Abstract
Introduction
We established a colony of dogs that harbor an X-linked MTM1 missense mutation. Muscle from affected male dogs exhibits reduction and altered localization of the MTM1 gene product, myotubularin, and provides a model analogous to X-linked myotubular myopathy (XLMTM).
Methods
We studied hind limb muscle function in age-matched canine XLMTM genotypes between ages 9 and 18 weeks.
Results
By the end of the study, affected dogs produce only ~15% of the torque generated by normals or carriers (0.023 ± 0.005 vs 0.152 ± 0.007 and 0.154 ± 0.003 N-m/Kg body mass, respectively, p <0.05) and are too weak to stand unassisted. At this age, XLMTM dogs also demonstrate an abnormally low twitch:tetanus ratio, a right-shifted torque-frequency relationship and an increase in torque during repetitive stimulation (p<0.05).
Conclusions
We hypothesize that muscle weakness results from impaired excitation-contraction (E-C) coupling. Interventions that improve E-C coupling might be translated from the XLMTM dog model to patients.
Keywords: myotubular myopathy, stretch, myotubularin, muscle damage, strength, function
Introduction
Mutations in MTM1 cause X-linked myotubular myopathy (XLMTM), a congenital muscle disorder estimated to affect 1 in 50,000 live male births worldwide.1–6 Affected children typically present with hypotonia and weakness and ultimately succumb to respiratory failure with only palliative care.3 Analogous to patients, Labrador retriever dogs that harbor an MTM1 mutation also display severe muscle weakness.7 Because of the overt phenotype and clinical similarities to patients, the XLMTM canine model provides a superb preclinical system to test therapeutic approaches.8 As a first step prior to testing interventions, we studied muscle function in affected dogs between ages 9 and 18 weeks, roughly equivalent to the age of a child between the ages of 6 months and 2 years.
MATERIALS AND METHODS
Dogs were used and cared for according to principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Newborn XLMTM dogs were identified using a rapid TaqMan genotyping assay as described.7 In-vivo muscle strength and responses to repeated contractions were performed as described previously.9,10 Fourteen animals were studied from 3 litters: normal (n=6); carrier (n=5); and affected XLMTM (n=3).
Isometric torque measures
Data were obtained from both hind limbs as described below. Anesthetized dogs were positioned in dorsal recumbency. One pelvic limb was immobilized in a stereotactic frame11 to align the tibia at a right angle to the femur. To place the cranial tibial muscles at L0, the tibiotarsal joint was positioned at 90 degrees. Adhesive wrap affixed the foot to a pedal mounted on the shaft of a servomotor to measure torque (model 310LR, Aurora Scientific Inc., Aurora ON, Canada). Percutaneous stimulation of the fibular nerve activated hindlimb muscles of the foot to pull against the pedal to generate tibiotarsal flexor torque. Supramaximal 150 V, 100 µs pulses were applied (Model S88X stimulator and Model SIU-C isolation unit, Grass Instruments, Quincy, MA, USA). First, stimulating electrodes were positioned until twitches (Pt) reached a maximum, and then a tetanus was induced by a 1 sec train of 50 Hz pulses. The limb was repositioned, and the sequence was repeated. After twitches and tetany were obtained with the limb repositioned, an isometric flexion torque-frequency relation was obtained at frequencies from 1–60 Hz. Then, an eccentric protocol was conducted with the stimulating electrode position unchanged. Dynamic Muscle Control computer software (DMC, Aurora Scientific, Inc.) controlled the servomotor, stimulation timing and capture of torque responses.
Flexor tibiotarsal eccentric (ECC) protocol
We used a repeated contraction protocol as described.10,9 During percutaneous fibular nerve stimulation (100µs square wave pulses, 50 Hz), the muscle was first held at constant length for 900 ms (i.e., isometric phase), followed by a stretch for 100 ms (i.e., eccentric phase). The servomotor rotated the lever arm 29 degrees9 opposite to contracting flexor muscles at a rate of ~0.7 muscle length/sec followed by a 1 sec rampback to baseline position. This procedure was repeated every 4 sec. To avoid fatigue, 4 min rest followed every series of 10 contractions, and a total of 30 contractions were performed by each animal.
