Abstract
Mental stress often begins with a sudden sensory (or internal) stimulus causing a brief arousal reaction, and is followed by a more long lasting stress phase. Both arousal and stress regularly induce blood pressure (BP) increases whereas effects on muscle sympathetic nerve activity (MSNA) are variable. Here we have compared responses of MSNA and BP during arousal induced by an electrical skin stimulus and mental stress evoked by a 3 min paced auditory serial arithmetic test (PASAT) in 30 healthy males aged 33 ± 10 years. In addition, recordings were made of ECG, respiratory movements, electrodermal activity and perceived stress. We also monitored corresponding effects of a cold test (CT: 2 min immersion of a hand in ice water). The arousal stimulus evoked significant inhibition of one or two MSNA bursts in 16 subjects, who were classified as responders; the remaining 14 subjects were non-responders. During mental stress responders showed a significant decrease of MSNA and a lesser BP increase compared to non-responders. In non-responders MSNA was unchanged or increased. Perceived stress was higher in non-responders (P= 0.056), but other measures were similar in the two groups. In non-responders mental stress and the cold test induced increases of BP that lasted throughout the subsequent rest period. During the cold test MSNA and BP increased equally in responders and non-responders. In the whole group of subjects, there was a significant correlation (r= 0.80, P < 0.001) between MSNA responses induced by arousal and by mental stress but not between responses evoked by arousal and the cold test (r < 0.1, P > 0.6). Additionally arousal-induced MSNA change was positively correlated with blood pressure changes during MS (systolic BP: r= 0.48; P < 0.01; diastolic BP: r= 0.42; P < 0.05) but not with blood pressure changes during CT. We conclude that in males the MSNA response to arousal predicts the MSNA and BP responses to mental stress.
Key points
Mental stress (MS) is often initiated by a sensory or cognitive stimulus, which induces a brief arousal reaction followed by a longer stress phase. Both phases induce blood pressure (BP) increases whereas effects on muscle sympathetic nerve activity (MSNA) vary: in approximately 50% of healthy subjects (responders) arousal induces a brief MSNA reduction, which is absent in the remaining 50% (non-responders).
We now report a link between the arousal response and neurovascular effects of MS in healthy males.
Our data show that during MS, responders to arousal exhibited a significant decrease of MSNA and a lesser BP increase compared to non-responders. The whole material displayed a positive correlation between MSNA responses induced by arousal and MS. In addition, arousal induced MSNA changes correlated positively with BP changes during MS.
We conclude that the MSNA response to arousal predicts MSNA and BP responses to MS.
Introduction
Acute mental stress is known to induce transient increases of blood pressure (BP) and heart rate (HR) in healthy subjects. Both in experimental animals and in humans, the BP increase is the result of sympathetic activation and vasoconstriction in several vascular beds (Brod et al. 1959; Forsyth, 1972; Caraffa-Braga et al. 1973; Yu & Blessing 1997). In muscle, however, the sympathetic response to mental stress is controversial. In some studies mental stress has been reported to increase leg muscle sympathetic nerve activity (MSNA) (Anderson et al. 1987, 1991; Callister et al. 1992; Ng et al. 1993; Carter et al. 2002), but in others a decrease or no change have been reported (Matsukawa et al. 1991; Wasmund et al. 2002; Carter et al. 2005, 2008). Callister et al. (1992) suggested that the perception of stress may modulate the MSNA response, but this hypothesis has been recently challenged (Carter et al. 2008) and an explanation for the highly variable MSNA response to mental stress is still lacking.
We have previously reported (Donadio et al. 2002a,b) that MSNA responses to surprising sensory stimuli (i.e. visual, electrical or auditory stimuli) also display marked interindividual variability. When sensory stimuli inducing arousal were delivered 200–400 ms after the R wave of the ECG, a short-lasting inhibition of MSNA occurred in approximately 50% of healthy subjects. Importantly, subjects in whom inhibition occurred, also had a lesser blood pressure increase to the stimulus than subjects without inhibition. In repeated recordings in the same subject, presence or absence of inhibition was reproducible over 6 months, suggesting that the response behaviour is characteristic for the individual. In subsequent studies we found that the reflex is influenced by the strength of the stimulus: no inhibition occurred after weak stimuli or after prepulse inhibition of startle (Eder et al. 2009). In contrast, the inhibition was exaggerated in syncope patients who suffered from phobia to blood/injury (Donadio et al. 2007). These findings suggest that the reflex can be modified in relation to the degree of stress.
Against this background the aim of the present study was to search for a link between MSNA responses induced by arousal and by mental stress. More specifically, we wanted to test the following hypotheses: (a) individuals who display an arousal induced inhibition of MSNA are more likely to respond with a reduction of MSNA also during mental stress, and (b) the stress evoked BP response will be smaller in subjects who display an arousal induced reduction of MSNA.
Methods
We studied 30 healthy males aged 33 ± 10 (range 21–52) years with arterial blood pressure ≤130/80 (measured with a sphygmomanometer on the upper arm after approximately 20 min supine rest). The recordings were performed approximately 3 h after a light meal. Tobacco, caffeine and alcohol were not allowed for 12 h before the examination.
The experimental procedures were approved by the Human Ethics Committees and performed at both the University of Bologna (16 subjects) and the University of Göteborg (14 subjects); they followed the Declaration of Helsinki regarding international clinical research on humans. All subjects gave their written informed consent to the study.
Experimental procedures
During microneurography all subjects underwent an arousal test and a stress protocol, consisting of a mental stress (MS) test and a cold test (CT).
In 20 subjects both procedures were made during the same microneurographic recording, the stress protocol starting 15 min after the end of the arousal stimulation: MS was the first test administered in 11 subjects whereas CT came first in nine subjects. The 15 min of rest before arousal stimulation was taken as baseline for both MS and CT (Fig. 1A).
Figure 1. Experimental procedures.
