ABSTRACT
DNA lesions in the template strand block synthesis by replicative DNA polymerases (Pols). Eukaryotic cells possess a number of specialized translesion synthesis (TLS) Pols with the ability to replicate through DNA lesions. The Epstein-Barr virus (EBV), a member of the herpesvirus family, infects human B cells and is maintained there as an extrachromosomal replicon, replicating once per cycle during S phase. Except for the requirement of the virus-encoded origin-binding protein EBNA1, replication of plasmids containing the EBV origin of replication (oriP) is controlled by the same cellular processes that govern chromosomal replication. Since replication of EBV plasmid closely mimics that of human chromosomal DNA, in this study we examined the genetic control of TLS in a duplex plasmid in which bidirectional replication initiates from an EBV oriP origin and a UV-induced cis-syn TT dimer is placed on the leading- or the lagging-strand DNA template. Here we show that TLS occurs equally frequently on both the DNA strands of EBV plasmid and that the requirements of TLS Pols are the same regardless of which DNA strand carries the lesion. We discuss the implications of these observations for TLS mechanisms that operate on the two DNA strands during chromosomal replication and conclude that the same genetic mechanisms govern TLS during the replication of the leading and the lagging DNA strands in human cells.
IMPORTANCE
Since replication of EBV (Epstein-Barr virus) origin-based plasmids appropriates the cellular machinery for all the steps of replication, our observations that the same genetic mechanisms govern translesion synthesis (TLS) on the two DNA strands of EBV plasmids imply that the requirements of TLS Pols are not affected by any of the differences in the replicative Pols or in other proteins that may be used for the replication of the two DNA strands in human cells. These findings also have important implications for evaluating the significance of results of TLS studies with the SV40 origin-based plasmids that we have reported previously, in which we showed that TLS occurs similarly on the two DNA strands. Since the genetic control of TLS in SV40 plasmids resembles that in EBV plasmids, we conclude that TLS studies with the SV40 plasmids are as informative of TLS mechanisms that operate during cellular replication as those with the EBV plasmids.
Introduction
Epstein-Barr virus (EBV), a member of the herpesvirus family, exists in a latent form in over 90% of the world’s population. The 165-kb genome of EBV is maintained as a nucleosome-coated nuclear plasmid in infected B cells, replicating once per cell cycle during S phase. EBV infection causes infectious mononucleosis, and it contributes to Burkitt’s lymphoma, T-cell lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma, and other cancers. Because of its ability to be maintained as an extrachromosomal plasmid in infected B lymphocytes, and because it uses the cellular machinery for all aspects of DNA replication, studies of EBV replication have been useful for understanding the DNA replication mechanisms in human chromosomes (1).
A 1.7-kb region of the EBV chromosome, oriP, supports the replication and maintenance of recombinant plasmids in human cells, and EBNA1 is the only virus-encoded protein that is required (2, 3). A dyad symmetry (DS) region of ~120 bp is the functional replicator of oriP, and this region is sufficient for initiation of bidirectional replication in the presence of EBNA1 (4–7). Because EBNA1 lacks DNA helicase or any other enzymatic activities, replication of EBV plasmid relies upon the host cellular DNA replication machinery for initial unwinding of DNA, for initiation of DNA replication, and for the other steps needed for replication of the entire plasmid (8, 9). EBNA1-dependent replication from the EBV origin is regulated to occur only once per cell cycle by the same licensing mechanism that is used for chromosome replication (10, 11). Following the binding of EBNA1 to oriP, the origin recognition complex (ORC1 to ORC6) and the regulatory protein Cdc6 are recruited, which together effect the recruitment of the MCM2 through MCM7 helicase complex (1, 6, 12–15). The association of these proteins at oriP with the licensing protein Cdt1 initiates the replication process.
Replication of the simian virus 40 (SV40) genome has been examined both in vivo and in vitro (16). In vivo studies with SV40 in mammalian cells and in vitro studies with reconstituted mammalian cell-free systems with circular plasmids have shown that bidirectional replication ensues from an SV40 origin sequence in the presence of T antigen (17–20), which functions both as an origin-binding protein and as a DNA helicase for the unwinding of duplex DNA (16, 21–23). In the reconstituted system, T antigen, replication protein A (RPA), DNA polymerase α, and topoisomerase I are sufficient for primer synthesis (24). The loading of proliferating cell nuclear antigen (PCNA) by clamp loader replication factor C (RFC) affects the switch from synthesis by Polα to highly processive synthesis by Polδ (25–27). Although studies with the purified proteins in reconstituted systems have been informative regarding how the initiation, elongation, and Okazaki fragment maturation processes could occur in vitro, it still remains unclear whether the replication of SV40-based plasmids in human cells primarily utilizes the cellular replication machinery or whether the use of T antigen as a DNA helicase precludes the need for many of the proteins such as the MCM2-7 DNA helicase. Also, because Polδ is sufficient for replicating both the DNA strands in the reconstituted SV40 system, whereas Polε may also be required for chromosomal replication (28, 29), the replication of SV40 plasmids may differ from chromosomal replication in significant ways.