Analysis
Isometric and eccentric contraction torque profiles were analyzed with Dynamic Muscle Analysis Software (Aurora Scientific). Isometric torque (N-m) for twitches and tetanus were normalized to body mass (kg). Tetanic torque data over time were analyzed by a 2-way ANOVA (Genotype and age); Torque-frequency and eccentric contraction data were analyzed by a 2-way ANOVA (genotyope and either frequency or contraction number, respectively) with repeated measures; and twitch-tetanus ratios were analyzed with a 1-way ANOVA. Differences in means were assessed by Tukey post hoc analysis. Values were considered statistically different when P < 0.05.
RESULTS
Hind limb strength and clinical deterioration
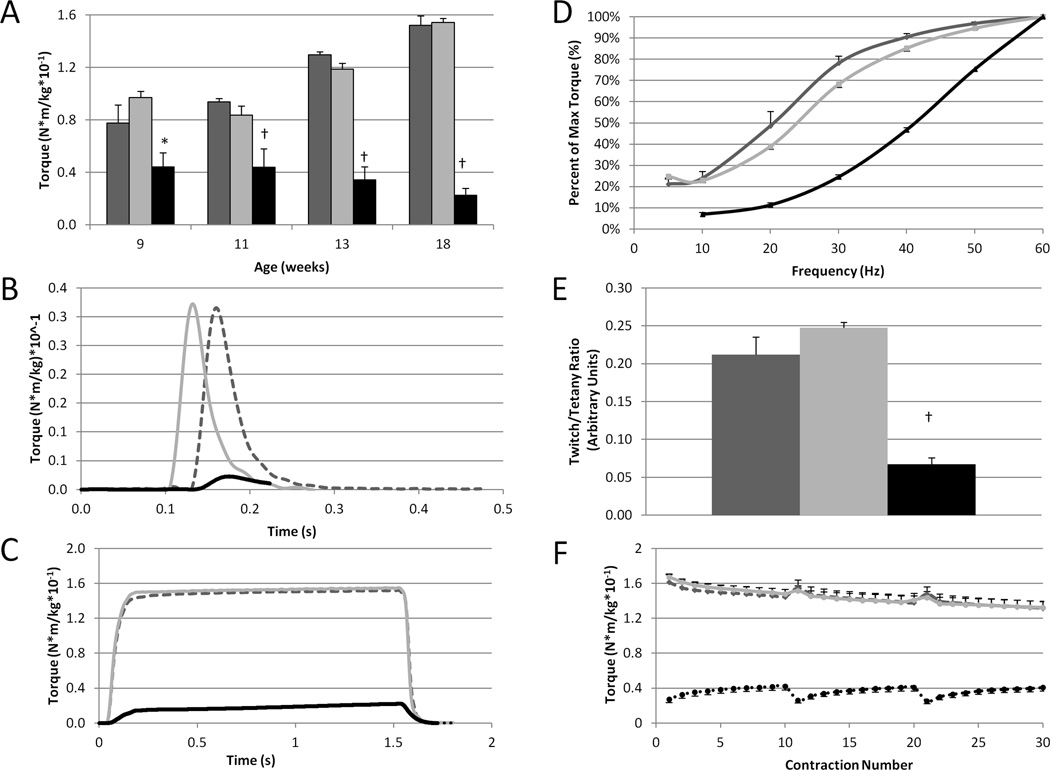
Fig 1A depicts the progression of hind limb weakness in XLMTM dogs measured by isometric torque. This physiologic assay of function shows that XLMTM dogs are always weaker than normal or carrier dogs, and that weakness becomes more pronounced with age. Near the end of life (18 weeks-of-age), affected dogs produce only ~15% of the torque generated by their age-matched normal and carrier littermates (0.023 ± 0.005 vs 0.152 ± 0.007 and 0.154 ± 0.003 N-m/Kg body mass, respectively, p <0.05). The clinical course of disease progression follows a pattern analogous to physiological assays. At the beginning of the study, 9 week-old XLMTM dogs appear relatively normal, but by age 13 weeks, affected dogs display abnormalities in posture and develop a slow and stiff-legged gait. By age 18 weeks, affected dogs are unable to stand without assistance and have difficulty feeding. There is a concomitant decline in weight gain, but body mass does not differ significantly between genotypes (not shown).
FIGURE 1. Hind limb muscles of XLMTM dogs develop progressive weakness, demonstrate a right-shifted torque-frequency relationship, and present an abnormal response to repeated eccentric contractions.