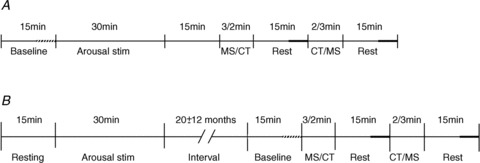
Schematic diagram illustrating the experimental procedures. A, in 20 subjects (8 responders and 12 non-responders) arousal stimulation as well as mental stress and cold tests were made in the same recording, first testing the effect of arousal and then (15 min after the end of arousal stimulation) the effects of stressors test. B, in 10 subjects (8 responders and 2 non-responders) two separate recordings were made, first evaluating the MSNA response to arousal and then 20 ± 12 months later, the effects of stressors. Period of analysis during baseline indicated by thick, interrupted horizontal line and during rest by thick, continuous horizontal line.
In 10 subjects two separate recordings were made, the first one testing arousal and the second one, 20 ± 12 months later, the stress protocol: MS was applied first in six and CT in four subjects. The 15 min of rest before the stress protocol was taken as baseline (Fig. 1B).
Measurements
Subjects were semi-reclining (upper body approximately 60 deg and lower legs approximately 45 deg from the horizontal plane, respectively) in a comfortable chair. ECG was recorded by Ag–AgCl electrodes on the chest and respiratory movements by a strain gauge belt around the lower part of the chest. Arterial finger blood pressure was monitored continuously by the volume-clamp method (Finometer model, Arnhem, The Netherlands), with the cuff around the middle phalanx of the third finger on the same side as the microneurography recording.
Electrodermal changes (EDCs) were recorded by Ag–AgCl surface electrodes placed on the palm (filter setting 0.2–100 Hz).
Multiunit post-ganglionic muscle sympathetic nerve activity (MSNA) was recorded with a tungsten microelectrode with a tip diameter of a few micrometres, inserted into a peroneal nerve, posterior to the fibular head. A low-impedance reference electrode was inserted subcutaneously a few centimetres away. The nerve signal was amplified (×50,000), filtered (band pass 700–2000 Hz) and fed through a discriminator for further noise reduction and audio monitoring. A mean voltage (integrated) display was obtained by passing the original signal through a resistance–capacitance circuit (time constant 0.1 s). During the experiment, neural activity and arterial pressure were monitored on a storage oscilloscope. When a muscle nerve fascicle had been identified, small electrode adjustments were made until a site was found in which sympathetic impulses with a good signal-to-noise ratio could be recorded. A recording of MSNA was considered acceptable when it revealed spontaneous, pulse-synchronous bursts of neural activity that fulfilled the criteria for MSNA, previously described (Sundlöf & Wallin, 1977). The filtered and integrated nerve signals were sampled (200 Hz) and stored together with other signals in a personal computer, using a locally produced data acquisition system.
Arousal stimulus
The arousal stimulus consisted of an electrical constant current square wave pulse (0.2 ms duration, 9–35 mA amplitude) triggered with a delay of 200 ms on the R-wave of the ECG and delivered to the index finger on the hand opposite to the microneurography recording (see Donadio et al. 2002a,b for detailed description). Prior to the insertion of the microneurography electrodes, the strength of the stimulus was adjusted to be as high as possible without causing pain, the aim being that each stimulus during the whole stimulation period would induce a high degree of arousal. The real stimuli were randomly interspersed with dummy stimuli, consisting of trigger pulse without subsequent electric shock (i.e. subjects were unaware of the dummy stimuli). Electrical or dummy stimuli were randomly delivered every 30 s in an irregular fashion, and the same order was used for all subjects. Intervals between real stimuli varied between 30 and 210 s. A total of 30 electrical and 30 dummy stimuli were applied in each subject.
Stress protocol
Mental stress
To elicit mental stress a 3 min paced auditory serial arithmetic test (PASAT) was used. The subject was required to add two sequentially presented single-digit numbers and to retain the latter of the two numbers in memory for subsequent addition to the next number presented (Willemsen et al. 1998). Numbers to add (1–9) were presented by a computer voice and were followed by a supposed sum number on a computer screen. Subjects, who were seated in front of the screen, were instructed to listen to the presented numbers and, after each number had been visualized on the screen, press with their right hand on either of two mouse buttons, indicating ‘right’ or ‘wrong’. The same sequence of numbers was used in all subjects. A total of 60 additions were presented.
Cold test
Subjects were instructed to immerse their right hand (with fingers spread) up to the wrist in an insulated container of ice water (4 °C) for 2 min.
Perceived stress
At the end of the experiment the experienced intensity of mental stress (PASAT test) or discomfort/pain (cold test) was assessed by a VAS scale. A subject was asked to indicate his perceived intensity of stress or discomfort/pain along a 100 mm horizontal line (0 = no stress or pain and 100 = maximum stress or pain). The rating was measured in millimetres from the left edge (= VAS score) (Myles et al. 1999).
Data analysis
Resting levels of MSNA are expressed as burst incidence (BI; bursts per 100 heart beats), burst frequency (BF; bursts per minute) The number of MSNA bursts was counted during the last 5 min of the baseline period as well as during the last 5 min of the rest after MS (rest 1) and CT (rest 2) (Fig. 1). The coefficient of variability of burst amplitude (CVA) was calculated as 100 × standard deviation of burst amplitude × (mean amplitude)−1.
MSNA effects induced by electrical stimuli
To analyse the effects of the electrical stimulus on MSNA, the following definitions were adopted (Fig. 2). The electrical stimulus was delivered in a cardiac cycle (between R waves 0 and 1) denoted cardiac interval (CI) 1. A sympathetic burst generated in the central nervous system during this cardiac interval is defined as burst 1 and will arrive at the recording electrode after a delay corresponding to the baroreflex latency (defined as the latency from the R wave of the ECG to the start of the inhibition (equal to the peak) of the appropriate burst in the mean voltage neurogram). When recording in the peroneal nerve at the fibular head, this delay is approximately 1.3 s (Fagius & Wallin, 1980). When present, a stimulus artefact was subtracted from the neurogram by the computer before starting the quantitative analysis (Donadio et al. 2002b). To quantify data the amplitudes of bursts 0 and 1 were normalized in relation to the mean amplitude (set to 100 units) of pre-stimulus bursts −10 to −2 (which were uncontaminated by artefacts from the electrical pulse) for either real or dummy stimulations in each subject. Then, in each subject the mean normalized amplitude for each of bursts 0 and 1 was compared to the mean amplitude of all bursts −10 to −2 both for real and dummy stimuli and expressed as a percentage. Absent bursts were included and given the value of zero (Donadio et al. 2007).