SV40 origin-based plasmid systems have been used extensively for DNA repair studies with mammalian cell-free extracts (30–33), and more recently, in our studies for analyzing the roles of translesion synthesis (TLS) DNA polymerases in human cells, we utilized a duplex plasmid system in which bidirectional replication initiates from an SV40 origin in the presence of T antigen (34–36). From these analyses, we inferred that TLS occurs very similarly on the leading and lagging DNA strands. However, since the SV40 system utilizes Polδ for the replication of both the leading and the lagging DNA strands, whereas genetic studies in Saccharomyces cerevisiae have suggested that Polδ replicates the lagging strand and Polε replicates the leading strand (37, 38), TLS on the two DNA strands could differ during mammalian chromosomal replication, if in mammalian cells Polε also replicates the leading strand and Polδ replicates the lagging strand. It is not known whether in human cells, Polδ replicates both the DNA strands or whether Polε and Polδ replicate the leading and lagging strands, respectively, as in yeast.
Because the EBV plasmid system uses the cellular machinery for all aspects of DNA replication in human cells, whereas such information has been lacking for the in vivo replication of SV40 origin-based plasmids, we have designed a duplex plasmid system in which bidirectional replication initiates from the EBV origin and the genetic control of TLS on both the leading and lagging DNA strands can be determined separately. Here, we present our analyses of TLS opposite a site-specific UV-induced cis-syn TT dimer present on the template for synthesis of the leading or the lagging DNA strand, and show that in the EBV plasmid also, similar genetic mechanisms control TLS on the two DNA strands. We discuss the implications of these observations for TLS during chromosomal replication in human cells.
RESULTS
Construction of heteroduplex target vectors containing an EBV origin of replication and a site-specific cis-syn TT dimer.
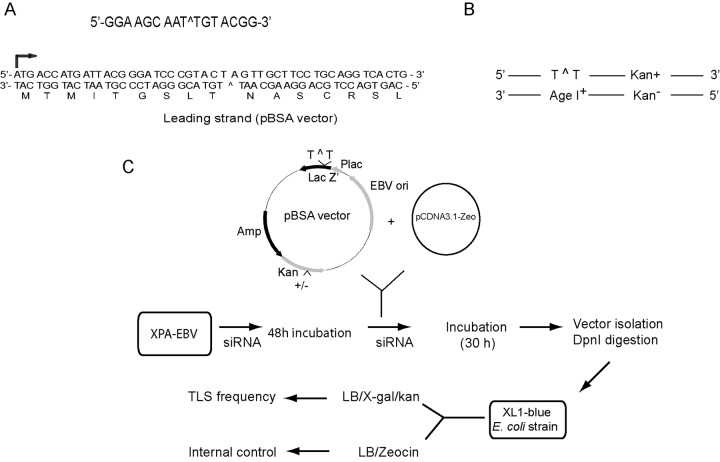
To construct the EBV plasmid for TLS studies, the 2-kb EBV replication origin sequence was PCR amplified from pCEP4 (Invitrogen) and used to replace the SV40 origin in the SV40-based plasmids that we have used for TLS studies. As shown in Fig. 1, the final EBV vector (pBSA/pSBA) contains an EBV origin and a heteroduplex adduct site, one strand of which carries a site-specific cis-syn TT dimer and the other of which contains an AgeI+ site. Since EBV replication requires the EBNA1 protein, we expressed EBNA1 in human cells. Because the DNA lesion located on either the leading strand DNA template or the lagging strand template is in frame with the LacZ′ sequence, and the lesion containing DNA strand contains the kan+ gene, replication through the DNA lesion by TLS will produce blue colonies among Kan+ colonies, whereas white colonies among Kan+ colonies would result from template switching. That is because the other strand has the AgeI+ site opposite the TT dimer, which puts the LacZ′ gene out of frame, and since template switching utilizes sequence information from the strand with the AgeI+ site for copying past the lesion site, this lesion bypass mechanism produces white colonies.
FIG 1 .
