.6) | 6.0 (7.4) | 45.0 (13.6) | 2.2 (8.7) | 3.7 (9.9) | 2.3 (−2.8, 7.3) |
| TC/HDL ratio | 4.1 (1.1) | −0.1 (0.9) | 0.1 (1.06) | 3.9 (1.0) | −0.1 (0.7) | −0.1 (0.75) | 0.2 (−0.3, 0.8) |
| ApoA1, mg/dl | 114.9 (25.7) | 10.7 (21.3) | 12 (16) | 127.6 (21.9) | −0.7 (17.8) | 3 (19) | 9.7 (−0.5, 19.8) |
| ApoB, mg/dl | 74.5 (19.0) | −0.4 (20.0) | 4 (21) | 81.7 (18.5) | −4.9 (16.2) | 2 (17) | 2.0 (−9.3, 13.4) |
| ApoB/ApoA1 ratio | 0.68 (0.20) | −0.06 (0.17) | −0.01 (0.20) | 0.65 (0.16) | −0.04 (0.16) | 0.01 (0.14) | −0.02 (−0.127, 0.079) |
| Glucose, insulin, and HOMA-IR,bmean (SD) | |||||||
| Glucose, mg/dl | 88.5 (12.37) | 1.5 (12.52) | 2.8 (9.10) | 89.7 (10.84) | 5.8 (14.55) | 6.4 (22.07) | −3.6 (−12.8, 5.6) |
| Insulin, μIU/ml | 6.0 (5.57) | −1.1 (4.97) | 1.0 (6.01) | 8.6 (14.28) | 0.7 (18.79) | −2.9 (16.73) | 3.8 (−3.0, 10.6) |
| HOMA-IR | 1.6 (1.70) | −0.5 (2.02) | 0.04 (2.26) | 2.9 (6.02) | 0.1 (7.51) | −1.24 (8.01) | 1.3 (−2.7, 5.2) |
| Biomarkers,bmean (SD) | |||||||
| IL-1 beta, pg/ml | 0.2 (0.32) | 0.01 (0.22) | 0.3 (1.45) | 0.3 (0.33) | −0.01 (0.28) | −0.1 (0.28) | 0.41 (−0.23, 1.04) |
| IL-6, pg/ml | 1.9 (1.90) | −0.6 (2.88) | 0.2 (7.30) | 1.0 (1.32) | 1.5 (6.34) | 0.3 (0.94) | −0.08 (−3.24, 3.08) |
| hs-CRP, mg/liter | 3.1 (5.16) | −0.6 (5.97) | 1.2 (11.20) | 2.2 (2.50) | 0.7 (4.17) | 0.6 (5.09) | 0.65 (−4.55, 5.85) |
| d-dimer, ng/ml | 373.0 (580.45) | −181.6 (580.91) | −192.1 (586.55) | 189.0 (111.43) | 51.0 (635.25) | −24.1 (143.98) | −168.0 (−432.2, 96.2) |
| Fibrinogen, g/liter | 3.3 (1.05) | −0.5 (1.12) | −0.3 (1.08) | 3.2 (0.70) | −0.1 (0.89) | −0.3 (0.90) | 0.02 (−0.57, 0.61) |
| TNF RII, pg/ml | 4207 (1701.7) | −1456 (1518.5) | −1384 (1722.3) | 2957 (727.2) | −562 (529.6) | −442 (722.2) | −942.1 (−1735.3, −149.0) |
| LPS, pg/ml | 85.3 (29.2) | −2.5 (38.4) | −18.4 (34.9) | 86.2 (30.0) | −6.9 (25.3) | −17.0 (50.6) | −1.4 (−25.6, 22.9) |
Sample size varies by time point and parameter.
Lipid-evaluable population.
Intent-to-treat population.
DRV/r, darunavir/ritonavir; ATV/r, atazanavir/ritonavir; CI, confidence interval; BL, baseline; SD, standard deviation; TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Apo, apolipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IL, interleukin; hs-CRP, high-sensitivity C-reactive protein; TNF RII, tumor necrosis factor receptor II; LPS, lipopolysaccharide.
Over the course of the study, the median duration of exposure to DRV or ATV was similar in the DRV/r (337 days) and ATV/r (336 days) arms. No subjects received lipid-lowering medications after week 12.
Lipid evaluations
Primary end point: week 12 change in triglyceride levels
From baseline to week 12, triglyceride levels increased by a mean of 22.0 mg/dl (SD: 62.7) in the DRV/r arm and 8.1 mg/dl (SD: 81.2; Table 2) in the ATV/r arm. The difference in week 12 mean change (95% CI) between the DRV/r and ATV/r arms was 13.8 (–25.8, 53.4). A sensitivity analysis was conducted on the changes in triglyceride levels using normalized (natural log) triglyceride data, yielding similar results.
Secondary end points
Small changes in other lipid parameters were noted with DRV/r therapy from baseline to week 12 (Table 2). Between arms, differences in lipid changes were seen only in TC and apoA1, with the DRV/r arm experiencing greater changes in both parameters than the ATV/r arm. The difference in week 12 mean change between arms (95% CI) was 15.7 mg/dl (0.0, 31.3) for TC (20.3 mg/dl in the DRV/r group and 4.6 mg/dl in the ATV/r group) and 11.4 mg/dl (0.7, 22.1) for apoA1 (10.7 mg/dl in the DRV/r group and –0.7 mg/dl in the ATV/r group) (Table 2). The actual mean TC values at week 12 were 161.5 mg/dl and 169.7 mg/dl for DRV/r and ATV/r, respectively, while the actual mean apoA1 values at week 12 were 125.6 mg/dl and 126.8 mg/dl for DRV/r and ATV/r, respectively. Consistent with the week 12 results, no clinically meaningful difference was seen between arms for mean changes in triglyceride levels at week 48. In contrast to the week 12 results, changes in TC and apoA1 were similar between arms by week 48 (Table 2). Additionally, no clinically relevant differences between arms were noted for changes in the other fasting lipid parameters, including the apoB/apoA1 ratio, at week 48 (Table 2). Changes from baseline for the lipid parameters were generally normally distributed.