Figure 2. Relationship between sympathetic bursts, cardiovascular parameters and arousal stimulus.
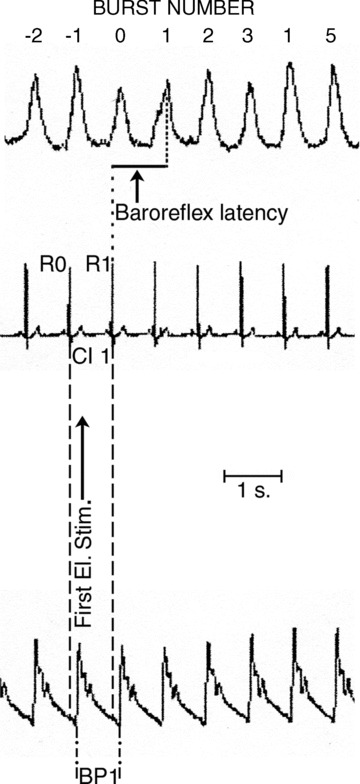
Definitions and terminology for the relationship between mean voltage neurogram, cardiovascular parameters and electrical stimulus (First El. Stim.) triggered with a delay of 200 ms from the R-wave of the ECG. The stimulus was delivered in cardiac interval 1 (CI 1). Baroreflex latency defined as time from R-wave 1 of the ECG (R1) to peak of burst 1 in mean voltage neurogram, i.e. burst 1 is terminated by the afferent baroreceptor discharge induced by the systolic pressure wave 2 occurring in CI 2; BP: blood pressure. The stimulus artefact was evident and subsequently removed before starting MSNA quantitative analysis.
MSNA changes during the stress protocol
Burst incidence, burst frequency and CVA were determined for the whole MS and CT periods. In addition, MSNA total activity (MSNA TA = burst/min × burst amplitude) was also compared between baseline and the whole period of the respective stresses. Due to large interindividual differences in resting activity, MSNA is presented in the text as normalized values in relation to baseline MSNA, which was set to 100. The effect of a test will then correspond to the deviation from 100. In Table 3 both absolute and normalized values are given. Two subjects were excluded from MS because of artefacts from muscle activation which prevented the identification of sympathetic bursts in the neurogram.
Table 3.
Absolute and normalized (%) sympathetic and cardiovascular values: statistical comparison between responders and non-responders
| Responders | Non-responders | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | MS | Rest 1 | CT | Rest 2 | Baseline | MS | Rest 1 | CT | Rest 2 | |
| RR (s) | 0.93±0.16 | 0.84±0.15 | 0.93±0.16 | 0.93±0.16 | 0.94±0.14 | 0.96±0.16 | 0.85±0.19 | 0.97±0.18 | 0.98±0.17 | 0.96±0.17 |
| RR (%) | 100 | 90±9 | 100±6 | 99±7 | 100±6 | 100 | 88±8 | 101±5 | 102±7 | 100±5 |
| SBP (mmHg) | 125±21 | 139±32 | 126±23 | 142±31 | 129±22 | 126±19 | 158±20 | 141±20 | 149±17 | 139±17 |
| SBP (%) | 100 | 111±15 | 101±9 | 114±17 | 103±9 | 100 | 126±11** | 112±8** | 119±14 | 111±10 |
| DBP (mmHg) | 70±13 | 76±20 | 71±17 | 78±21 | 71±16 | 71±19 | 84±18 | 76±19 | 82±15 | 76±16 |
| DBP (%) | 100 | 108±12 | 101±10 | 111±16 | 102±10 | 100 | 121±10** | 109±8* | 119±17 | 109±10 |
| BI (b/100HB) | 60±12 | 40±13 | 57±13 | 64±14 | 58±15 | 59±15 | 56±18* | 61±15 | 66±14 | 60±16 |
| BI (%) | 100 | 58±27 | 100±18 | 113±21 | 102±19 | 100 | 97±16*** | 107±15 | 118±20 | 105±14 |
| BF (b/min) | 41±14 | 31±13 | 38±12 | 43±14 | 39±14 | 36±7 | 40±10* | 38±8 | 41±8 | 38±7 |
| BF (%) | 100 | 76±16 | 100±14 | 114±17 | 101±16 | 100 | 113±12*** | 106±14 | 115±16 | 105±11 |
| MSNA TA (a.u.) | 16±18 | 11±16 | 14±11 | 37±52 | 29±52 | 22±10 | 22±12 | 24±9* | 27±10 | 23±10 |
| MSNA TA (%) | 100 | 56±29 | 108±40 | 192±85 | 135±67 | 100 | 102±28** | 114±30 | 135±41 | 112±28 |
| EDC (resp min−1) | 4±2 | 14±3 | 4±2 | 6±4 | 4±2 | 3±2 | 13±5 | 5±2 | 4±3 | 4±2 |
| EDC (%) | 100 | 518±250 | 144±53 | 226±134 | 138±90 | 100 | 602±418 | 213±191 | 160±136 | 192±161 |
| RF (breaths/min) | 15±2 | 20±3 | 15±2 | 16±3 | 15±3 | 16±3 | 21±3 | 16±3 | 18±3 | 16±3 |
| RF (%) | 100 | 135±27 | 96±9 | 109±23 | 97±10 | 100 | 138±22 | 100±6 | 115±18 | 103±9 |
EDCs were expressed as frequency (responses per minute). To be included, the amplitude of an electrodermal change had to exceed 5% of the biggest spontaneous deflection occurring during the baseline period (Donadio et al. 2005). EDC data are presented in the text as normalized values using the same convention as for MSNA.
Cardiovascular variables
For each analysis period, the computer determined systolic (SBP) and diastolic (DBP) blood pressures and R-R interval of all individual cardiac cycles. Data are presented as normalized values in the text using the same convention as for MSNA.
Respiration frequency (RF)
The number of breaths was counted manually for each period of analysis and expressed as breaths min−1. Only normalized data are reported in the text.
Statistics
All values are expressed as means ± SD.