Assay for determining the genetic control of TLS on the leading and lagging strands of an EBV origin-based plasmid. (A) The target 16-mer sequence containing a cis-syn TT dimer (T^T) is shown at the top. The sequence of the N-terminal part of the lacZ′ gene in the pBSA vector (leading strand), including the TT dimer, is shown. (B) Strategy for TLS. In the duplex plasmid, the DNA strand containing the TT dimer carries the wild-type kanamycin resistance gene (kan+) so that TLS opposite the UV lesion will result in a blue colony on LB/Kan plates containing IPTG and X-Gal. (C) Assay for TLS and for determining replication efficiency of damage-containing plasmids in siRNA-treated human cells. The purified DNA lesion-containing plasmid, undamaged pCDNA3.1-Zeocin plasmid, and siRNA are cotransfected into human cells that have been pretreated with siRNA for 48 h. After 30 h incubation, the rescued plasmid DNA is treated with DpnI to remove any unreplicated plasmid, and then transformed into XL-1 Blue E. coli cells. TLS frequency is determined from the frequency of blue colonies among kan+ colonies. The replication efficiency of undamaged EBV plasmid relative to that of the zeocin resistance plasmid was determined by the number of colonies that grew on LB/Amp plates, indicative of the EBV plasmid, and the number of colonies that grew on LB/Zeo plates, indicative of the zeocin plasmid.
Replication efficiency of EBV plasmid in human cells.
We first carried out control experiments to verify that the replication of the EBV plasmids we constructed was strictly dependent upon the presence of the EBV origin sequence and the EBNA1 protein. For this purpose, we used SV40-transformed 293T cells, EBV-transformed 293E cells, and NER-defective xeroderma pigmentosum group A (XPA) human fibroblasts stably expressing the EBNA1 protein and examined the replication efficiency of undamaged plasmids bearing the SV40 or the EBV origin sequence relative to the replication of the pCDNA3.1 zeocin resistance (Zeor) plasmid, which has the SV40 origin. As shown in Table 1, in SV40-transformed 293T cells, neither of the pBSA or pSBA EBV plasmid was able to replicate, whereas the SV40 origin-bearing plasmids PBS and PSB and the pCDNA3.1 Zeor plasmid replicated. In contrast, in EBV-transformed 293 E cells, only the plasmids pBSA or pSBA carrying the EBV origin replicated, and in SV40-transformed XPA cells in which the EBNA1 protein is also expressed, all the plasmids replicated. As indicated from the relative numbers of ampicillin-resistant colonies, which represent the replication of EBV origin- or SV40 origin-bearing plasmid, and zeocin-resistant colonies, the EBV plasmids replicated ~80% as efficiently as the SV40 origin-based pCDNA3.1 Zeor plasmid. We conclude from these observations that the EBV origin-containing plasmids we have constructed replicate efficiently in human cells and that their replication requires the EBV origin and the EBNA1 protein.
TABLE 1 .
Replication efficiency of undamaged (ND) duplex plasmids in which bidirectional replication initiates from an SV40 or EBV origin in SV40- or EBV-transformed human cell lines
| Cell type | Plasmid (origin) | No. of colonies resistant to: |
|
|---|---|---|---|
| Ampicillin | Zeocin | ||
| pBSA-ND (EBV) | None | 508 | |
| 293T (SV40 transformed) | pSBA-ND (EBV) | None | 489 |
| pBS-ND (SV40) | 583 | 524 | |
| pSB-ND (SV40) | 536 | 528 | |
| pBSA-ND (EBV) | 486 | None | |
| 293E (EBV transformed) | pSBA-ND (EBV) | 502 | None |
| pBS-ND (SV40) | None | None | |
| pSB-ND (SV40) | None | None | |
| XPA (SV40 transformed and expressing EBNA1 protein) | pBSA-ND (EBV) | 418 | 489 |
| pSBA-ND (EBV) | 397 | 524 | |
| pBS-ND (SV40) | 496 | 428 | |
| pSB-ND (SV40) | 463 | 508 | |
Genetic control of TLS opposite a cis-syn TT dimer carried on the leading- or lagging-strand DNA template of EBV plasmid.
In our previous studies with a cis-syn TT dimer carried on the leading- or the lagging-strand DNA template of SV40 plasmids, we showed that on both strands, TLS occurs almost equally frequently and the same TLS Pols contribute to lesion bypass (36). For determining the genetic control of TLS on the two DNA strands of EBV plasmids, and to be certain of the similarities or differences between the SV40 and EBV plasmids, we carried out TLS studies in which we examined TLS in both plasmid systems concurrently. As is shown in Table 2, on both the DNA strands of the SV40 plasmid carried in XPA cells, TLS occurred with a frequency of ~35% in cells treated with control (NC) small interfering RNA (siRNA), and the frequency of TLS was reduced upon the depletion of Polη, Polκ, or Polζ but not depletion of Polι. These independent sets of data resemble closely the more extensive TLS results we published previously (36).