Other laboratory evaluations and biomarkers
No clinically relevant changes were seen from baseline to week 12, or baseline to week 48, in glucose, insulin, and insulin sensitivity with DRV/r or ATV/r therapy (Table 2). Biomarkers of inflammation, coagulation and microbial translocation generally decreased from baseline to week 12 in both treatment arms (Table 2). From baseline to week 48, fibrinogen, d-dimer, TNF RII, and LPS decreased in both treatment arms, while hs-CRP and IL-6 demonstrated small increases in both arms (Table 2). Small increases in IL-1 beta were seen in the DRV/r arm, whereas small decreases were observed in the ATV/r arm, from baseline to week 48. At both time points, differences were noted between arms for changes in TNF RII, with the DRV/r arm experiencing greater decreases than the ATV/r arm.
Adipose tissue distribution
At baseline, subjects in the ATV/r arm had higher mean values for all CT scan parameters compared with subjects in the DRV/r arm (Table 3). Changes in TAT, VAT, SAT, and the SAT/VAT ratio from baseline to week 48 were small and comparable between the DRV/r and ATV/r arms (Table 3). Although the mean change in PAT over 48 weeks was larger in the DRV/r arm than in the ATV/r arm (Table 3), none of the changes in adipose tissue distribution over 48 weeks was considered clinically relevant.
Table 3.
Change in Adipose Tissue Distribution from Baseline to Week 48
| |
DRV/r n=34 |
ATV/r n=31 |
|
||
|---|---|---|---|---|---|
| BL | Change from BL to week 48 | BL | Change from BL to week 48 | Difference in mean change between arms (95% CI) | |
| Abdomen | n=29 | n=20 | n=30 | n=24 | |
| TAT, mean (SD), cm2 | 258.7 (89.38) | 24.2 (98.40) | 285.1 (120.80) | 29.4 (38.32) | −5.20 (−49.19, 38.79) |
| VAT, mean (SD), cm2 | 97.6 (47.10) | −0.6 (49.20) | 102.5 (44.60) | 3.8 (24.48) | −4.38 (−27.43, 18.67) |
| SAT, mean (SD), cm2 | 161.1 (74.06) | 24.8 (56.50) | 182.7 (108.37) | 25.6 (28.20) | −0.82 (−27.31, 25.67) |
| VAT/SAT ratio, mean (SD) | 0.50 (0.281) | 0.00 (0.135) | 0.53 (0.289) | −0.04 (0.104) | 0.04 (−0.03, 0.11) |
| Mid-thigh | n=29 | n=21 | n=27 | n=21 | |
| PAT, mean (SD), cm2 | 55.4 (25.74) | 7.0 (17.32) | 61.0 (38.06) | −1.9 (9.53) | 8.88 (0.16, 17.60) |
DRV/r, darunavir/low-dose ritonavir; ATV/r, atazanavir/low-dose ritonavir; CI, confidence interval; BL, baseline; TAT, total adipose tissue; SD, standard deviation; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; PAT, peripheral adipose tissue.
Assessment of Body Change and Distress questionnaire