Arousal stimulation
Significant effect on MSNA (i.e. responder) was defined in each individual by Student's two-tailed t test for paired data comparing mean normalized amplitude of bursts −10 to −2 with each of bursts 0 and 1. Bonferroni corrections for two repeated tests (Statistica, StatSoft Inc., Tulsa, OK, USA) were made using a nominal level of significance at P= 0.05. Separate analyses were made for real and dummy stimuli (cf. Donadio et al. 2002a).
Stress protocol
Repeated paired t tests with the Bonferroni correction for multiple comparisons were used to test time-dependent changes during the stress protocol from the baseline in MSNA, EDC, SBP, DBP, R-R interval and RF. Unpaired t tests were used to detect significant difference between responders and non-responders. Linear regression analysis was used to correlate: (1) arousal-induced change of the normalized MSNA burst 0 or 1 amplitude (in each subject the burst showing the lowest amplitude was selected for the analysis) with the normalized MSNA and BP changes during the stress protocol; (2) MSNA change during stress protocol with normalized BP parameters; (3) VAS score or percentage of correct answers at the PASAT test with sympathetic or cardiovascular changes during the stress protocol.
P < 0.05 was considered significant.
Results
Resting levels
Sympathetic and cardiovascular activities during baseline, MS and CT showed no differences between subjects performing different experimental procedures (Fig. 1A and B), and hence only pooled data from the whole material will be presented.
Baseline MSNA, EDCs, SBP, DBP, R-R interval and RF did not differ between responders and non-responders (Table 1). CVA was, however, higher in responders than in non-responders (57 ± 10 and 50 ± 7% respectively; P < 0.05). In the text below, results regarding the effects of the different manoeuvres will be given as normalized data but in Table 3 absolute values are also presented.
Table 1.
Demographic characteristics and baseline values in responders and non-responders
| MSNA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | Age (years) | Incidence (bursts (100 HB−1)) | Frequency (bursts min−1) | EDC (resp min−1) | SBP (mmHg | DBP (mmHg) | RR (s) | RF (breaths min−1) | |
| Responders | 16 | 33 ± 10 | 60 ± 12 | 41 ± 14 | 3 ± 3 | 125 ± 21 | 70 ± 13 | 0.9 ± 0.2 | 15 ± 2 |
| Non-responders | 14 | 34 ± 12 | 59 ± 15 | 36 ± 7 | 3 ± 2 | 126 ± 19 | 71 ± 19 | 1 ± 0.2 | 16 ± 3 |
| P | 0.8 | 0.9 | 0.3 | 0.4 | 0.9 | 0.9 | 0.6 | 0.7 | |
MSNA, muscle sympathetic nerve activity; EDC, electrodermal change; SBP, systolic BP; DBP, diastolic BP; RR, cardiac interval; RF, respiratory frequency.
Arousal responses
Of the 30 studied subjects 16 were classified as responders, i.e. they displayed a significant arousal induced reduction of the averaged amplitude of MSNA burst 0 or 1 or both (Fig. 3 upper panel). In the remaining 14 subjects there was no significant decrease of bursts 0 or 1 and these subjects were classified as non-responders (Fig. 3 lower panel). Dummy stimuli induced no changes of burst amplitudes in any subject.
Figure 3. Sympathetic changes induced by arousal stimulation and stress protocol in a responder and a non-responder subject, respectively.
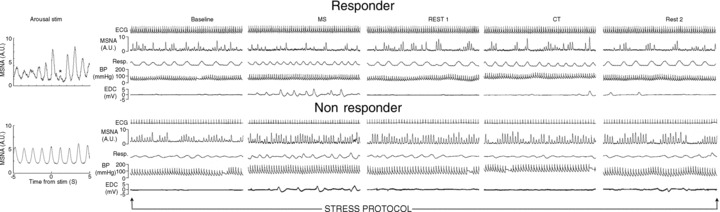
Arousal-induced effect on MSNA burst amplitude (left) and MSNA changes induced during the stress protocol (right) in a responder and a non-responder subject. The stimulus artefact during arousal stimulation was removed in both subjects. Responder showed significant decrease of MSNA following arousal stimulation (*) and during mental stress whereas cold test produced an MSNA increase. Non-responder displayed no change of MSNA amplitude following arousal stimulation and both mental stress and cold test produced increases of MSNA and BP. Additionally MSNA was still increased in the post-mental stress recovery period.
Mental stress induced effects
Changes from baseline in responders
On a group basis MSNA BI, BF and MSNA TA decreased during MS (P < 0.01) and returned to the baseline level during Rest 1 (Tables 2 and 3; Fig. 3). On an individual basis both BI and BF decreased in 13 subjects (93%) and increased in only one subject.
Table 2.
Statistical evaluation of changes from baseline in responders and non-responders (for numerical data see Table 3)
| Responders | Non-responders | |||||||
|---|---|---|---|---|---|---|---|---|
| MS | Rest 1 | CT | Rest 2 | MS | Rest 1 | CT | Rest 2 | |
| RR (%) | §§ | — | — | — | §§ | — | — | — |
| SBP (%) | * | — | * | — | *** | *** | *** | ** |
| DBP (%) | — | — | — | — | *** | ** | ** | * |
| BI (%) | §§§ | — | — | — | — | — | * | — |
| BF (%) | §§§ | — | * | — | ** | — | * | — |
| MSNA TA (%) | §§§ | — | ** | — | — | — | * | — |
| EDC (%) | *** | * | ** | — | ** | — | — | — |
| RF (%) | ** | — | — | — | *** | — | * | — |
Repeated paired t tests, Bonferroni corrected for multiple comparisons, were used to test time-dependent changes during the stress protocol from the baseline in MSNA, EDC, SBP, DBP, R-R interval and RF. Significant increase of considered parameter indicated by *P < 0.05; **P < 0.01; ***P < 0.001. Significant decrease indicated by §§P < 0.01 and §§§P < 0.001; no significant effect represented by —. BI, MSNA burst incidence; BF, MSNA burst frequency; MSNA TA, MSNA total activity; MS, mental stress; CT, cold test; other abbreviations as in Table 1.
Cardiovascular variables also showed significant changes during mental stress: SBP increased (P < 0.01) whereas R-R interval decreased (P < 0.001). DBP did not show a significant change during MS. All these values returned to baseline during Rest 1 (Tables 2 and 3; Fig. 4). Respiratory frequency increased to 135 ± 27% during MS (P < 0.001). EDCs showed a pronounced increase during MS (P < 0.001) which persisted slightly also during Rest 1 (Tables 2 and 3).