TABLE 2 .
Effects of siRNA knockdowns of Pols on TLS opposite a cis-syn TT dimer located on the leading- or lagging-strand DNA template of SV40 plasmid carried in XPA human fibroblasts
| siRNA | Leading strand |
Lagging strand |
||||
|---|---|---|---|---|---|---|
| No. of kan+ colonies | No. of blue colonies among kan+ colonies |
TLS (%) | No. of kan+ colonies | No. of blue colonies among kan+ colonies |
TLS (%) | |
| NC | 421 | 150 | 35.6 | 326 | 105 | 32.2 |
| Polη | 340 | 57 | 16.8 | 368 | 52 | 14.1 |
| Polι | 486 | 169 | 34.8 | 456 | 136 | 29.8 |
| Polκ | 429 | 102 | 23.8 | 322 | 69 | 21.4 |
| Rev3 | 321 | 72 | 22.4 | 416 | 86 | 20.7 |
| Rev7 | 360 | 77 | 21.4 | 425 | 91 | 21.4 |
The data for the effects of siRNA depletions of TLS Pols on promoting replication through a cis-syn TT dimer carried on the leading- or the lagging-strand DNA template of EBV plasmids are shown in Table 3. In XPA cells treated with control siRNA, TLS on both the strands occurred with a frequency of ~30%. For both the DNA strands, Polη depletion conferred an ~50% reduction in the frequency of TLS compared to that in control cells, and depletion of either Polκ or Polζ resulted in an ~30% reduction in TLS frequency. In contrast, Polι depletion had no effect on TLS frequency for the lesion carried on either DNA strand. In our previous study with SV40 plasmids, we showed that Polη, -κ, and -ζ function independently of one another in mediating TLS opposite a cis-syn TT dimer, as TLS was further reduced upon the simultaneous depletion of any two of these Pols, and TLS was almost completely inhibited upon the simultaneous depletion of both Polκ and Polζ in xeroderma pigmentosum variant (XPV) cells, which lack Polη (36). Similar to our observations with the SV40 plasmid, the frequency of TLS opposite a cis-syn TT dimer carried on either the leading- or the lagging-strand DNA template in the EBV plasmid shows a further reduction upon the simultaneous knockdown of Polη and Polκ, Polη and Polζ, or Polκ and Polζ beyond that observed upon the depletion of any of these Pols individually. We conclude from these observations that TLS opposite a cis-syn TT dimer carried on the leading- and the lagging-strand DNA templates of the EBV plasmid occur with similar frequencies and utilize the same TLS Pols.
TABLE 3 .
Effects of siRNA knockdowns of Pols on TLS opposite a cis-syn TT dimer located on the leading- or lagging-strand DNA template of EBV plasmid carried in XPA human fibroblasts
| siRNA | Leading strand |
Lagging strand |
||||
|---|---|---|---|---|---|---|
| No. of kan+ colonies | No. of blue colonies among kan+ colonies |
TLS (%) | No. of kan+ colonies | No. of blue colonies among kan+ colonies |
TLS (%) | |
| NC | 678 | 194 | 28.6 | 621 | 175 | 28.2 |
| Polη | 484 | 69 | 14.3 | 523 | 69 | 13.2 |
| Polι | 580 | 175 | 30.2 | 589 | 174 | 29.5 |
| Polκ | 525 | 102 | 19.4 | 535 | 104 | 19.4 |
| Rev3 | 496 | 98 | 19.8 | 498 | 96 | 19.3 |
| Rev7 | 514 | 96 | 18.8 | 547 | 111 | 20.3 |
| Polη + Polι | 620 | 90 | 14.5 | 426 | 60 | 14.1 |
| Polη + Polκ | 423 | 39 | 9.2 | 465 | 38 | 8.2 |
| Polη + Rev3 | 396 | 35 | 8.8 | 536 | 46 | 8.6 |
| Polη + Rev7 | 367 | 34 | 9.3 | 478 | 40 | 8.4 |
| Polκ + Rev3 | 469 | 76 | 16.2 | 356 | 60 | 16.9 |
| Polκ + Rev7 | 566 | 89 | 15.7 | 412 | 63 | 15.3 |
Error-free and mutagenic TLS opposite the lesion carried on the leading- or lagging-strand DNA template of the EBV plasmid.