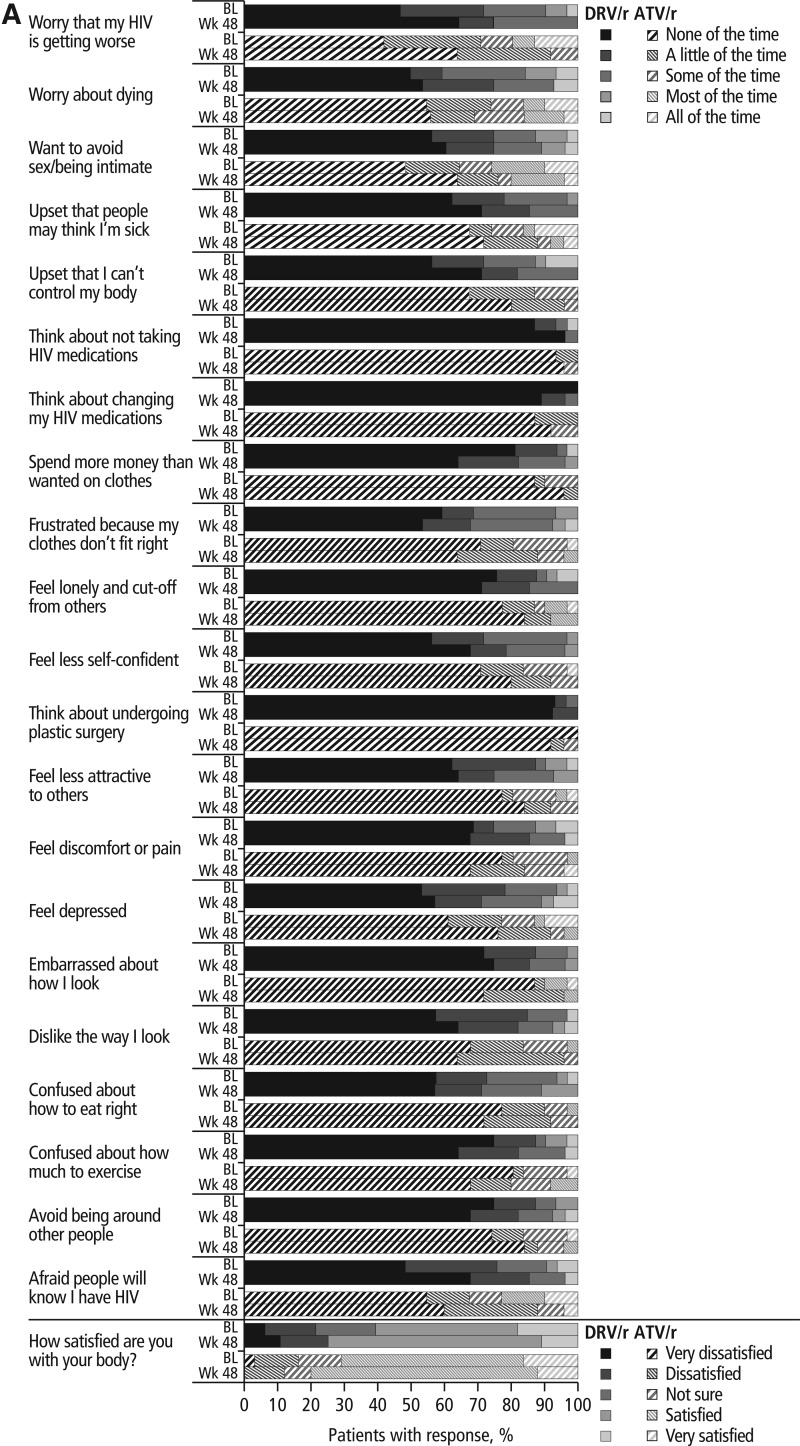
Subjects' perceptions of body changes generally improved from baseline to week 48 in both study arms. Overall, higher proportions of subjects in both arms were “satisfied” or “very satisfied” with their bodies after 48 weeks compared with baseline (Fig. 1A). Within each study arm, responses to the categorical portion of the ABCD questionnaire were similar at baseline and at week 48, with few subjects switching categories. Furthermore, these categorical responses were also similar between the DRV/r and ATV/r arms at both time points.
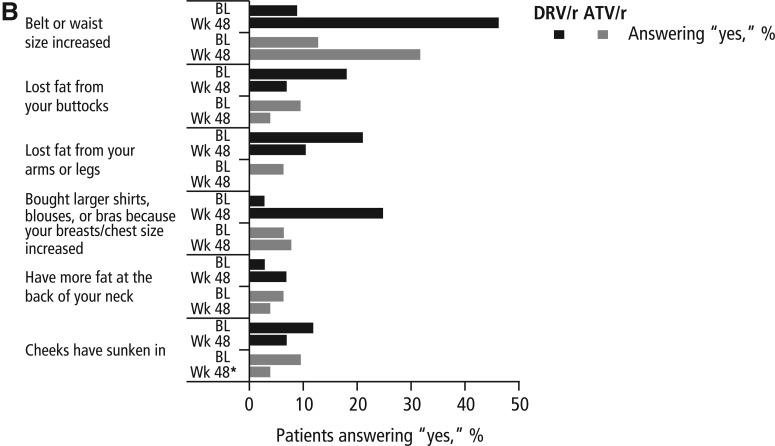
FIG. 1.
Results of the Assessment of Body Change and Distress questionnaire: (A) categorical and (B) yes versus no. *Data were missing from one ATV/r subject at week 48; DRV/r, darunavir/lowdose ritonavir; ATV/r, atazanavir/low-dose ritonavir; BL, baseline; Wk, week.
In the yes/no portion of the ABCD questionnaire (Fig. 1B), more subjects in both the DRV/r and ATV/r arms reported increases in waist size at week 48 compared with baseline (DRV/r, 46.4% vs. 9.1%; ATV/r, 32.0% vs. 12.9%). Fewer subjects in both the DRV/r and ATV/r arms reported that they had lost fat in their buttocks or in their arms and legs or that their cheeks had sunken in at week 48, compared with baseline. Slightly more subjects in the DRV/r arm and slightly fewer subjects in the ATV/r arm reported gaining fat at the back of their neck at week 48 compared with baseline. Although responses to this portion of the questionnaire were generally similar between arms, more subjects in the DRV/r arm reported increases in waist and chest size at week 48 compared with subjects in the ATV/r arm (Fig. 1B).
Efficacy evaluations
Viral load and CD4+ cell count improved over the course of the study. Mean viral load decreased and mean CD4+ count increased from baseline to week 48 in both the DRV/r (change in log10 viral load, –3.3 copies/ml; change in CD4+ count, +217.4 cells/mm3) and ATV/r arms (change in log10 viral load, –2.9 copies/ml; change in CD4+ count, +205.3 cells/mm3). At week 48, 76.5% of DRV/r and 71.0% of ATV/r subjects (CVR) achieved virologic response.
Cellular activation and senescence
The proportion of CD4+ and CD8+ cells displaying markers of cellular activation (HLADR+/CD38+), proliferation (Ki67+), and senescence (CD28−/CD57+) generally decreased from baseline to week 48 (Table 4). There was also a general increase in the percent of naive CD4+ and CD8+ T cells in both treatment arms.
Table 4.