Figure 4. Normalized time-dependent cardiovascular changes during stress protocol.
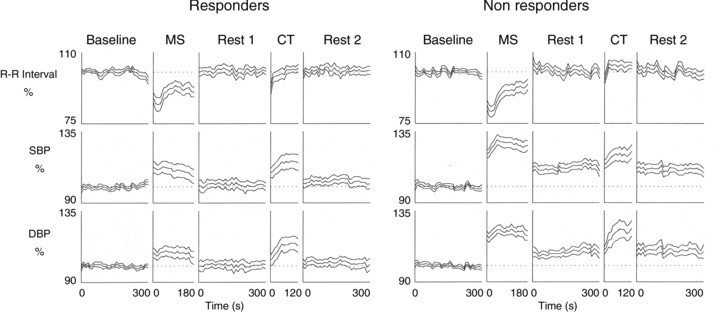
Time-dependent changes of cardiovascular parameters in responders and non-responders. Mental stress (MS) and cold test (CT) induced significant increases of blood pressure and heart rate in both groups. Dotted line during MS, Rest 1, CT and Rest 2 represents mean normalized (= 100) value during baseline. Values are reported as mean (middle line) ± SEM change from baseline. Note, greater increases of BP during and no return to baseline after MS in non-responders compared to responders
The number of correct answers during the PASAT test was 88 ± 11% and showed no correlation with changes of MSNA, EDC, BP and R-R interval. The VAS score of perceived stress (group mean value 45 ± 20 mm) showed a positive relationship to MSNA, which was close to statistical significance (P= 0.08), but there was no correlation to BP or R-R interval.
Changes from baseline in non-responders
On a group basis MSNA BI and TA were unchanged during MS whereas BF displayed a significant increase (P < 0.01) (Tables 2 and 3; Fig. 3). Individually, BF increased in 11 (79%) and BI in eight individuals (57%); in one subject MSNA BF was unchanged (7%) and MSNA decreased in two (BF) or five (BI) subjects.
SBP and DBP increased during MS (P < 0.001) and the increase was present also during Rest 1. R-R interval decreased during MS (P < 0.001) and returned to baseline during Rest 1 (Tables 2 and 3; Fig. 4). RF showed a pronounced increase during MS (138 ± 22%; P < 0.001). EDCs increased during mental stress (P < 0.001) and returned to the baseline level during Rest 1.
The number of correct answers in the PASAT test (group mean 89 ± 12%) and the VAS score of perceived stress (group mean 59 ± 16 mm) did not correlate with changes of sympathetic and cardiovascular parameters.
Responders vs. non-responders (Table 3)
During MS there were significant differences in MSNA BI, BF and TA (P < 0.01), normalized SBP and DBP (P < 0.01) between responders and non-responders. The difference was sustained over Rest 1 for MSNA TA (P < 0.01), normalized SBP (P < 0.01) and DBP (P < 0.05).
During MS there was a weak correlation between MSNA and SBP (r= 0.5, P= 0.02) but not for DBP.
No differences were found between responders and non-responders for CVA (59 ± 11 and 64 ± 13%, respectively), EDCs, change of R-R interval or change of RF. The PASAT test of correct answers did not show any difference between responders and non-responders whereas the difference in VAS score was close to significance (P= 0.059).
Cold test effects
Changes from baseline in responders and non-responders
Changes of sympathetic and cardiovascular changes were similar in both groups. MSNA BF and TA were characterized by pronounced increases during CT with a return to baseline values during Rest 2 (Table 3; Fig. 3). In both groups SBP increased significantly during CT (P < 0.05) whereas DBP increased significantly only in non-responders. In responders blood pressures returned to the baseline level during Rest 2 whereas non-responders showed a sustained, small increase also during Rest 2 (P < 0.05) (Tables 2 and 3; Fig. 4). A small but significant increase of RF was found in non-responders. EDC did not change significantly in non-responders but showed a slight but significant increase in responders (Tables 2 and 3). There was no correlation between MSNA and BP changes during the CT.
VAS scale data (38 ± 19 and 35 ± 11 mm in responders and non-responders, respectively) did not correlate with changes of MSNA, BP or R-R interval in either group of subjects.
Responders vs. non-responders
No significant differences were found between the groups for MSNA, EDC, SBP, DBP, R-R interval and RF during CT and rest 2 (Table 3).
Relationship between MSNA responses to arousal and stress protocol
The percentage change of MSNA amplitude induced by arousal stimulation showed a fairly close correlation to normalized MSNA BI and BF during MS (r= 0.8 for both) (Fig. 5A). A similar correlation during MS was also found with normalized MSNA TA (r= 0.44; P < 0.05). In contrast, no corresponding correlations were found during the CT (Fig. 5B).
Figure 5. Relationship between MSNA amplitude change following arousal stimulation and MSNA change during stress protocol.
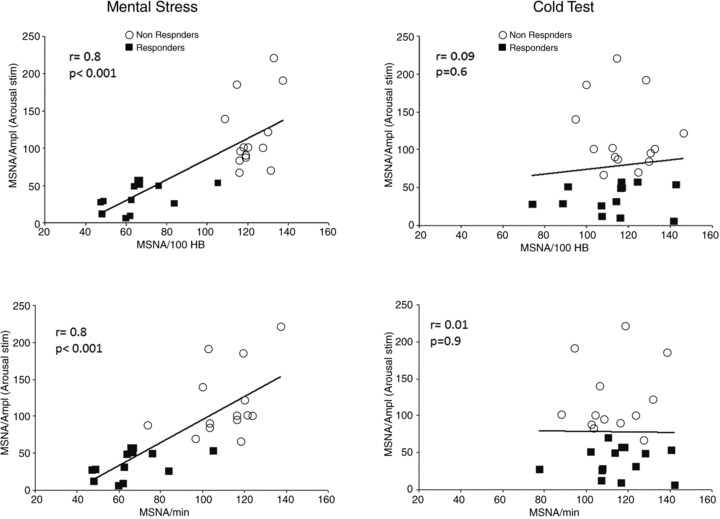
Linear correlations between normalized MSNA amplitude change following arousal stimulation and changes of MSNA BI (upper panel) and BF (lower panel) during mental stress and cold test.
