Abstract
Background
Neuroanatomic studies have not yet addressed how subtle phenotypic distinctions in psychosis alter the underlying brain changes, and whether there is evidence for psychosis as a dimensional construct. We explored the relationship of cortical GM thickness to psychotic phenotypes in children.
Methods
Cross-sectional comparison of anatomic brain imaging between patients referred as childhood-onset schizophrenia (COS) but ruled out after a drug free inpatient observation. Groups included: patients with no evidence of psychosis (n=22) after drug free observation, patients with psychosis not otherwise specified (PNOS; total n=29) further divided into those without other axis I diagnoses (n=13) and those with other axis I comorbidities (n=16), age/sex matched COS patients (n=48), and 51 matched healthy controls. GM cortical thickness was compared between the groups, and regressed on patients’ SAPS, SANS and GAS scores.
Results
Patients with no evidence of psychosis showed no cortical GM deficits. Presence of psychosis (PNOS with or without co-morbidities) showed some areas of temporal and prefrontal deficits, more subtle compared to the extensive bilateral cortical deficits seen for COS. GAS SAPS and SANS scores showed a relationship with cortical GM thickness although it did not survive adjustment for multiple comparisons.
Conclusions
These results highlight the need for careful phenotypic characterization, as subtle diagnostic distinctions appear to reflect distinct underlying patterns of brain deficits. The incremental nature of cortical deficits from no psychosis to PNOS to COS may further support dimensional model for psychosis.
Keywords: adolescence, psychosis, brain imaging, schizophrenia, gray matter, pediatrics
Introduction
Anatomic magnetic resonance imaging (MRI) studies in schizophrenia have established structural brain deficits including reduced cortical gray matter (GM) volume, lateral ventricular enlargement (Ho et al., 2003; Wright et al., 2000), reduced volumes of the superior temporal gyrus (STG)(Barta et al., 1990) and medial temporal lobe structures (Shenton et al., 2001). These cortical GM deficits are more pronounced in childhood-onset schizophrenia (COS) which, defined as onset of psychosis before age 13, is a rare, severe, and treatment-refractory form of adult onset schizophrenia (AOS)(Rapoport and Gogtay, 2008). COS shows an exaggerated pattern of the normal GM maturational trajectory which appears as a wave of parieto-frontal GM loss during adolescence (Gogtay et al., 2004; Thompson et al., 2001) that merges into the AOS pattern of prefrontal and temporal GM deficits as these patients become young adults(Greenstein et al., 2006).
Epidemiologic studies show that psychotic symptoms are surprisingly common in non-schizophrenic clinical samples (Biederman et al., 2004; Garralda, 1984; Ulloa et al., 2000) as well as in otherwise healthy children (McGee et al., 2000; Scott et al., 2009; Yoshizumi et al., 2004) with some reports of psychotic symptoms in as many as 19% otherwise healthy children by age 12 (Johns et al., 2004; Polanczyk et al., 2010; van Os et al., 2009; Wiles et al., 2006). Furthermore, psychotic symptoms in adults (van Os et al., 2009) and in children(Polanczyk et al., 2010), appear to be associated with the same risk factors for schizophrenia and other psychotic disorders (Lataster et al., 2009; Polanczyk et al., 2010). These findings have increasingly raised the possibility that psychosis could be a dimensional rather than a categorical construct as currently described (Dutta et al., 2007).
The diagnostic specificity and dimensionality of brain abnormalities associated with psychotic symptoms, however, has not been explored. If psychosis is indeed a dimensional construct, then it is possible that the underlying brain abnormalities may reflect the presence or absence of psychosis as well as the severity of psychosis. Existing literature provides some evidence. GM deficits in COS, with a more severe phenotype, are more profound compared to the adult onset illness (Gogate et al., 2001). In adults, studies on bipolar I disorder that include patients who have psychotic symptoms (Lopez-Larson et al., 2002; Takahashi et al., 2009; Tost et al.) also show fronto-temporal GM abnormalities as seen in schizophrenia, while studies on non psychotic bipolar patients show no cortical GM abnormalities (Koo et al., 2008; McDonald et al., 2006; Scherk et al., 2008). Similarly, more recent early psychosis studies also support the psychosis continuum as patients with milder/prodromal phases of psychosis appear to show some subtle functional (Fusar-Poli et al.; Fusar-Poli et al.) and structural(Fusar-Poli et al.; Fusar-Poli et al.) alterations in fronto-striatal circuits.