On both the DNA strands of the SV40 plasmid, TLS incurs only ~2% mutational events, to which Polκ and Polζ contribute equally, and depletion of both Pols results in an almost complete absence of mutagenic TLS (36). In contrast, Polη replicates through the cis-syn TT dimer in an error-free manner, and mutation frequencies rise ~2 to 3-fold upon Polη depletion. TLS on both the DNA strands of the EBV plasmid carried in XPA cells also occurs in a predominantly error-free manner, as we found only ~1% mutational events, and the genetic control of error-free and mutagenic TLS is the same for the two DNA strands of the EBV plasmid (Tables 4 and 5). On both DNA strands, mutation frequencies increase to ~3% in Polη-depleted cells, and mutagenic TLS was reduced in cells depleted of Polκ or Polζ. Also, as expected, the increase in mutation frequencies in Polη-depleted cells showed a reduction if Polκ or Polζ was also depleted. Hence, on both DNA strands of EBV plasmid, Polη carries out error-free TLS, whereas TLS by Polκ and -ζ generates a low frequency of mutations.
TABLE 4 .
Effects of TLS Pols on mutation frequencies and nucleotides inserted opposite a cis-syn TT dimer carried on the leading-strand template of EBV plasmid in XPA human fibroblasts
| siRNA(s) | No. of kan+ blue colonies sequenceda | No. with nucleotide insertedb |
Mutation frequency (%) | |||
|---|---|---|---|---|---|---|
| A | G | C | T | |||
| NC | 288 (4) | 284 | 1 (5′ T) | 0 | 1 (5′ T) | 1.4 |
| 1 (3′ T) | 1 (3′ T) | |||||
| Polη | 190 (5) | 185 | 1 (5′ T) | 1 (3′ T) | 2 (3′ T) | 2.6 |
| 1 (3′ T) | ||||||
| Polκ | 240 (1) | 239 | 1 (3′ T) | 0 | 0 | 0.4 |
| Rev3 | 196 (0) | 196 | 0 | 0 | 0 | 0 |
| Rev7 | 278 (1) | 277 | 1 (3′ T) | 0 | 0 | 0.4 |
| Polη + Polκ | 178 (1) | 177 | 0 | 0 | 1 (3′ T) | 0.6 |
| Polη + Rev3 | 232 (2) | 230 | 1 (3′ T) | 0 | 1 (3′ T) | 0.9 |
| Polκ + Rev3 | 288 (0) | 288 | 0 | 0 | 0 | 0 |
Numbers of mutant colonies are in parentheses.
The site where mutation occurred (the 3′ T or the 5′ T of the TT dimer) is shown in parentheses.
TABLE 5 .
Effects of TLS Pols on mutation frequencies and nucleotides inserted opposite a cis-syn TT dimer carried on the lagging-strand template of EBV plasmid in XPA human fibroblasts
| siRNA(s) | No. of kan+ blue colonies sequenceda | No. with nucleotide insertedb |
Mutation frequency (%) | |||
|---|---|---|---|---|---|---|
| A | G | C | T | |||
| NC | 190 (2) | 188 | 1 (5′ T) | 0 | 1 (3′ T) | 1.1 |
| Polη | 142 (4) | 138 | 1 (5′ T), 2 (3′ T) |
0 | 1 (3′ T) | 2.8 |
| Polκ | 192 (1) | 191 | 1 (3′ T) | 0 | 0 | 0.5 |
| Rev3 | 186 (0) | 186 | 0 | 0 | 0 | 0 |
| Rev7 | 190 (1) | 189 | 0 | 0 | 1 (3′ T) | 0.5 |
| Polη + Polκ | 178 (1) | 177 | 1 (3′ T) | 0 | 0 | 0.6 |
| Polη + Rev3 | 196 (2) | 194 | 1 (3′ T) | 0 | 1 (3′ T) | 1.0 |
| Polκ + Rev3 | 194 (0) | 194 | 0 | 0 | 0 | 0 |
Numbers of mutant colonies are in parentheses.
The site where mutation occurred (the 3′ T or the 5′ T of the TT dimer) is shown in parentheses.
DISCUSSION
SV40 plasmids have been used extensively for DNA repair studies in mammalian cells, but it remains unclear whether this plasmid uses the host cellular machinery for the various steps of replication or whether its replication differs in important aspects from chromosomal replication. This becomes particularly relevant because SV40 T antigen has a DNA helicase activity; hence, the question of whether SV40 plasmid requires the MCM2-7 proteins or whether the DNA helicase function of T antigen is sufficient for its replication in human cells arises. A lack of requirement for MCM2-7 would imply that SV40 plasmid replication differs from chromosomal replication in major ways, and that would suggest that any replication associated processes such as TLS would occur differently in SV40 plasmid replication from that during chromosomal replication.