Additionally arousal-induced MSNA change was positively correlated with blood pressure changes during MS (SBP: r= 0.48; P < 0.01; DBP: r= 0.42; P < 0.05) but not with blood pressure changes during CT.
Discussion
The main finding of the present study is that in young healthy males, changes of muscle sympathetic nerve activity in response to arousal are linked to neural and cardiovascular responses during mental stress. Specifically, our results show (1) that subjects who responded to arousal with a short lasting MSNA inhibition (responders), also displayed a decrease of sympathetic activity during 3 min of mental stress; in contrast, non-responders to arousal showed an increase of MSNA during mental stress; (2) that the mental stress resulted in greater and sustained BP increases in non-responders than in responders whereas increases in breathing frequency were similar in the two groups; and (3) that the results are specific to mental stress, since responders and non-responders to arousal showed similar neural and cardiovascular responses to a different stressor (cold test).
Sympathetic responses
Naturally occurring mental stress is often initiated by a sudden sensory (auditory, visual, touch, etc.) or internal (e.g. a sudden thought or insight) stimulus, which induces a short lasting arousal or alarm reaction, followed by a more long lasting phase of mental stress, the strength of which depends on the estimated seriousness of the problem. We have previously shown that the immediate MSNA response to surprising sensory stimuli displays marked interindividual variability: in approximately 50% of studied subjects the stimulus led to an inhibition of one or two sympathetic bursts (Donadio et al. 2002a,b, 2007, Eder et al. 2009) whereas in the remaining 50% of the subjects there was no inhibition. Previous studies of the mental stress phase have also shown a high degree of variability, and MSNA has (for unknown reasons) been found to either increase (Callister et al. 1992; Carter et al. 2002; Ng et al. 1993), or decrease or remain unchanged (Matsukawa et al. 1991; Carter et al. 2008; Carter & Ray 2009) during mental stress.
In most previous mental stress studies the test has been either the Stroop test (Callister et al. 1992) or mental arithmetics using forced mental subtractions (Callister et al. 1992; Carter et al. 2008; Carter & Ray, 2009). Our present results confirm these previous findings, and indicate that the PASAT test gives similar response variability to the other two mental stress tests. Importantly, however, we also found that the variations of results with the arousal and the mental stress tests are not random: the correlation between changes of MSNA during arousal and mental stress suggests that underlying neural mechanisms are similar or coupled. Previously, we have shown that the arousal induced effects on MSNA display intraindividual reproducibility over at least 6 months (Donadio et al. 2002b), indicating that the arousal response is characteristic for the individual. This finding, together with the correlation between arousal and stress responses, suggests (1) that an individual's MSNA response to mental stress also is reproducible over time and characteristic for the individual and (2) that an individual's change of MSNA during arousal provides a marker of his or her MSNA and BP changes during mental stress.
Interestingly, during baseline responders showed a higher variability of burst amplitude than non-responders, perhaps as evidence of ongoing environmental attention. If so, the lack of difference between the groups in burst amplitude variability during mental stress may be a consequence of subjects focusing on the ongoing task.
Cardiovascular responses
Mental stress has previously been found to increase cardiac output and vascular resistance in several vascular beds except skeletal muscle (Brod et al. 1959), leading to a redistribution of blood towards the muscles. This effect is functionally relevant in terms of fight or flight and can probably be regarded as a human counterpart of a defence reaction (Hilton 1982), which may be orchestrated by hypothalamic neurons expressing orexin (Zhang et al. 2006; Kuwaki 2011).
Our present results show that these cardiovascular events display systematic interindividual differences: in subjects who responded with MSNA inhibition to surprising sensory stimuli, mental stress was associated with MSNA reduction and a lesser BP increase than in non-responders. Presumably, the attenuation of the BP response is a consequence of the MSNA reduction inducing a decrease of vascular resistance in skeletal muscle. In a fight or flight situation this effect may give responders a functional advantage over non-responders because of an earlier and more efficient oxygen supply to the muscles before metabolic vasodilatation becomes active. Furthermore, in addition to having a greater BP increase during mental stress, the BP of the non-responders (as well as their total MSNA) remained higher than in responders also during the succeeding rest period. Prolonged sympathetic and cardiovascular activation after mental stress has been reported previously (Callister et al. 1992; Carter et al. 2005) but the present results indicate that this applies to non-responders only; responders returned to the control level almost immediately after the end of the stress period (Fig. 4). If these findings are extrapolated to everyday life in modern society with its multitude of arousing stimuli and stressful situations, it means that individuals who are non-responders will be subjected to repeated episodes when their sympathetic outflow is stronger and their blood pressure higher for longer times periods than in responders.
Stress level and perception
Sudomotor nerve activity is known to increase during mental stress (Delius et al. 1972; Oshima et al. 2001), and our measurements of EDC provided a measure of the stress induced activation of skin sympathetic nerve activity. EDC frequencies did not differ between responders and non-responders, indicating that this type of cognitive activation (cf. Critchley 2002) was similar in the two groups. This agrees with the two groups having similar frequencies of correct answers during the mental stress test. In addition, we found that mental stress tachypnoea and tachycardia were similar in the two groups. Our only finding suggesting a difference in the type of mental activation between the two groups was a tendency for a higher (P= 0.059) VAS score of perceived stress in non-responders. Thus, non-responders had higher MSNA, BP and VAS score than responders, and in this sense our results agree with Callister et al. (1992), who found that MSNA increased during mental stress in proportion to the perception of stress. Several other studies, however, have not found a relationship between perceived stress and MSNA during mental stress (Carter et al. 2008; Carter & Ray 2009). A possible reason for this discrepancy between studies may be related to the design of the stress tests. In the study of Callister et al. (1992) the subjects had to grade the perceived stress in several tests with increasing levels of difficulty whereas in other studies, only a single test was used. The psychological reactions induced during mental stress include discomfort, insufficiency, anxiety and anger (Wahlström et al. 2002; Williams et al. 2009). In addition, it should be remembered that immobilization is a well defined model of stress (Wang et al. 2011) and since microneurography requires subjects to lie still and avoid muscle tension during the different manoeuvres, this may also be a factor contributing to the degree of stress. Therefore, it is hardly surprising that the subjects have difficulties quantifying this wide range of feelings by a single test score. The repeated comparisons in the study of Callister et al. (1992) may have improved the reliability of the judgements in that study as compared to that of a single assessment in other studies.