Here we compare regional cortical GM thickness for three unique groups of pediatric patients referred for evaluation for childhood onset schizophrenia, but excluded after an inpatient medication free diagnostic observation. In the NIMH COS study, the COS diagnosis is finalized after an inpatient evaluation that usually includes medication washout with up to three week drug-free observation. We have used three subgroups of ‘ruled out’ patients as follows: i) those who show no evidence of psychosis after inpatient evaluation but have other axis I diagnosis (e.g. anxiety disorder etc), ii) those who show transient, mild psychotic symptoms (and received the label psychosis not otherwise specified (PNOS)) but had no other axis I diagnosis, and iii) those who had psychotic symptoms in addition to other axis I diagnoses (PNOS with comorbidities). Since all these children were originally referred with the diagnosis of COS, they had similar medication/treatment histories. We also selected an age/sex matched group of COS patients and a common matched group of healthy controls, and compared GM cortical thickness across the five groups. We hypothesized that, if the brain involvement in psychosis was dependent on accurate diagnostic characterization and if the psychosis was indeed a dimensional construct then; i) the ‘non-psychotic’ ‘rule-out’ patients would show no GM deficits; and ii) the presence of psychosis in the ‘PNOS’ groups may result in some cortical GM deficits, most probably in prefrontal and temporal cortices, that would be less severe than typically seen for COS.
Methods and Materials
Since 1991, out of over 3,000 nationwide referrals, over 200 cases have been admitted for inpatient evaluation of which 117 to date have received the COS diagnosis, in most cases after medication washout. The inclusion and exclusion criteria have been described elsewhere (McKenna et al., 1994). Almost all patients that are offered inpatient observation undergo a complete medication washout followed by drug free observation up to 3 weeks. This is done mainly to achieve a diagnostic clarity, and only in rare instances final diagnosis of COS is offered without a complete washout. Among those that were ruled out as having schizophrenia, a group of patients showed ‘no evidence’ of psychosis during their entire stay at the NIMH (n= 22, age: 12.4±2.4). These patients, however, had other Axis I diagnoses which are shown in Table 1. 29 other patients who were ruled out as having schizophrenia, did have psychotic symptoms and were given a diagnosis of psychosis not otherwise specified (PNOS). Of these, 13 (average age: 11.6±2.9) had no other comorbid axis I diagnoses while 16 (average age: 13.2±2.8) had psychotic symptoms associated with other axis I diagnoses (see Table 1). In order to evaluate the effect of co-morbidities on brain development (if any), these two groups were considered separately during the analyses. A comparison group of age- and sex-matched COS patients (n=48, average age: 12.5±2.2) was identified from the COS cohort and a sample of healthy controls (n= 51, average age: 12.5±2.2) was identified that matched with the three patient groups. The recruitment details for healthy controls are described elsewhere (Giedd et al., 1999). The study was approved by the NIMH institutional review board and written consents and assents were obtained from parents of minor patients, and patients themselves respectively.