Since except for EBNA1, the EBV-based plasmids are known to use the host cellular machinery at all the steps of its replication, it provides a useful system for determining whether or not the information obtained from TLS analysis with the SV40-based plasmids is reflective of cellular TLS processes. For this reason, we constructed a duplex plasmid system which carries a site-specific DNA lesion on the leading-strand or the lagging-strand DNA template and in which bidirectional replication initiates from an EBV origin sequence and the origin-binding protein EBNA1 is expressed in human cells. Our observations that opposite a cis-syn TT dimer carried on either DNA strand of the EBV plasmid, TLS occurs equally frequently, and that the same Pols carry out TLS on both DNA strands have provided strong evidence that there is a close correspondence between the information gleaned for TLS with the SV40 plasmid system and that obtained with the EBV system. In our previous TLS studies, we have shown that for both the UV-induced DNA lesions, cyclobutane pyrimidine dimers (CPDs), and 6–4 photoproducts, the genetic control of error-free and mutagenic TLS in the SV40 plasmid is the same as that determined from analyses of UV-induced mutagenesis resulting from replication through CPDs or 6–4 photoproducts in the cII gene carried in the mouse chromosome (35, 36). Although these studies have been important for verifying that overall, the genetic control of TLS in SV40 plasmid in human cells resembles that for TLS in a chromosomal gene in mouse cells, the question still remained whether the same genetic mechanisms are employed for TLS on the two DNA strands during chromosomal replication as those indicated from our studies with the SV40 plasmid or whether they occur differently in the SV40 plasmid and during chromosomal replication. Our findings that TLS on the two DNA strands in the EBV plasmid resembles that in the SV40 plasmid provide strong support to the inference that TLS studies with the SV40 plasmid are as informative regarding TLS processes in chromosomal DNA as those gleaned from TLS analyses with the EBV plasmid.
The requirement of Polε for the replication of the leading strand and of Polδ for the replication of the lagging strand as inferred from genetic studies in yeast (37, 38) might suggest that in humans also, Polε and Polδ act similarly. In that case, the TLS mechanisms for the two DNA strands could be expected to differ because of the likelihood that the TLS Pols and their associated proteins would interact differently with Polε versus Polδ. Since we find that the EBV and SV40 plasmids use similar TLS mechanisms on the two DNA strands, and since Polδ is known to replicate both the DNA strands of SV40 plasmid, that raises the possibility that Polδ replicates both DNA strands in human cells. The alternative possibility that the requirements of TLS Pols are not affected by any of the differences in the replicative Pols or in other proteins that may be used for the replication of the two DNA strands, however, cannot be excluded.
Regardless of the above-noted considerations, our observations that the genetic control of TLS in the SV40 plasmid resembles that in the EBV plasmid provide strong support for the premise that TLS studies with the SV40 plasmid system are highly revealing of cellular TLS mechanisms. Furthermore, since the information derived from studies with these plasmid systems resembles that gleaned from studies of the genetic control of error-free and mutagenic TLS in human and mouse cells, the combination of these various studies has yielded conclusive evidence for the appropriateness of the SV40 plasmid system for the elucidation of TLS mechanisms in human cells (34–36).
MATERIALS AND METHODS
Construction of the plasmid vector containing the EBV origin and a site-specific cis-syn TT dimer.
To construct the plasmid carrying an EBV origin, the EBV replication origin sequence (~2 kb) was amplified from pCEP4 (Invitrogen) by PCR and used for replacing the SV40 origin sequence at the AflIII and SapI sites in pBS/pSB TLS vectors (Fig. 1C). The heteroduplex target sequence containing a cis-syn TT dimer in one strand and an AgeI site opposite the TT dimer on the other strand is shown in Fig. 1A. The heteroduplex target sequence is placed into the lacZ′ sequence such that the lesion-containing strand is in frame with the lacZ′ sequence, whereas in the other strand, the AgeI site puts the lacZ′ sequence out of frame (Fig. 1A). The wild-type kanamycin resistance gene (kan+) was placed on the same strand with the UV lesion, which is in frame with lacZ′ an and MfeI site (Fig. 1B). The rest of the procedure for the construction of the final lesion-containing EBV vector (Fig. 1C) is identical to that described previously (36).
In vivo translesion synthesis assays in human cells.