Markers of Immune Activation and Senescence over 48 Weeks (Intent-to-Treat Population, Observed Values)
| |
DRV/r |
ATV/r |
||
|---|---|---|---|---|
| Parameter, mean % (SD) | BL | Week 48 | BL | Week 48 |
| CD4+ | ||||
| Naive (CCR7+/CD45RA+) | 47.9 (21.14) | 54.7 (19.55) | 38.9 (21.10) | 49.1 (20.91) |
| Central memory (CCR7+/CD45RA−) | 11.9 (8.45) | 14.2 (9.37) | 19.1 (14.49) | 15.0 (7.22) |
| Terminal effectors (CCR7−/CD45RA+) | 10.9 (5.50) | 9.4 (3.43) | 12.1 (8.78) | 11.4 (7.88) |
| Effector memory (CCR7−/CD45RA−) | 29.3 (18.85) | 21.7 (16.76) | 30.0 (12.03) | 24.5 (15.00) |
| CD38+/HLADR+ | 13.6 (7.10) | 8.5 (4.50) | 13.0 (10.82) | 10.9 (5.44) |
| Ki67+ | 1.9 (1.52) | 0.9 (0.86) | 1.6 (1.55) | 1.0 (0.97) |
| CD28−/CD57+ | 7.7 (12.75) | 1.7 (1.50) | 9.1 (14.72) | 3.5 (5.57) |
| CD8+a | ||||
| Naive (CCR7+/CD45RA+) | 13.6 (10.09) | 25.4 (15.63) | 15.4 (9.89) | 26.1 (12.33) |
| Central memory (CCR7+/CD45RA−) | 12.37 (12.43) | 8.7 (8.70) | 16.2 (10.32) | 8.5 (7.11) |
| Terminal effectors (CCR7−/CD45RA+) | 26.4 (14.91) | 27.2 (11.07) | 26.0 (16.67) | 33.9 (14.19) |
| Effector memory (CCR7−/CD45RA−) | 47.7 (14.85) | 38.7 (14.42) | 42.4 (14.85) | 31.4 (11.40) |
| CD38+/HLADR+ | 43.8 (17.75) | 19.4 (12.49) | 40.5 (17.51) | 22.2 (11.05) |
| Ki67+ | 2.8 (1.75) | 1.2 (0.75) | 2.3 (2.06) | 1.2 (0.78) |
| CD28−/CD57+ | 32.1 (11.81) | 19.6 (12.32) | 31.8 (15.02) | 22.8 (15.06) |
Sample size varies by time point and parameter.
CD8+ cell subpopulations were based on CD8+ percentages and were not directly measured.
SD, standard deviation; DRV/r, darunavir/ritonavir; ATV/r, atazanavir/ritonavir; BL, baseline; HLADR, human leukocyte antigen-DR.
Safety evaluations
Over 48 weeks, rates of AEs were generally low and comparable between the DRV/r and ATV/r arms (Table 5), except grade 2–4 hyperbilirubinemia (considered at least possibly related to study drug), which was observed in more ATV/r subjects than DRV/r subjects. Increased total bilirubin reported as a grade 2–4 laboratory abnormality was observed far more frequently in the ATV/r arm compared with the DRV/r arm (Table 5). No other major differences in grade 2–4 safety parameters were noted between arms. A total of 10 subjects (Table 5) experienced 16 SAEs. Three events, chronic obstructive pulmonary disease with respiratory distress (DRV/r arm), diabetes mellitus (DRV/r arm), and mitral valve incompetence (ATV/r arm), were grade 4 in severity; all three were considered not related to study medication. Of the 16 SAEs, only one event, grade 3 pancreatitis in a subject in the DRV/r arm, was considered possibly related to study medication. No clinically relevant changes in creatinine clearance were seen from baseline to week 48 in the DRV/r [mean (SD) change: –0.00 (0.288) ml/min] or ATV/r [mean (SD) change: –0.03 (0.244) ml/min] arm.
Table 5.
Safety Parameters over 48 Weeks
| DRV/r n=34 | ATV/r n=31 | |
|---|---|---|
| Adverse events, n (%) | ||
| Subjects with ≥1 AE | 31 (91.2) | 29 (93.5) |
| Subjects with ≥1 SAE | 5 (14.7) | 5 (16.1) |
| Subjects with ≥1 grade 3–4 AE | 3 (8.8) | 13 (41.9) |
| Subjects with ≥1 AE at least possibly related to study drug | 15 (44.1) | 22 (71.0) |
| Grade 2–4 AEs at least possibly related to study drug,an (%) | ||
| Hyperbilirubinemia | 0 | 3 (9.7) |
| Ocular icterus | 0 | 2 (6.5) |
| Pollakiuria | 0 | 2 (6.5) |
| Neutropenia | 0 | 1 (3.2) |
| Diarrhea | 1 (2.9) | 0 |
| Pancreatitis | 1 (2.9) | 0 |
| Dehydration | 1 (2.9) | 0 |
| Hypercholesterolemia | 1 (2.9) | 0 |
| Joint swelling | 0 | 1 (3.2) |
| Acute renal failure | 1 (2.9) | 0 |
| Dermatitis | 0 | 1 (3.2) |
| Leukocytoclastic vasculitis | 0 | 1 (3.2) |
| Grade 2–4 laboratory abnormalities, n (%) | ||
| Glucose | 4 (11.8) | 3 (9.7) |
| Total cholesterol | 3 (8.8) | 1 (3.2) |
| Neutrophils | 2 (5.9) | 4 (12.9) |
| Low-density lipoprotein (direct) | 2 (5.9) | 3 (9.7) |
| Aspartate aminotransferase | 2 (5.9) | 0 |
| Total bilirubin | 1 (2.9) | 27 (87.1) |
| Creatinine | 1 (2.9) | 1 (3.2) |
| Sodium | 1 (2.9) | 0 |
| Triglycerides | 0 | 1 (3.2) |
Adverse events reported as laboratory abnormalities are not included.
DRV/r, darunavir/ritonavir; ATV/r, atazanavir/ritonavir; AE, adverse event; SAE, serious adverse event.