Table 1.
| Sample Demographics | No Psychosis | PNOS | PNOS with Comorbidities | COS | Healthy controls | Test Statistic (df) | P value |
|---|---|---|---|---|---|---|---|
| N | 22 | 13 | 16 | 48 | 51 | ||
| Age at scan | 12.42±2.38 | 11.63±2.85 | 13.23±2.82 | 12.54±2.16 | 12.53±2.16 | F(4,145) = 0.85 | 0.49 |
| Sex(M/F) | (14/8) | (9/4) | (13/3) | (31/17) | (33/18) | χ2(4) =1.84 | 0.76 |
| Medication at scan | |||||||
| Atypical Antipsychotics | 17 | 4 | 8 | 36 | - | χ2(3) = 11.9 | <0.01 |
| Typical Antipsychotics | 4 | 7 | 6 | 19 | - | χ2(3)=5.08 | 0.16 |
| Mood Stabilizers | 7 | 5 | 6 | 19 | - | χ2(3) = 0.40 | 0.94 |
| Unknown | 2 | 0 | 3 | 0 | - | χ2(3) = 10.2 | 0.02 |
| Ethnicity | χ2(16) = 13.4 | 0.64 | |||||
| Caucasian | 18 | 10 | 13 | 27 | 31 | ||
| African American | 1 | 2 | 2 | 13 | 13 | ||
| Asian | 0 | 0 | 0 | 3 | 2 | ||
| Hispanic | 1 | 0 | 1 | 1 | 1 | ||
| Other | 2 | 1 | 0 | 4 | 4 | ||
| Comorbid Diagnoses | |||||||
| Affective Disorder | 9 | - | 2 | 2 | - | χ2(2) = 15.9 | <0.01 |
| Behavioral Disorder | 4 | - | 2 | 2 | - | χ2(2) = 3.75 | 0.15 |
| Anxiety Disorder | 6 | - | 2 | 5 | - | χ2(2) = 3.44 | 0.17 |
| ASD | 4 | - | 1 | 8 | - | χ2(2) = 1.23 | 0.54 |
| ADHD | 8 | - | 7 | 5 | - | χ2(2) = 10.3 | <0.01 |
| LD | 8 | - | 2 | 13 | - | χ2(2) = 2.69 | 0.25 |
| No diagnosis or Unknown | 0 | 1 | 1 | χ2(2) =1.61 | 0.44 | ||
| Handedness | χ2(12)= 19.3 | 0.08 | |||||
| Right | 19 | 9 | 12 | 34 | 46 | ||
| Left | 0 | 3 | 2 | 5 | 3 | ||
| Ambidextrous | 0 | 1 | 1 | 5 | 2 | ||
| Unknown | 3 | 0 | 1 | 4 | 0 | ||
| GAS Scores* | 42.5±13.1 | 37.3±15.6 | 34.8±17.2 | 23.2±13.7 | - | Kruskal Wallis χ2(2) = 25.6 | <0.01 |
| SAPS Scores** | 13.9±16.2 | 16.3±23.3 | 21.3±25.1 | 48.9±21.5 | - | Kruskal Wallis χ2(2) =19.3 | <0.01 |
| SANS Scores*** | 21.4±21.4 | 9.3±1.1 | 24.8±28.0 | 54.7±26.1 | - | Kruskal Wallis χ2(2) =18.2 | <0.01 |
PNOS not included (only 3 observations); Wilcoxon rank sum post hoc vs COS: COS < No psychosis, PNOS plus comorbidities
PNOS not included (only 3 observations); Wilcoxon rank sum post hoc vs COS: COS > No psychosisp < 0.001
PNOS not included (only 3 observations); Wilcoxon rank sum post hoc vs COS: COS < No psychosis, PNOS plus comorbidities p≤0.03
MRI acquisition and analysis
All magnetic resonance scans were obtained on the same 1.5-T GE Signa MRI scanner (General Electric Medical Systems, Milwaukee, Wisconsin) with previously described standardized head placement (Giedd et al., 1996). T1-weighted, 3D (256×256×124) fast spoiled gradient echo volumes were obtained with contiguous 1.5-mm slices in the axial plane and 2.0-mm slices in the coronal plane. These resolution parameters have been maintained throughout the study for continuity. Imaging parameters were: echo time of 5ms, repetition time of 24ms, 45° flip angle, number of excitations equaled 1, acquisition matrix of 256×192, and 24-cm field of view.