A. Loss of isometric strength (torque) of the hind limb flexors (muscles that pull the toes up) from ages 9 to 18 weeks. B–F: Functional assays are shown at age 18 weeks when affected dogs lose the ability to stand independently and walk; representative torque responses for twitch (B) and tetanus (C) for the 3 genotypes; compared to normal or carriers, affected dogs demonstrate a right-shifted torque frequency curve (D) and an abnormally low twitch:tetanus ratio (E); isometric torque produced by the hind leg flexor muscles during a series of 10 eccentric contractions repeated 3 times with rest periods of 4 min (broken lines in x axis) between each (F; see Childers et al., 2011 for method). Following each rest period, XLMTM compared to normal or carriers initially generate abnormally low isometric torque that increases with subsequent contractions (F). Legend: dark grey = normal (n=6); light grey = carrier (n=5); black = XLMTM (n=3). Means ± SEM. *Significantly different compared to the other genotypes (p<0.05) at age 18 weeks. Note: Torque responses are normalized to body mass (N-m/kg).
Torque-frequency relationship
Compared to normal controls, affected dogs display a rightward shift in the torque-frequency relationship. This abnormal response was observed at age 9 weeks (not shown), and as the disease progressed, the shift became more pronounced over time. Fig 1D,E demonstrates this relationship measured at age 18 weeks.
Response to repeated activations
Affected dogs exhibit lower-than-normal isometric torque but generate a progressive increase (summation) in the isometric phase during successive repeated stretch-activations measured at ages 9,11, and 13 weeks (not shown). Fig 1F depicts this response at age 18 weeks, and it also shows that despite a progressive increase during repeated activations, maximum isometric torque never exceeded values observed in normal controls or carriers.
DISCUSSION
Findings in myotubularin-deficient animals can translate to application of diagnostic and therapeutic strategies for XLMTM patients. Although zebrafish12 and mouse13,14 models of XLMTM have been established, the discovery of an XLMTM canine model provides distinct advantages for preclinical applications, particularly for strategies with significant risk. For example, the overall body mass of dogs approaches that of young patients, and compared to rodents or fish, dogs provide clearer therapeutic endpoints and allow for repeated blood draws for serum chemistry analysis. In addition, measures of respiratory and immunological function are readily performed in dogs but are not easily applied in rodents or fish. Finally, because of similarities in disease progression and clinical course, the XLMTM dog model is analogous to patients with XLMTM and is therefore a superb preclinical model for investigation of high-risk, high-benefit therapeutics.
Our findings in this natural history study of the XLMTM dog provide objective measures of hind limb strength for preclinical endpoints. Our data also provide insight into the underlying pathophysiology resulting from a mutation in MTM1. Mutant dogs generate a gradual increase in isometric torque during a series of eccentric contractions until activations cease. Following a brief rest, the next activation of 10 eccentric contractions begins at a lower initial torque (Fig 1F). The pronounced torque summation from repetitive stimulation in XLMTM dogs is in keeping with recent findings in ryanodine-receptor (RyR1) mutant mice.15 Both mutant dogs and mice display similar responses to repetitive stimulation by increased force summation in vivo. While the precise molecular mechanism responsible for these observations is unknown, we hypothesize that a defect in the physiological process of converting an electrical stimulus to a mechanical response [known as excitation-contraction (E-C) coupling] accounts for these findings in the XLMTM dog. The right-shifted torque-frequency responses16 of the XLMTM muscles compared to those of the normal and carrier dogs, along with the gradual increase in the torque produced during a series of eccentric contractions, are in keeping with E-C coupling impairment as the underlying pathophysiology in this mutant animal.14,12,16,15 Interventions that improve contractile function can be tested in the XLMTM dog, and positive findings may translate into benefits for patients with XLMTM.
Acknowledgements
The Muscular Dystrophy Association, Association Française contre les Myopathies, and the Joshua Frase Foundation, the Anderson Family Foundation, and NIH R01 AR044345.