Discussion
These results illustrate that changes in triglyceride levels over 12 weeks of treatment were similar in subjects treated with DRV/r-based and ATV/r-based regimens. Additionally, small changes in lipids and insulin sensitivity, decreases in biomarkers, small changes in body fat, improvements in efficacy parameters, and a low incidence of AEs were seen over 48 weeks in both treatment arms. In the DRV/r arm, the increase in apoA1, the major component of HDL, indicates favorable lipid changes that support the return-to-health phenomenon observed in subjects with lower CD4+ cell counts initiating ARV therapy.38 The small changes in lipids seen in this trial with DRV/r are in agreement with results from the ARTEMIS (AntiRetroviral Therapy with TMC114 ExaMined In naïve Subjects) trial of 689 treatment-naive subjects, which showed that once-daily DRV/r had a more favorable metabolic profile compared with that of lopinavir (LPV)/r.39
Subjects infected with HIV-1 have increased levels of hs-CRP, TNF alpha (TNF-α), IL-6, d-dimer, and other biomarkers compared with HIV-negative subjects.2,40–42 These elevations in biomarkers persist even after virologic suppression, likely due to HIV-induced activation of inflammation and coagulation pathways.2 Levels of TNF-α, IL-6, hs-CRP, and other proinflammatory cytokines are associated with HIV-1 viral load and may predict disease progression, as well as correlate with a higher risk of CVD and all-cause mortality.42–45 Our findings corroborate another recent analysis, which reported that only d-dimer, and not IL-6 or hs-CRP, is reduced over the short term in those initiating ARV therapy.46 Additionally, the reductions in LPS observed here are similar in magnitude to those seen in a previous study47; the similar reductions in the DRV/r and ATV/r arms suggest that these ARV agents do not result in differential levels of LPS, a trigger of persistent immune activation in ARV-treated individuals. In contrast, no decreases in hs-CRP were seen in either arm of this study over 48 weeks; this observation is in agreement with the ACTG (AIDS Clinical Trials Group) A5095 study, which demonstrated similar results in subjects receiving efavirenz-based regimens over 96 weeks.48
During HIV infection, chronic viremia induces progressive immune dysregulation characterized broadly by a decrease in CD4+ cells and an increase in CD8+ cells and specifically by elevated expression of CD38 and HLADR. This persistent immune activation yields rapid and elevated lymphocyte turnover and a shift to an immunosenescent phenotype (CD28−/CD57+ T cells).49,50 As expected, based on the results of previous studies demonstrating the mitigating effect of ARV therapy on these processes,51–53 the current study found reductions in the proportion of activated T cells (CD38−/HLADR+) in both the DRV/r and ATV/r arms. Furthermore, the decline in the proportion of cells with an immunosenescent phenotype (CD28−/CD57+) illustrates the relationship between immune activation and immunosenescence and a critical immunologic benefit of ARV therapy.
Individuals infected with HIV-1 have a higher risk of serious, non-AIDS conditions than do uninfected subjects.1 Given that HIV infection is now considered a manageable chronic disease, there is a growing level of attention focused on the need for identification of metabolically favorable ARVs. Atazanavir has generally been considered to have the most favorable metabolic profile among PIs34 and was, therefore, chosen as a comparator drug for this study. In the study presented here, changes in metabolic parameters and biomarkers from baseline with DRV/r were comparable to changes observed with ATV/r. These results are in agreement with those seen in the TMC114-C159 trial, which investigated metabolic changes in healthy subjects treated with DRV/r or ATV/r over 28 days and observed similar mean changes in lipid and glucose parameters between treatment groups.33 The TMC114-C159 trial did report significant differences in the changes between treatment arms for insulin and the TC/HDL ratio; these results, however, were not observed in METABOLIK, which noted differences between arms only in changes in TNF RII over 48 weeks.
The effect of some boosted PIs on insulin sensitivity remains controversial. In contrast to some other PIs,8,10,11 no clinically significant changes were seen in insulin sensitivity in either the DRV/r or ATV/r arm of this study. Treatment with indinavir and treatment with LPV/r have both been associated with the development of insulin resistance in healthy subjects9–11; however, other studies have demonstrated that LPV/r does not affect insulin sensitivity in healthy subjects.12,13 Atazanavir has generally had little effect on insulin sensitivity in previous trials of HIV-negative subjects,10,12 and switching from other PI-based therapies to ATV/r has been shown to improve insulin sensitivity in HIV-1-infected subjects.54 The results from this trial suggest that DRV/r, likewise, has little impact on insulin sensitivity. It should be noted that unlike the other studies cited here, which used the euglycemic, hyperinsulinemic clamp technique, this study used HOMA-IR as a measure of insulin resistance. However, several studies have demonstrated that results obtained using HOMA-IR correlate well with results using the euglycemic, hyperinsulinemic clamp technique.36,55
Slight reductions in creatinine clearance were seen from baseline to week 12 in both treatment arms of this study; however, these reductions were no longer apparent after 48 weeks of treatment. A previous study suggested an association between the use of ATV, TDF, or indinavir and creatinine clearance, as indicated by a persistent reduction in glomerular filtration rate over time.56 The biological explanations for these findings are unclear, but may include glomerular dysfunction, high renal excretion rates, and/or crystalluria.56 Though the current study observed modest changes over 48 weeks, the ARTEMIS study did not show any changes in creatinine clearance over 96 weeks,39 suggesting that long-term use of DRV/r has little effect on creatinine clearance.
The small increases in TAT and SAT seen in this study are similar to those seen in previous trials of boosted PIs and nucleoside reverse transcriptase inhibitors (NRTIs) in treatment-naive subjects.19–22,24 Increases in abdominal fat and waist size have been associated with increased cardiovascular risk factors in HIV-1-infected subjects, suggesting a need to monitor even small changes in fat distribution in this population. The increase in PAT seen with DRV/r treatment in this study was in contrast to data from other studies, which have reported decreases in PAT in HIV-1-infected individuals receiving other ARVs.19,20,22 Although some studies have demonstrated an association between lipoatrophy and use of specific PIs or NRTIs,19,22 others have shown no ARV-specific effects,20 and it has been observed that HIV-1-infected subjects, in general, have lower levels of PAT compared with uninfected control subjects.57 The increase in PAT in the DRV/r arm of this study, which had more advanced disease at baseline compared with the ATV/r arm, can be considered as potentially favorable; however, the small sample size and short duration of this trial limit clinical interpretation of these data.