Image analyses were done using a fully automated, validated cortical surface extraction pipeline (Shaw et al., 2009). MRI scans were registered into standardized stereotaxic space (MNI-ICBM152 non-linear sixth generation symmetric target) with a nine-parameter linear transformation and were corrected for non-uniformity artifacts (Sled et al., 1998; Zijdenbos et al., 2002). Volumes were then divided into gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), and background using an advanced neural net classifier (Tohka et al., 2004). The Constrained Laplacian Anatomic Segmentation Using Proximities (CLASP) surface extraction procedure(Kim et al., 2005) was used to generate surface meshes determining the WM/GM interface. CT was calculated using the root mean square thickness between corresponding nodes on the surface meshes in native space. Surface registration was used to align CT measurements to maximize thickness value in terms of corresponding gyral patterning between subjects. We used a 30-mm surface based blurring kernel that maximizes statistical power and minimizes false positives (Lerch and Evans, 2005) for noise reduction in thickness measurements. CT was then calculated in native space at 40,962 homologous points per hemisphere. Results were projected onto a three-dimensional template where positive and negative values indicated relative increases and decreases in CT, respectively. Parcellation also generates measures of total lobar cortical volume, total surface area and mean cortical thickness (MCT).
Statistical analysis
Demographic data
Group differences were tested with analyses of variance (ANOVA) for continuous measures and chi-square tests of independence for categorical measures. ANOVA assumptions of homogeneity of variance and normality were assessed and data were visually screened for outliers.
Image Analyses
We used a general linear model to test for cortical thickness differences from controls at 40,962 points in each cerebral hemisphere. The independent variables included group (COS, PNOS, clean rule-outs, and psychosis with other diagnoses, and healthy controls) age and sex. Healthy controls were set as the reference group/intercept, and other groups’ differences from controls were tested with t-tests. A mean cortical thickness value of less than 2.5 mm was considered unrealistic and 1 scan below this threshold was excluded as an outlier. The results were then projected onto a brain template to visualize thickness group differences compared to healthy controls. We used the False Discovery Rate procedure to control for type I errors and we set the alpha at q=0.05.
To explore the relationships between dimensional measures of psychosis such as illness severity and cortical thickness, we combined all patient groups and then regressed cortical thickness on SAPS, SANS and GAS scores (obtained at drug free baseline).
Statistical analyses were performed using R and SPSS version 16.0.
Results
Sample demographics are shown in Table 1.
There were no group differences in age at scan, sex, ethnicity, or handedness; however results were covaried for age and sex to reduce error variance.
The GM deficit pattern in patient groups compared to matched, common set of controls is shown in Figure 1. Mean cortical thickness (MCT) per hemisphere was significantly different across groups and hence MCT was used as a covariate.
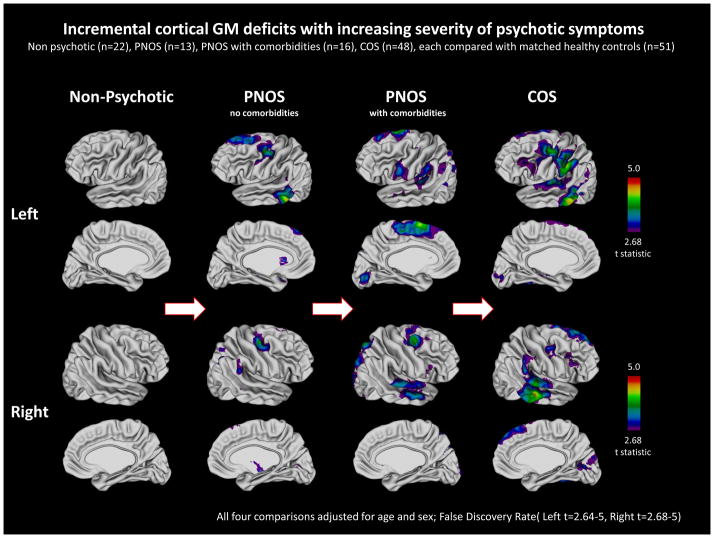
Figure 1. Incremental cortical GM deficits with increasing severity of psychotic symptoms.