The cold test has previously been found to be associated with increases in MSNA and BP without consistent changes of EDC and R-R interval (Victor et al. 1987; Fagius et al. 1989; Cui et al. 2002) or with a mild increase of EDC (Fagius & Blumberg, 1985). Our present results confirm these previous findings. Importantly, there was no difference between responders and non-responders during the cold stress, thereby indicating that the difference between the two groups is specific for mental stress.
Limitations
A limitation of the present study is the long time delay between the rest period and subsequent periods of stress in subjects who underwent the experimental protocol described in Fig. 1A. The delay was introduced to avoid using a resting blood pressure value which, in non-responders, still had not returned to the control level after the stress tests. Therefore, to avoid a false resting value we decided to use the last 5 min of the initial basal recording as rest period for mental stress and cold tests in subjects in whom effects of arousal stimulation and the stress protocol were studied in the same experimental session. Since resting MSNA is known to be highly reproducible over time (Sundlöf & Wallin, 1977), we considered the 45 min delay between rest and manoeuvre to be a lesser source of error than an increase of resting blood pressure induced by the stress tests.
The fact that 10 subjects were studied, first in a session in which only arousal stimulation was performed and then, many months later, in a session during which the stressor protocol was applied, may be seen as a limitation of the study. However, in view of our finding of a fairly close correlation between responses to arousal and mental stress, it may also be seen as a strength, confirming that arousal induced MSNA responses show intra-individual reproducibility over a long time (cf. Donadio et al. 2002b).
Clinical perspectives
Several factors may contribute to the development of chronic hypertension and there is little doubt that both genetic mechanisms and chronic mental stress can be involved (Garcia et al. 2003; Esler et al. 2008; Schwatz et al. 2011). For example, it is known that offspring to hypertensive patients run a greater risk of developing hypertension than offspring of non-hypertensive patients (Goldstein et al. 2006). In this context it is interesting to note that our finding of differences between non-responders and responders regarding responses to mental stress and the cold test have a counterpart in studies of offspring of hypertensive patients: Noll et al. (1996) found that offspring of hypertensive patients had stronger MSNA responses to mental stress than the control group. In contrast, this was not the case for the cold pressor test (Lambert & Schleich 2004). In other words, in relative terms the response to mental stress in offspring to hypertensive patients was similar to that of our non-responders.
With regard to chronic mental stress Timio et al. (1997) have provided convincing evidence that females, living in a western style environment, develop age related increases of blood pressure and die at younger ages compared to nuns living in a secluded environment in the same geographical area.
There is also evidence suggesting that subjects with high blood pressure reactivity are at higher risk of developing hypertension (Matthews et al. 1993; Everson et al. 1996; Carroll et al. 2001; Flaa et al. 2008; Chida & Steptoe 2010) and/or atherosclerosis (Jennings et al. 2004) later in life. The present finding of a correlation between the sympathetic contribution to a subject's arousal and mental stress responses may provide a physiological link to these clinical experiences: subjects who are non-responders to arousal stimuli will have the largest sympathetic and BP responses both to arousal and mental stress, and thereby be more likely than responders to develop later hypertension and cardiovascular disease.
In conclusion, the present findings show that a young male subject's MSNA response to arousal predicts his MSNA and BP responses to mental stress. Subjects who respond with arousal induced MSNA inhibition reduce their MSNA and get lesser blood pressure increases during mental stress than subjects who don't respond to arousal with MSNA inhibition. The results may have implications for the development of stress related cardiovascular disease.
Acknowledgments
We are grateful to Massimo Armaroli for excellent technical collaboration. Supported by the Swedish Research Council (proj 12170) and the Gothenburg Medical Faculty.
Glossary
- BI
burst incidence
- BF
burst frequency
- BP
blood pressure
- CI
cardiac interval
- CT
cold test
- DBP
diastolic blood pressure
- CVA
coefficient of variability of burst amplitude
- EDC
electrodermal change
- HR
heart rate
- MSNA
muscle sympathetic nerve activity
- MS
mental stress
- PASAT
paced auditory serial arithmetic test
- RF
respiration frequency
- SBP
systolic blood pressure
- TA
total activity
Author contributions
V.D.: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, acquisition of data; R.L.: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, study supervision; M.E.: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, study supervision; T.K.: study concept and design, analysis and interpretation of data, acquisition of data; M.P.G.: analysis and interpretation of data, acquisition of data; G.P.: analysis and interpretation of data, acquisition of data; F.G.: acquisition of data; B.G.W.: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, acquisition of data, study supervision. The authors have reported no conflicts of interest. All authors have approved the final version for publication.