In this study, despite slight increases in VAT and SAT, subjects' perceptions of their body changes generally improved or remained constant over time. The results from the ABCD questionnaire are in line with those from a previous study, which demonstrated improvements in ABCD scores over 48 weeks of DRV/r-based therapy.30 In both treatment arms, the question “In the past 4 weeks has your belt or waist size increased?” had the greatest increase in subjects answering “yes” at 48 weeks compared with baseline; this result is consistent with the increases in VAT and SAT seen during the trial. Although changes in adipose tissue distribution over the study period were similar between arms, more DRV/r subjects reported increases in waist and chest size compared with ATV/r subjects. This discrepancy may indicate subtle differences in lipodystrophy and lipoatrophy between the two treatment regimens, or may have been partially due to the differences in racial distribution between the two study arms.
Both regimens had favorable safety profiles, with low incidences of AEs and laboratory abnormalities; the increased incidence of grade 2–4 hyperbilirubinemia or grade 2–4 increased total bilirubin in the ATV/r arm was expected, as they are known side effects of ATV/r-based therapy.34,58 Similar to the results obtained here, safety results from ARTEMIS suggested low rates of grade 2–4 AEs, including gastrointestinal and renal AEs, in subjects receiving DRV/r over 96 weeks.39 Subjects receiving DRV/r in ARTEMIS had a significantly lower rate of diarrhea compared with LPV/r subjects (4% vs. 11%, respectively) and, in line with results from this trial, no clinically relevant changes were seen in creatinine clearance in either treatment arm.
Interpretation of the data reported in this pilot study may be limited by the small sample size, as well as the fact that the trial was not powered to test for statistical significance, but was rather intended to be an exploratory analysis. The variation seen between arms in certain baseline characteristics, despite the randomized study design, is likely related to the small sample size. Somewhat larger changes in TC and apoA1 with DRV/r versus ATV/r, particularly at week 12, are likely due to lower baseline values of these parameters in the DRV/r arm. Likewise, the larger reduction in TNF RII is likely due to the higher baseline value in the DRV/r arm. Additionally, it should be noted that subjects with abnormal lipid or glucose levels were excluded from the trial and may not, therefore, be reflective of the overall HIV-1-infected population. Despite these limitations, the equally favorable metabolic profile observed with DRV/r-based therapy when compared with ATV/r-based therapy warrants further investigation. It is noteworthy that the ACTG is conducting a large study of approximately 1800 treatment-naive, HIV-1-infected subjects receiving DRV/r, ATV/r, or raltegravir (all receiving fixed-dose FTC/TDF in the background regimen); end points from this study include changes in metabolic parameters over 144 weeks (clinicaltrials.gov identifier: NCT00811954). This study is statistically powered to definitively evaluate whether DRV/r-based therapy and ATV/r-based therapy are characterized by similarly favorable metabolic profiles.
Acknowledgments
The authors would like to thank the subjects and their families, the study sites, and the principal investigators for their participation in the trial. The authors would like to acknowledge Gilead for supplying emtricitabine, tenofovir, and emtricitabine/tenofovir. The authors would additionally like to acknowledge internal study support staff, as well as Cali Howitt, PhD, Medicus International New York, for her editorial assistance. Funding for the study and for editorial support was provided by Janssen Therapeutics.
Original Presentations of These Data
Aberg J, et al.: METABOLIK (Metabolic Evaluation in Treatment-naïves Assessing the impact of two BOosted protease inhibitors on LIpids and other marKers): Comparison of the Metabolic Effects of Darunavir/Ritonavir versus Atazanavir/Ritonavir over 12 Weeks. XVIII International AIDS Conference (IAC), Vienna, Austria, July 18–23, 2010. Poster WEPE011.
Overton T, et al.: METABOLIK: Week 48 Comparison of Metabolic Parameters and Biomarkers in Subjects Receiving Darunavir/Ritonavir or Atazanavir/Ritonavir. Presented at the 10th International Congress on Drug Therapy and HIV Infection (HIV10), Glasgow, UK, November 7–11, 2010. Poster P74
Tebas P, et al.: METABOLIK (Metabolic Evaluation in Treatment-naïves Assessing the impact of two BOosted protease inhibitors on LIpids and other marKers): Week 48 Comparison of Body Fat Changes in ARV-Naïve Subjects Receiving Darunavir/Ritonavir- or Atazanavir/Ritonavir-Based Therapy. Presented at the 12th International Workshop on Adverse Drug Reactions and Co-morbidities in HIV (ADRL), London, UK, November 4–6, 2010. Poster P07.