Gray Matter (GM) cortical deficits among four patients groups compared to the common group of healthy controls. Colors show areas of significant cortical GM deficits. Cortical deficits show qualitative increase with the increasing severity of psychosis with non psychotic patients showing no deficits while COS group showing the most extensive cortical deficits.
As seen in Figure 1, patients who had no evidence of psychosis after the medication washout had no cortical deficits compared to controls across the entire hemisphere. The children who had some evidence of psychosis (PNOS) showed mild cortical deficits. When divided further ‘PNOS only (no other axis I comorbidities)’ sample showed mainly prefrontal (left superior frontal, and bilateral posterior middle frontal) and temporal (left middle and inferior, and a small area of right posterior-superior) cortical thinning, qualitatively smaller compared to those seen for COS. Similarly, the group of ‘PNOS with other comorbidities’ also showed prefrontal (bilateral superior prefrontal) and temporal (bilateral superior, and right middle temporal) cortical thinning, but additionally also showed some deficits in parietal cortex and left medial prefrontal cortex. When the two PNOS groups were combined, the degree or the quality of deficit pattern remained the same with mostly small areas of prefrontal and temporal deficits. The COS patients, on the other hand, had more pervasive deficits over prefrontal, parietal and temporal cortices bilaterally as have been described previously in our other studies(Gogtay, 2008).
We attempted to quantify cortical GM deficits by calculating the effect size (ES) at each cortical point (Supplemental Figure 1). These analyses were limited by the small sample sizes, which made the power inconsistent (with the largest COS group having the most power for a more precise ES map). Despite this limitation, the ES results showed a more or less similar pattern with minimal ES in non-psychotic subjects, which increased with the psychosis and morbidities. All findings survived multiple comparisons using the False Discovery Rate procedure.
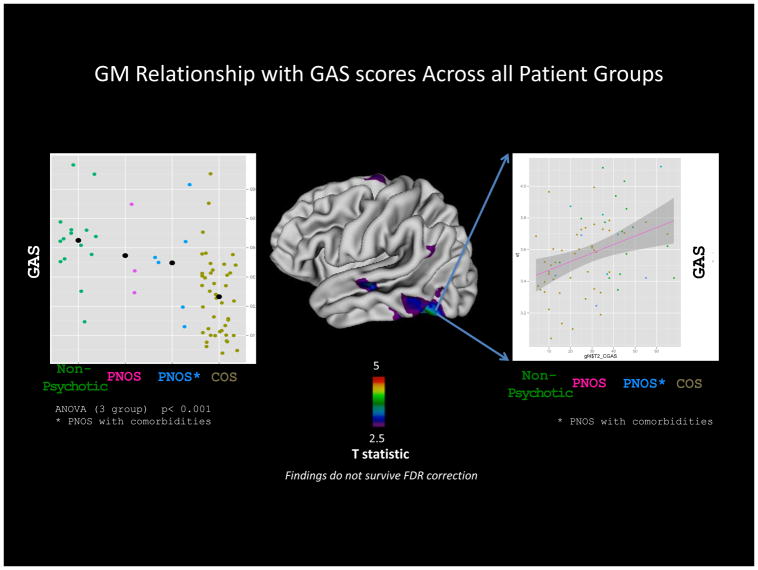
GAS scores (when available) were significantly different between the patient groups (ANOVA p < 0.05), which was driven by the difference between ‘ non-psychotic’ and COS groups. When all patient groups were combined and cortical thickness was regressed on GAS scores, areas of significant positive relationship were seen throughout the cortex but more prominently on the left temporal cortex (Figure 2). However, this relationship did not survive adjustment for multiple comparisons using false discovery rate statistic (FDR). The SAPS (ANOVA p< 0.01) and SANS (ANOVA p<0.01) scores also were significantly different between patient groups, which was also driven mainly by the difference between ‘non psychotic’ and COS groups (Table 1). SAPS and SANS scores however, did not show significant relationship with GM cortical thickness.