Since EBV replication requires Epstein-Barr nuclear antigen 1 (EBNA1), the host cell has to be EBV transformed or express the EBNA1 protein in trans. To test replication efficiency, we used HEK293T cells (American Type Culture Collection [ATCC]), EBV-transformed HEK 293 cells (ATCC), and XPA-deficient human fibroblasts (XP12DE) stably expressing EBNA1. The siRNA knockdown efficiencies of TLS Pols have been shown previously (35, 36). For in vivo TLS assays, XPA cells were plated in six-well plates at 70% confluence (approximately 3 × 105 cells per well) and transfected with 100 pmol siRNAs. For the simultaneous siRNA knockdown of two genes, 100-pmol amounts of siRNAs for each gene were mixed and transfected. After a 48-h incubation, the heteroduplex target vector DNA (1 µg), 1 µg of pCDNA3.1-Zeor (Invitrogen) and 50 pmol of siRNA (second transfection) were cotransfected with Lipofectamine 2000 (Invitrogen) (Fig. 1C). The pCDNA3.1-Zeocin DNA was used as an internal control. Since pCDNA3.1-Zeocin has an SV40 replication origin, it can replicate in human cells expressing the T antigen along with the EBV plasmid if the EBNA1 protein is also expressed. The replication efficiency of the EBV plasmid can then be determined relative to that of the SV40 origin-based zeocin plasmid by selection for EBV plasmid on LB medium containing kanamycin (Kan) and for the zeocin resistance plasmid on LB medium containing zeocin (Zeo). After a 30-h incubation, plasmid DNA was rescued from cells by the alkaline lysis method and digested with DpnI to remove unreplicated plasmid DNA. The plasmid DNA was then transformed into Escherichia coli XL1-Blue supercompetent cells (Stratagene). Transformed bacterial cells were diluted in 1 ml super optimal broth with catabolite repression (SOC) medium, and then 150 µl of diluted bacterial cells was plated on LB/Zeo (50 µg/ml zeocin; Invitrogen) and 300 µl of cells was plated on LB/Kan (25 µg/ml kanamycin; Sigma) plates containing 1 µM isopropyl-1-thio-β-d-galactopyranoside (IPTG) (Roche) and 100 µg/ml of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Roche), respectively. After 16 h incubation at 37°C, blue and white colonies were counted on kanamycin plates. The TLS frequency was determined from the number of blue colonies out of total colonies on LB/Kan plates. Plasmid DNA obtained from blue colonies was analyzed to determine the mutation frequency and the mutational changes incorporated during TLS. Replication efficiency of undamaged EBV plasmid was determined by comparing the number of colonies that grew on LB plates containing ampicillin (Amp) versus LB/Zeo and LB/Kan plates. For details of these methods, see reference 37.
ACKNOWLEDGMENT
This work was supported by the National Institute of Environmental Health Sciences grant ES012411.
Footnotes
Citation: Yoon J-H, Prakash S, Prakash L. 2012. Genetic control of translesion synthesis on leading and lagging DNA strands in plasmids derived from Epstein-Barr virus in human cells. mBio 3(5):e00271-12. doi:10.1128/mBio.00271-12.
REFERENCES
- 1. Lindner SE, Sugden B. 2007. The plasmid replicon of Epstein-Barr virus: mechanistic insights into efficient, licensed, extrachromosomal replication in human cells. Plasmid 58:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yates J, Warren N, Reisman D, Sugden B. 1984. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. U. S. A. 81:3806–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yates JL, Warren N, Sugden B. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812–815 [DOI] [PubMed] [Google Scholar]
- 4. Gahn TA, Schildkraut CL. 1989. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell 58:527–535 [DOI] [PubMed] [Google Scholar]
- 5. Harrison S, Fisenne K, Hearing J. 1994. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. J. Virol. 68:1913–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shirakata M, Hirai K. 1998. Identification of minimal oriP of Epstein-Barr virus required for DNA replication. J. Biochem. 123:175–181 [DOI] [PubMed] [Google Scholar]
- 7. Yates JL, Camiolo SM, Bashaw JM. 2000. The minimal replicator of Epstein-Barr virus oriP. J. Virol. 74:4512–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frappier L, O’Donnell M. 1991. Overproduction, purification, and characterization of EBNA1, the origin binding protein of Epstein-Barr virus. J. Biol. Chem. 266:7819–7826 [PubMed] [Google Scholar]
- 9. Middleton T, Sugden B. 1992. EBNA1 can link the enhancer element to the initiator element of the Epstein-Barr virus plasmid origin of DNA replication. J. Virol. 66:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blow JJ, Laskey RA. 1988. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332:546–548 [DOI] [PubMed] [Google Scholar]
- 11. Yates JL, Guan N. 1991. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J. Virol. 65:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhar SK, et al. 2001. Replication from oriP of Epstein-Barr virus requires human ORC and is inhibited by geminin. Cell 106:287–296 [DOI] [PubMed] [Google Scholar]