Author Disclosure Statement
J.A.A. has served as a scientific advisor to Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline, Merck & Co, Inc., Pfizer Inc., Theratechnologies Inc., Janssen Therapeutics, and ViiV Healthcare and has received research support from Gilead Sciences, Inc., GlaxoSmithKline, Merck & Co, Inc., Pfizer Inc., Schering-Plough Corp, Theratechnologies Inc., Janssen Therapeutics, Virco Lab, Inc., and Wyeth. P.T. has received grant support from Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline, Inovio Pharmaceuticals, Inc., Merck & Co, Inc., Janssen Therapeutics, and VIRxSYS. E.T.O. has served as a consultant, on a speakers bureau or on an advisory board for Gilead Sciences, Inc., Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck & Co., Inc., Monogram Sciences, and Boehringer Ingelheim and has received research support from Abbott Laboratories, Gilead Sciences, Inc., Bavarian Nordic, GlaxoSmithKline, Boehringer Ingelheim, and Janssen. S.K.G. has received grant/research support from Janssen Therapeutics, Merck & Co., Inc., and Gilead Sciences, Inc. and is the site Principal Investigator on trials sponsored by TaiMed, Pfizer Inc., and GlaxoSmithKline. P.E.S. has served as a consultant for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Inc., GlaxoSmithKline, Merck & Co, Inc., and Janssen Therapeutics and has received grant support from Gilead Sciences, Inc., GlaxoSmithKline, Merck & Co, Inc., and Janssen Therapeutics. A.L. has served as a scientific advisor to Merck, Pfizer, and Tobira and has received research support from Merck & Co., Inc., Abbott Laboratories, and Janssen Therapeutics. R.F. is an employee of Janssen Therapeutics and a Johnson & Johnson stockholder. R.R. is an employee of Janssen R&D and is a Johnson & Johnson stockholder. G.D.L.R. is an employee of Janssen Global Services and is a Johnson & Johnson stockholder.
References
- 1.Phillips AN. Neaton J. Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhaus J. Jacobs DR. Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Moller N. Reiss P. Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 4.Flint OP. Noor MA. Hruz PW, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: Cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37:65–77. doi: 10.1177/0192623308327119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haubrich RH. Riddler SA. DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein JH. Komarow L. Cotter BR, et al. Lipoprotein changes in HIV-infected antiretroviral-naive individuals after starting antiretroviral therapy: ACTG Study A5152s Stein: Lipoprotein Changes on Antiretroviral Therapy. J Clin Lipidol. 2008;2:464–471. doi: 10.1016/j.jacl.2008.08.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H. Jarujaron S. Gurley EC, et al. HIV protease inhibitors increase TNF-alpha and IL-6 expression in macrophages: Involvement of the RNA-binding protein HuR. Atherosclerosis. 2007;195:e134–143. doi: 10.1016/j.atherosclerosis.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Carper MJ. Cade WT. Cam M, et al. HIV-protease inhibitors induce expression of suppressor of cytokine signaling-1 in insulin-sensitive tissues and promote insulin resistance and type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2008;294:E558–567. doi: 10.1152/ajpendo.00167.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noor MA. Lo JC. Mulligan K, et al. Metabolic effects of indinavir in healthy HIV-seronegative men. AIDS. 2001;15:F11–18. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor MA. Parker RA. O'Mara E, et al. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 2004;18:2137–2144. doi: 10.1097/00002030-200411050-00005. [DOI] [PubMed] [Google Scholar]
- 11.Noor MA. Seneviratne T. Aweeka FT, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: A randomized, placebo-controlled study. AIDS. 2002;16:F1–8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dube MP. Shen C. Greenwald M. Mather KJ. No impairment of endothelial function or insulin sensitivity with 4 weeks of the HIV protease inhibitors atazanavir or lopinavir-ritonavir in healthy subjects without HIV infection: A placebo-controlled trial. Clin Infect Dis. 2008;47:567–574. doi: 10.1086/590154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee GA. Seneviratne T. Noor MA, et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS. 2004;18:641–649. doi: 10.1097/00002030-200403050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui DY. Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res. 2003;42:81–92. doi: 10.1016/s0163-7827(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 15.Melroe NH. Kopaczewski J. Henry K. Huebsch J. Lipid abnormalities associated with protease inhibitors. J Assoc Nurses AIDS Care. 1999;10:22–30. doi: 10.1016/S1055-3290(06)60296-3. [DOI] [PubMed] [Google Scholar]
- 16.Periard D. Telenti A. Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 17.Purnell JQ. Zambon A. Knopp RH, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 18.Riddler SA. Li X. Otvos J, et al. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–288. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 19.Hammond E. McKinnon E. Nolan D. Human immunodeficiency virus treatment-induced adipose tissue pathology and lipoatrophy: Prevalence and metabolic consequences. Clin Infect Dis. 2010;51:591–599. doi: 10.1086/655765. [DOI] [PubMed] [Google Scholar]
- 20.Martinez E. Mocroft A. Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: A prospective cohort study. Lancet. 2001;357:592–598. doi: 10.1016/S0140-6736(00)04056-3. [DOI] [PubMed] [Google Scholar]
- 21.Tsiodras S. Mantzoros C. Hammer S. Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: A 5-year cohort study. Arch Intern Med. 2000;160:2050–2056. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley S. Cheung AM. Fantus G, et al. A prospective study of body fat redistribution, lipid, and glucose parameters in HIV-infected patients initiating combination antiretroviral therapy. HIV Clin Trials. 2008;9:314–323. doi: 10.1310/hct0905-314. [DOI] [PubMed] [Google Scholar]