Abbreviations
- ANOVA
analysis of variance
- XLMTM
X-linked myotubular myopathy
- ECC
eccentric-contractions
- L0
muscle length at which tetanic torque is maximal
- N-m
Newton-meter
- Pt
twitches
References
- 1.Heckmatt JZ, Sewry CA, Hodes D, Dubowitz V. Congenital centronuclear (myotubular) myopathy. A clinical, pathological and genetic study in eight children. Brain. 1985;108(Pt 4):941–964. doi: 10.1093/brain/108.4.941. [DOI] [PubMed] [Google Scholar]
- 2.Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, Klauck SM, Poustka A, Dahl N. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet. 1996;13:175–182. doi: 10.1038/ng0696-175. [DOI] [PubMed] [Google Scholar]
- 3.Herman GE, Finegold M, Zhao W, de Gouyon B, Metzenberg A. Medical complications in long-term survivors with X-linked myotubular myopathy. J Pediatr. 1999;134:206–214. doi: 10.1016/s0022-3476(99)70417-8. [DOI] [PubMed] [Google Scholar]
- 4.Hu LJ, Laporte J, Kress W, Dahl N. Prenatal diagnosis of X-linked myotubular myopathy: strategies using new and tightly linked DNA markers. Prenat Diagn. 1996;16:231–237. doi: 10.1002/(SICI)1097-0223(199603)16:3<231::AID-PD842>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Jungbluth H, Wallgren-Pettersson C, Laporte J. Centronuclear (myotubular) myopathy. Orphanet J Rare Dis. 2008;3:26. doi: 10.1186/1750-1172-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laporte J, Blondeau F, Buj-Bello A, Mandel JL. The myotubularin family: from genetic disease to phosphoinositide metabolism. Trends Genet. 2001;17:221–228. doi: 10.1016/s0168-9525(01)02245-4. [DOI] [PubMed] [Google Scholar]
- 7.Beggs AH, Bohm J, Snead E, Kozlowski M, Maurer M, Minor K, Childers MK, Taylor SM, Hitte C, Mickelson JR, Guo LT, Mizisin AP, Buj-Bello A, Tiret L, Laporte J, Shelton GD. MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14697–14702. doi: 10.1073/pnas.1003677107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goddard MA, Mitchell EL, Smith BK, Childers MK. Establishing clinical end points of respiratory function in large animals for clinical translation. Physical medicine and rehabilitation clinics of North America. 2012;23:75–94. xi. doi: 10.1016/j.pmr.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Childers MK, Grange RW, Kornegay JN. In vivo canine muscle function assay. J Vis Exp. 2011 doi: 10.3791/2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tegeler CJ, Grange RW, Bogan DJ, Markert CD, Case D, Kornegay JN, Childers MK. Eccentric contractions induce rapid isometric torque drop in dystrophin-deficient dogs. Muscle & nerve. 2010;42:130–132. doi: 10.1002/mus.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornegay JN, Bogan DJ, Bogan JR, Childers MK, Cundiff DD, Petroski GF, Schueler RO. Contraction force generated by tarsal joint flexion and extension in dogs with golden retriever muscular dystrophy. J Neurol Sci. 1999;166:115–121. doi: 10.1016/s0022-510x(99)00118-5. [DOI] [PubMed] [Google Scholar]
- 12.Dowling JJ, Vreede AP, Low SE, Gibbs EM, Kuwada JY, Bonnemann CG, Feldman EL. Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet. 2009;5:e1000372. doi: 10.1371/journal.pgen.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buj-Bello A, Laugel V, Messaddeq N, Zahreddine H, Laporte J, Pellissier JF, Mandel JL. The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci U S A. 2002;99:15060–15065. doi: 10.1073/pnas.212498399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Qusairi L, Weiss N, Toussaint A, Berbey C, Messaddeq N, Kretz C, Sanoudou D, Beggs AH, Allard B, Mandel JL, Laporte J, Jacquemond V, Buj-Bello A. T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci U S A. 2009;106:18763–18768. doi: 10.1073/pnas.0900705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi N, Prosser BL, Ghassemi F, Xu L, Pasek DA, Eu JP, Hernandez-Ochoa EO, Cannon BR, Wilder PT, Lovering RM, Weber D, Melzer W, Schneider MF, Meissner G. Modulation of sarcoplasmic reticulum Ca2+ release in skeletal muscle expressing ryanodine receptor impaired in regulation by calmodulin and S100A1. Am J Physiol Cell Physiol. 2011;300:C998–C1012. doi: 10.1152/ajpcell.00370.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang W, Ingalls CP, Durham WJ, Snider J, Reid MB, Wu G, Matzuk MM, Hamilton SL. Altered excitation-contraction coupling with skeletal muscle specific FKBP12 deficiency. Faseb J. 2004;18:1597–1599. doi: 10.1096/fj.04-1587fje. [DOI] [PubMed] [Google Scholar]