- 13. Ritzi M, et al. 2003. Complex protein-DNA dynamics at the latent origin of DNA replication of Epstein-Barr virus. J. Cell Sci. 116:3971–3984 [DOI] [PubMed] [Google Scholar]
- 14. Schepers A, et al. 2001. Human origin recognition complex binds to the region of the latent origin of DNA replication of Epstein-Barr virus. EMBO J. 20:4588–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J, Lindner SE, Leight ER, Sugden B. 2006. Essential elements of a licensed, mammalian plasmid origin of DNA synthesis. Mol. Cell. Biol. 26:1124–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fanning E, Zhao K. 2009. SV40 DNA replication: from the A gene to a nanomachine. Virology 384:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bullock PA, Seo YS, Hurwitz J. 1991. Initiation of simian virus 40 DNA synthesis in vitro. Mol. Cell. Biol. 11:2350–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danna KJ, Nathans D. 1972. Bidirectional replication of simian virus 40 DNA. Proc. Natl. Acad. Sci. U. S. A. 69:3097–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fareed GC, Garon GF, Salzman NP. 1972. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J. Virol. 10:484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li JJ, Kelly TJ. 1985. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol. Cell. Biol. 5:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borowiec JA, Dean FB, Bullock PA, Hurwitz J. 1990. Binding and unwinding—how T antigen engages the SV40 origin of DNA replication. Cell 60:181–184 [DOI] [PubMed] [Google Scholar]
- 22. Dodson M, Dean FB, Bullock P, Echols H, Hurwitz J. 1987. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science 238:964–967 [DOI] [PubMed] [Google Scholar]
- 23. Mastrangelo IA, et al. 1989. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature 338:658–662 [DOI] [PubMed] [Google Scholar]
- 24. Matsumoto T, Eki T, Hurwitz J. 1990. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc. Natl. Acad. Sci. U. S. A. 87:9712–9716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsurimoto T, Melendy T, Stillman B. 1990. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature 346:534–539 [DOI] [PubMed] [Google Scholar]
- 26. Tsurimoto T, Stillman B. 1990. Functions of replication factor C and proliferating-cell nuclear antigen: functional similarity of DNA polymerase accessory proteins from human cells and bacteriophage T4. Proc. Natl. Acad. Sci. U. S. A. 87:1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberg DH, et al. 1990. Reconstitution of simian virus 40 DNA replication with purified proteins. Proc. Natl. Acad. Sci. U. S. A. 87:8692–8696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waga S, Stillman B. 1998. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 67:721–751 [DOI] [PubMed] [Google Scholar]
- 29. Zlotkin T, et al. 1996. DNA polymerase epsilon may be dispensable for SV40 but not cellular DNA replication. EMBO J. 15:2298–2305 [PMC free article] [PubMed] [Google Scholar]
- 30. Cordeiro-Stone M, Makhov AM, Zaritskaya LS, Griffith JD. 1999. Analysis of DNA replication forks encountering a pyrimidine dimer in the template to the leading strand. J. Mol. Biol. 289:1207–1218 [DOI] [PubMed] [Google Scholar]
- 31. Cordeiro-Stone M, Zaritskaya LS, Price LK, Kaufmann WK. 1997. Replication fork bypass of a pyrimidine dimer blocking leading strand DNA synthesis. J. Biol. Chem. 272:13945–13954 [DOI] [PubMed] [Google Scholar]
- 32. Nikolaishvili-Feinberg N, Cordeiro-Stone M. 2001. Bypass replication in vitro of UV-induced photoproducts blocking leading or lagging strand synthesis. Biochemistry 40:15215–15223 [DOI] [PubMed] [Google Scholar]
- 33. Svoboda DL, Vos J-M. 1995. Differential replication of a single, UV-induced lesion in the leading or lagging strand by a human cell extract: fork uncoupling or gap formation. Proc. Natl. Acad. Sci. U. S. A. 92:11975–11979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoon J-H, Bhatia G, Prakash S, Prakash L. 2010. Error-free replicative bypass of thymine glycol by the combined action of DNA polymerases κ and ζ in human cells. Proc. Natl. Acad. Sci. U. S. A. 107:14116–14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoon JH, Prakash L, Prakash S. 2010. Error-free replicative bypass of (6-4) photoproducts by DNA polymerase ζ in mouse and human cells. Genes Dev. 24:123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon JH, Prakash L, Prakash S. 2009. Highly error-free role of DNA polymerase η in the replicative bypass of UV induced pyrimidine dimers in mouse and human cells. Proc. Natl. Acad. Sci. U. S. A. 106:18219–18224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McElhinny SA, et al. 2008. Division of labor at the eukaryotic replication fork. Mol. Cell 30:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pursell ZF, Isoz I, Lundström EB, Johansson E, Kunkel TA. 2007. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science 317:127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]