- 23.Innes S. Levin L. Cotton M. Lipodystrophy syndrome in HIV-infected children on HAART. South Afr J HIV Med. 2009;10:76–80. doi: 10.4102/sajhivmed.v10i4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safrin S. Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–2505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 25.Tsiodras S. Perelas A. Wanke C. Mantzoros CS. The HIV-1/HAART associated metabolic syndrome–novel adipokines, molecular associations and therapeutic implications. J Infect. 2010;61:101–113. doi: 10.1016/j.jinf.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham WE. Crystal S. Bozzette S. Hays RD. The association of health-related quality of life with survival among persons with HIV infection in the United States. J Gen Intern Med. 2005;20:21–27. doi: 10.1111/j.1525-1497.2005.30402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer-van der Kolk IM. Sprangers MA. Prins JM. Smit C. de Wolf F. Nieuwkerk PT. Health-related quality of life and survival among HIV-infected patients receiving highly active antiretroviral therapy: A study of patients in the AIDS Therapy Evaluation in the Netherlands (ATHENA) Cohort. Clin Infect Dis. 2010;50:255–263. doi: 10.1086/649216. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan R. Laitinen D. Dietz B. Impact of lipoatrophy on quality of life in HIV patients receiving anti-retroviral therapy. AIDS Care. 2008;20:1197–1201. doi: 10.1080/09540120801926993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter VM. Hoy JF. Bailey M. Colman PG. Nyulasi I. Mijch AM. The prevalence of lipodystrophy in an ambulant HIV-infected population: It all depends on the definition. HIV Med. 2001;2:174–180. doi: 10.1046/j.1468-1293.2001.00073.x. [DOI] [PubMed] [Google Scholar]
- 30.Currier JS. Martorell C. Osiyemi O, et al. Effects of darunavir/ritonavir-based therapy on metabolic and anthropometric parameters in women and men over 48 weeks. AIDS Patient Care STDS. 2011;25:333–340. doi: 10.1089/apc.2010.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly JS. Langdon D. Serpell L. The phenomenology of body image in men living with HIV. AIDS Care. 2009;21:1560–1567. doi: 10.1080/09540120902923014. [DOI] [PubMed] [Google Scholar]
- 32.Baraldi E. Morales-Ramírez J. Schneider S, et al. Effects of once-daily darunavir/ritonavir versus lopinavir/ritonavir on lipid parameters, anthropometrics in treatment-naive, HIV-1-infected ARTEMIS patients at Week 96. Presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. Jul 19–22;2009 ; Poster MOPEB034. [Google Scholar]
- 33.Tomaka F. Lefebvre E. Sekar V, et al. Effects of ritonavir-boosted darunavir vs. ritonavir-boosted atazanavir on lipid and glucose parameters in HIV-negative, healthy volunteers. HIV Med. 2009;10:318–327. doi: 10.1111/j.1468-1293.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 34.Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2008;53:323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 36.Borai A. Livingstone C. Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem. 2007;44:324–342. doi: 10.1258/000456307780945778. [DOI] [PubMed] [Google Scholar]
- 37.Neidig JL. Holmes W. Reynolds NR, et al. ACTG 5089: Development of assessment of body change distress (ABCD) questionnaire for evaluation of fat redistribution. Presented at the XIV International AIDS Conference; Barcelona, Spain. Jul 7–12;2002 ; Abstract B10609. [Google Scholar]
- 38.Riddler SA. Smit E. Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 39.Mills AM. Nelson M. Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 40.Baker J. Ayenew W. Quick H, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201:285–292. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norris PJ. Pappalardo BL. Custer B. Spotts G. Hecht FM. Busch MP. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses. 2006;22:757–762. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stacey AR. Norris PJ. Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts L. Passmore JA. Williamson C, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24:819–831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachdeva RK. Wanchu A. Bagga R. Malla N. Sharma M. Effect of non-nucleoside reverse transcriptase inhibitors on cytokine, chemokine, and immunoglobulin profiles in serum and genital secretions of HIV-infected women. J Interferon Cytokine Res. 2009;30:13–24. doi: 10.1089/jir.2009.0056. [DOI] [PubMed] [Google Scholar]
- 45.Ford ES. Greenwald JH. Richterman AG, et al. Traditional risk factors and d-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker JV. Neuhaus J. Duprez D, et al. Changes in inflammatory and coagulation biomarkers: A randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2010;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenchley JM. Price DA. Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 48.Shikuma CM. Ribaudo HS. Zheng E. 96 week effects of suppressive efavirenz-containing antiretroviral therapy, abacavir, sex on hs-CRP in ACTG A5095. Presented at the 16th Conference on Retroviruses and Opportunistic Infections; Montréal, Canada. Feb 8–11;2009 ; Poster 736. [Google Scholar]
- 49.Kestens L. Vanham G. Gigase P, et al. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–797. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Kestens L. Vanham G. Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–441. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angel JB. Kumar A. Parato K, et al. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]
- 52.Ondoa P. Koblavi-Deme S. Borget MY. Nolan ML. Nkengasong JN. Kestens L. Assessment of CD8 T cell immune activation markers to monitor response to antiretroviral therapy among HIV-1-infected patients in Cote d'Ivoire. Clin Exp Immunol. 2005;140:138–148. doi: 10.1111/j.1365-2249.2005.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steel A. John L. Shamji MH, et al. CD38 expression on CD8 T cells has a weak association with CD4 T-cell recovery and is a poor marker of viral replication in HIV-1-infected patients on antiretroviral therapy. HIV Med. 2008;9:118–125. doi: 10.1111/j.1468-1293.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 54.Busti AJ. Bedimo R. Margolis DM. Hardin DS. Improvement in insulin sensitivity and dyslipidemia in protease inhibitor-treated adult male patients after switch to atazanavir/ritonavir. J Investig Med. 2008;56:539–544. doi: 10.2310/JIM.0b013e3181641b26. [DOI] [PubMed] [Google Scholar]
- 55.Noor M. Maa J. Witek J. Falutz J. Predictive accuracy of surrogate indices for predicting early insulin resistance after treatment with HIV protease inhibitors (PI). Poster presented at the 44th Annual Meeting of the Infectious Diseases Society of America (IDSA); Toronto, Canada. Oct 12–15;2006 ; Poster 986. [Google Scholar]
- 56.Mocroft A. Kirk O. Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 57.Grunfeld C. Saag M. Cofrancesco J, Jr, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24:1717–1726. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.REYATAZ (atazanavir): Full prescribing Information. http://packageinserts.bms.com/pi/pi_reyataz.pdf. [Aug 17;2010 ]. http://packageinserts.bms.com/pi/pi_reyataz.pdf