Figure 2. Relationship between cortical thickness and GAS scores in patients. Individual dots represent GAS scores at medication washout and are color coded to represent groups on X-axis.
Comparison of effect sizes (ES) for cortical GM deficits across patient groups relative to controls. Incremental ES from non psychotic group to psychotic groups suggest increasing GM involvement with psychosis. The lack of exact overlap between figures 1 and 2 is likely to be due to sample size limitations especially in non-psychotic groups.
Discussion
These results highlight the need for clear diagnostic characterization of psychosis and also indirectly support a dimensional approach to cortical brain deficits in psychosis. The ‘non psychotic rule out’ patients showed no cortical deficits, ‘PNOS’ patients with relatively milder, transient, and non schizophrenic psychotic symptoms showed smaller areas of prefrontal and temporal deficits, while the most severely psychotic COS patients has the most profound deficits. This was also seen by the increasing effect size for the deficits (from non psychotic to psychotic groups) in an incremental manner. Since all patient groups were treated with similar medications, this is unlikely to be due to treatment.
Epidemiological and pharmacological data support a dimensional model of psychosis. In the cortex, presence of psychosis correlated with the presence of brain deficits regardless of other comorbidities. Moreover, increasing severity of psychosis appeared to cause increasing brain deficits; enhanced by the presence of other co-morbidities (Figure 1), which was also reflected by significant positive correlations with GAS (Figure 2). Clinically, it is logical that psychosis could best be investigated on a continuum rather than through categorical diagnoses. Self reported psychotic symptoms in the general population are more common than previously perceived with prevalence estimates ranging from 5.5% (Johns et al., 2004), to 17.5% in the Dutch cohort(van Os et al., 2001), to as high as 28% in the national co-morbidity study in the US(Kendler et al., 1996). However, it appears that only a subgroup of patients go on to develop the illness (Kendler et al., 1996), which was also evident in the Dunedin birth cohort study, where 25% of the sample had reported at least one delusional or hallucinatory experience that was not related to drug use or other illness by age 26, but only 3.7% fulfilled criteria for schizophreniform disorder. The presence of hallucinatory experiences by age 11 was a strong predictor of developing schizophrenia by age 26, suggesting a continuum between psychotic experience to full illness(Poulton et al., 2000). Of particular importance, Polanczyk et al showed that self-reported positive symptoms in non-schizophrenic populations by age 12 correlated with known risk factors for schizophrenia at a later age showing a continuum of risk(Polanczyk et al., 2010).
The psychosis and functionality measures reflected increasing severity of illness as expected (Table 1). For example, non-psychotic group had the lowest SAPS scores while the COS group had the highest SAPS scores as expected. Although the SAPS and SANS measures in this study did not show significant correlations with cortical thickness, GAS scores (which are a crude index of functional outcome and thus reflect the severity of illness and treatment response) did show a positive correlation with cortical thickness in prefrontal and temporal regions (Figure 3). Furthermore, qualitatively there were some areas of overlap between the cortical deficit patterns and the regions where GAS scores correlated with cortical thickness. (e.g. left temporal regions and superior frontal cortex; Figures 1 and 2). The specific regions involved could reflect the psychosis pathology (e.g. involvement of temporal cortex in auditory hallucinations), but perhaps more reflect that overall functionality influences cortical thickness in a regionally specific manner, thus supporting the dimensionality of phenotypic severity (not just psychosis). However, it is important to note that these findings are weak at best as they did not survive adjustment for multiple comparisons.