References
- Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;6(Suppl III):114–119. doi: 10.1161/01.hyp.9.6_pt_2.iii114. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Mark AL. Mental stress increases sympathetic nerve activity during sustained baroreceptor stimulation in humans. Hypertension. 1991;17(Suppl III):43–49. doi: 10.1161/01.hyp.17.4_suppl.iii43. [DOI] [PubMed] [Google Scholar]
- Brod J, Fencl V, Hejl Z, Jirka J. Circulatory changes underlying blood pressure elevation during acute emotional stress (mental arithmetic) in normotensive and hypertensive subjects. Clin Sci. 1959;18:269–279. [PubMed] [Google Scholar]
- Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–387. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraffa-Braga E, Granata L, Pinotti O. Changes in blood-flow distribution during acute emotional stress in dogs. Pflugers Arch. 1973;339:203–216. doi: 10.1007/BF00587372. [DOI] [PubMed] [Google Scholar]
- Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med. 2001;63:737–743. doi: 10.1097/00006842-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Carter JR, Ray CA, Cooke WH. Vestibulosympathetic reflex during mental stress. J Appl Physiol. 2002;93:1260–1264. doi: 10.1152/japplphysiol.00331.2002. [DOI] [PubMed] [Google Scholar]
- Carter JR, Durocher JJ, Kern RP. Neural and cardiovascular responses to emotional stress in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1898–R1903. doi: 10.1152/ajpregu.90646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol. 2005;564:321–327. doi: 10.1113/jphysiol.2004.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol. 2009;296:H847–H853. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist. 2002;8:132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol. 2002;282:H1717–H1723. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Donadio V, Kallio M, Karlsson T, Nordin M, Wallin BG. Inhibition of human muscle sympathetic nerve activity by sensory stimulation. J Physiol. 2002a;544:285–292. doi: 10.1113/jphysiol.2002.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio V, Karlsson T, Elam M, Wallin BG. Interindividual differences in sympathetic and effector responses to arousal in humans. J Physiol. 2002b;544:293–302. doi: 10.1113/jphysiol.2002.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio V, Lenzi P, Montagna P, Falzone F, Baruzzi A, Liguori R. Habituation of sympathetic sudomotor and vasomotor skin responses: neural and non-neural components in healthy subjects. Clin Neurophysiol. 2005;116:2542–2549. doi: 10.1016/j.clinph.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Donadio V, Liguori R, Elam M, Karlsson T, Montagna P, Cortelli P, Baruzzi A, Wallin BG. Arousal elicits exaggerated inhibition of sympathetic nerve activity in phobic syncope patients. Brain. 2007;130:1653–1662. doi: 10.1093/brain/awm037. [DOI] [PubMed] [Google Scholar]
- Eder DN, Elam M, Wallin BG. Sympathetic nerve and cardiovascular responses to auditory startle and prepulse inhibition. Int J Psychophysiol. 2009;71:149–155. doi: 10.1016/j.ijpsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol. 2008;35:498–502. doi: 10.1111/j.1440-1681.2008.04904.x. [DOI] [PubMed] [Google Scholar]
- Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Anticipatory blood pressure responses to exercise predicts future high blood pressure in middle-age men. Hypertension. 1996;27:1059–1064. doi: 10.1161/01.hyp.27.5.1059. [DOI] [PubMed] [Google Scholar]
- Fagius J, Karhuvaara S, Sundlöf G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand. 1989;137:25–34. doi: 10.1111/j.1748-1716.1989.tb08760.x. [DOI] [PubMed] [Google Scholar]
- Fagius J, Blumberg H. Sympathetic outflow to the hand in patients with Raynaud's phenomenon. Cardiovasc Res. 1985;19:249–253. doi: 10.1093/cvr/19.5.249. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure : An 18-year follow-up study. Hypertension. 2008;52:336–341. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- Forsyth RP. Sympathetic nervous system control of distribution of cardiac output in unanesthetized monkeys. Fed Proc. 1972;31:1240–1244. [PubMed] [Google Scholar]
- Garcia EA, Newhouse S, Caulfield MJ, Munroe PB. Genes and hypertension. Curr Pharm Des. 2003;9:1679–1689. doi: 10.2174/1381612033454513. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Shapiro D, Guthrie D. Ambulatory blood pressure and family history of hypertension in healthy men and women. Am J Hypertens. 2006;19:86–91. doi: 10.1016/j.amjhyper.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Hilton SM. The defence-arousal system and its relevance for circulatory and respiratory control. J Exp Biol. 1982;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarack TW, Everson-Rose SA, Kaplan A, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middleaged Finnish men. Circulation. 2004;110:2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2011;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lambert EA, Schlaich MP. Reduced sympathoneural responses to the cold pressor test in individuals with essential hypertension and in those genetically predisposed to hypertension. No support for the ‘pressor reactor’ hypothesis of hypertension development. Am J Hypertens. 2004;17:863–868. doi: 10.1016/j.amjhyper.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand. 1991;141:157–165. doi: 10.1111/j.1748-1716.1991.tb09064.x. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Woodall KL, Michael T. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–485. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear. Anesth Analg. 1999;89:1517–1520. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, Weidmann P, Lüscher TF. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation. 1996;93:866–869. doi: 10.1161/01.cir.93.5.866. [DOI] [PubMed] [Google Scholar]
- Oshima A, Miyano H, Yamashita S, Owashi T, Suzuki S, Sakano Y, Higuchi T. Psychological, autonomic and neuroendocrine responses to acute stressors in the combined dexamethasone/CRH test: a study in healthy subjects. J Psychiatr Res. 2001;35:95–104. doi: 10.1016/s0022-3956(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Durocher JJ, Carter JR. Neurovascular responses to mental stress in prehypertensive humans. J Appl Physiol. 2011;110:76–82. doi: 10.1152/japplphysiol.00912.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timio M, Lippi G, Venanzi S, Gentili S, Quintaliani G, Verdura C, Monarca C, Saronio P, Timio F. Blood pressure trend and cardiovascular events in nuns in a secluded order: a 30-year follow-up study. Blood Press. 1997;6:81–87. doi: 10.3109/08037059709061804. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- Wahlström J, Hagberg M, Johnson PW, Svensson J, Rempel D. Influence of time pressure and verbal provocation on physiological and psychological reactions during work with a computer mouse. Eur J Appl Physiol. 2002;87:257–263. doi: 10.1007/s00421-002-0611-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Spiess J, Wong PT, Zhu YZ. Blockade of CRF1 and CCK2 receptors attenuated the elevated anxiety-like behavior induced by immobilization stress. Pharmacol Biochem Behav. 2011;98:362–368. doi: 10.1016/j.pbb.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Wasmund WL, Westerholm EC, Watenpaugh DE, Wasmund SL, Smith ML. Interactive effects of mental and physical stress on cardiovascular control. Appl Physiol. 2002;92:828–834. doi: 10.1152/japplphysiol.00019.2001. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Ring C, Carroll D, Evans P, Clow A, Hucklebridge F. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic and cold pressor. Psychophysiology. 1998;35:252–259. [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Ann Behav Med. 2009;37:26–40. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Yu YH, Blessing WW. Cutaneous vasoconstriction in conscious rabbits during alerting responses detected by hippocampal theta-rhythm. Am J Physiol Regul Integr Comp Physiol. 1997;272:R208–R216. doi: 10.1152/ajpregu.1997.272.1.R208. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1654–R1663. doi: 10.1152/ajpregu.00704.2005. [DOI] [PubMed] [Google Scholar]