The study also highlights the importance of careful phenotypic characterization, as the underlying brain changes may reflect relatively subtle clinical features. Although the cortical deficit pattern in the three psychotic patient groups did not exactly overlap, the GM deficits were largely localized to prefrontal and temporal cortices, as has been described in adult onset schizophrenia (Kuperberg et al., 2003; Narr et al., 2005; White et al., 2003; Wiegand et al., 2004), as well as in COS as young (Greenstein et al., 2006). Healthy young siblings and high-risk populations at risk for schizophrenia also show deficits in these regions (Pantelis et al., 2003) (Gogtay et al., 2007). Functional imaging studies are ongoing to examine more subtle correlates of abnormal circuitry development.
This study partially addresses the effect of antipsychotic medication on brain morphology. Studies in non-human primates chronically exposed to either typical or atypical antipsychotic medications reveal substantial reduction in gray matter volume, glial cell number, and increased neuronal density in the parietal lobes(Konopaske et al., 2007) as well as reduced fresh brain weight (Dorph-Petersen et al., 2005), although a recent meta-analysis of 25 longitudinal volumetric studies was equivocal (Moncrieff and Leo, 2010). Although this study cannot rule out the effect of medications on cortical GM, the fact that all four patient groups had similar medication exposure at the time of MRI scan suggests that the cortical deficits are less likely to be medication related.
There are several limitations to these data. First, a limitation of this cross-sectional study is the lack of longer-term information, as many of these excluded subjects were lost to follow up. Second, although this study provides some evidence for the dimensional nature of psychosis, it is qualitative and indirect at best. Furthermore, rather than the dimensional nature of psychosis, it could be the ‘illness burden’ that may be reflective of the brain changes as all cases had significant morbidities in the first place. The effect size maps do not exactly replicate the statistical maps (Figures 1 and Supplemental Figure 1) especially in PNOS groups, most likely due to sample size limitations. Similarly, although the GAS scores showed positive correlations with cortical thickness, we did not find significant correlations of GM cortical thickness and positive and negative symptoms as continuous measures, which could also be the result of small sample sizes. Finally, the clinically severe nature of the phenotype results in frequent medication changes and variable medication exposure, which could still influence the GM changes. The medication exposure is hard to accurately quantify in this population, which in turn makes it difficult to explore the effects of medications on GM findings. However, despite these limitations, these findings make a strong case for careful phenotypic characterization of psychosis and provide neurobiological support for a dimensional model for psychosis. Efforts to acquire longitudinal data on PNOS and other early psychosis subjects, combined with other functional imaging modalities to detect more subtle neurodevelopmental changes are currently underway.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147(11):1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty C, Faraone SV, Seidman L. Phenomenology of childhood psychosis: findings from a large sample of psychiatrically referred youth. J Nerv Ment Dis. 2004;192(9):607–614. doi: 10.1097/01.nmd.0000138228.59938.c3. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Dutta R, Greene T, Addington J, McKenzie K, Phillips M, Murray RM. Biological, life course, and cross-cultural studies all point toward the value of dimensional and developmental ratings in the classification of psychosis. Schizophr Bull. 2007;33(4):868–876. doi: 10.1093/schbul/sbm059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Matthiasson P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, McGuire P. Spatial working memory in individuals at high risk for psychosis: Longitudinal fMRI study. Schizophr Res. doi: 10.1016/j.schres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, McGuire P. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: Longitudinal VBM-fMRI study. J Psychiatr Res. doi: 10.1016/j.jpsychires.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Grasby PM, McGuire PK. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 67(7):683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- Garralda ME. Hallucinations in children with conduct and emotional disorders: I. The clinical phenomena. Psychol Med. 1984;14(3):589–596. doi: 10.1017/s0033291700015191. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogate N, Giedd J, Janson K, Rapoport JL. Brain imaging in normal and abnormal brain development: new perspectives for child psychiatry. Clinical Neuroscience Research. 2001;1:283–290. doi: 10.1046/j.1440-1614.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34(1):30–36. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, Butler P, Evans A, Rapoport J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64(7):772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Sporn A, Clasen LS, Nugent TF, 3rd, Greenstein D, Nicolson R, Giedd JN, Lenane M, Gochman P, Evans A, Rapoport JL. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61(1):17–22. doi: 10.1001/archpsyc.61.1.17. [DOI] [PubMed] [Google Scholar]
- Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, Rapoport J, Gogtay N. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47(10):1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Johns LC, Cannon M, Singleton N, Murray RM, Farrell M, Brugha T, Bebbington P, Jenkins R, Meltzer H. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996;53(11):1022–1031. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27(1):210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32(6):1216–1223. doi: 10.1038/sj.npp.1301233. [DOI] [PubMed] [Google Scholar]
- Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65(7):746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lataster T, Myin-Germeys I, Derom C, Thiery E, van Os J. Evidence that self-reported psychotic experiences represent the transitory developmental expression of genetic liability to psychosis in the general population. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(8):1078–1084. doi: 10.1002/ajmg.b.30933. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24(1):163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163(3):478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- McGee R, Williams S, Poulton R. Hallucinations in nonpsychotic children. J Am Acad Child Adolesc Psychiatry. 2000;39(1):12–13. doi: 10.1097/00004583-200001000-00006. [DOI] [PubMed] [Google Scholar]
- McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33(5):636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010:1–14. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15(6):708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Moffitt TE, Arseneault L, Cannon M, Ambler A, Keefe RS, Houts R, Odgers CL, Caspi A. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67(4):328–338. doi: 10.1001/archgenpsychiatry.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57(11):1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology. 2008;33(1):181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- Scherk H, Kemmer C, Usher J, Reith W, Falkai P, Gruber O. No change to grey and white matter volumes in bipolar I disorder patients. Eur Arch Psychiatry Clin Neurosci. 2008;258(6):345–349. doi: 10.1007/s00406-007-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Martin G, Bor W, Sawyer M, Clark J, McGrath J. The prevalence and correlates of hallucinations in Australian adolescents: results from a national survey. Schizophr Res. 2009;107(2–3):179–185. doi: 10.1016/j.schres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JN, Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66(8):888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66(4):366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tost H, Ruf M, Schmal C, Schulze TG, Knorr C, Vollmert C, Bosshenz K, Ende G, Meyer-Lindenberg A, Henn FA, Rietschel M. Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. J Affect Disord. 120(1–3):54–61. doi: 10.1016/j.jad.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Ulloa RE, Birmaher B, Axelson D, Williamson DE, Brent DA, Ryan ND, Bridge J, Baugher M. Psychosis in a pediatric mood and anxiety disorders clinic: phenomenology and correlates. J Am Acad Child Adolesc Psychiatry. 2000;39(3):337–345. doi: 10.1097/00004583-200003000-00016. [DOI] [PubMed] [Google Scholar]
- van Os J, Hanssen M, Bijl RV, Vollebergh W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry. 2001;58(7):663–668. doi: 10.1001/archpsyc.58.7.663. [DOI] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 2003;54(4):418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Wiegand LC, Warfield SK, Levitt JJ, Hirayasu Y, Salisbury DF, Heckers S, Dickey CC, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Prefrontal cortical thickness in first-episode psychosis: a magnetic resonance imaging study. Biol Psychiatry. 2004;55(2):131–140. doi: 10.1016/j.biopsych.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles NJ, Zammit S, Bebbington P, Singleton N, Meltzer H, Lewis G. Self-reported psychotic symptoms in the general population: results from the longitudinal study of the British National Psychiatric Morbidity Survey. Br J Psychiatry. 2006;188:519–526. doi: 10.1192/bjp.bp.105.012179. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yoshizumi T, Murase S, Honjo S, Kaneko H, Murakami T. Hallucinatory experiences in a community sample of Japanese children. J Am Acad Child Adolesc Psychiatry. 2004;43(8):1030–1036. doi: 10.1097/01.chi.0000126937.44875.6b. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21(10):1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.