Abstract
BACKGROUND: Clinical evidence points to patient-perceived difficulties and compliance problems in implementing early insulin therapy. Therefore, individual treatment aims are necessary to optimize diabetes therapy, as currently acknowledged by the new ADA/EASD guidelines. Better characterization of patient-perceived difficulties in the implementation of early insulin treatment may contribute to improved compliance and optimal tailoring of treatment regimens for the individual patient. OBJECTIVES: To assess differences in quality of life (QoL) and patient-perceived difficulties in health care with every addition of oral hypoglycemic agents (OHAs) and insulin therapy. METHODS: The analysis was conducted on a cross-sectional sample of 714 diabetic patients treated with OHAs or with insulin once or twice daily. Differences in diabetes-specific QoL, overall QoL, and perception of difficulties associated with specific diabetes treatment attributes were evaluated using trend analysis and comparisons between groups. The contribution of each diabetes treatment attribute to QoL measures and glycemic control was also assessed. RESULTS: No significant differences were found in QoL measures among patients treated exclusively with OHAs when these patients were assessed by the number of oral agents, irrespective of the degree of glycemic control. Better controlled patients treated with 2 OHAs, compared with poorly controlled patients treated with a single OHA, had a lower perception of difficulties associated with diabetes treatment attributes. Poorly controlled patients treated with 2 OHAs and better controlled patients treated with 3 OHAs had similar QoL and perceived difficulties with care. However, the insulin-based alternative was consistently associated with a significantly higher perception of pain and lower overall QoL when compared with the oral regimens. Multivariate models accounted for 52% and 32% of the variance in QoL measures. CONCLUSIONS: From the patients’ perspective, oral therapy is the preferred strategy for attaining the treatment goals since the addition of OHAs was not associated with lower QoL or patient-perceived difficulties with care. If early insulin treatment is considered, physicians should address specific diabetes treatment characteristics, mainly the issue of pain, to promote improved QoL and disease control.
Keywords: type 2 diabetes, patient perception, quality of life, oral hypoglycemic agent, insulin
Abbreviations: ANOVA - analysis of variance; DQOL-BCI - Diabetes Quality of Life Brief Clinical Inventory; HbA1c - glycated hemoglobin; MODY - maturity onset diabetes of the young; OHA - oral hypoglycemic agent; PDDT - patient-perceived difficulties in diabetes treatment; QoL - quality of life; SD - standard deviations; UKPDS - United Kingdom Prospective Diabetes Study; VAS - visual analogue scale
Introduction
Type 2 diabetes is a ubiquitous chronic illness associated with high rates of comorbidity and mortality [1]. Many years after the development of the first oral hypoglycemic agents (OHAs) optimal treatment is still disputed. Optimal treatment requires the patients' motivation and cooperation, which can be difficult to achieve, mainly owing to the changes it necessitates in important aspects of the patients' daily routine [2, 3]. Present knowledge indicates that optimal treatment is a mixture of measures and includes components such as appropriate diet, physical activity, oral medications, home blood glucose testing, and insulin injections.
As the disease progresses, the dosage and number of OHAs are increased, and many patients ultimately need to introduce insulin to maintain adequate glycemic control [4]. Increased resistance to oral agents is attributed to ongoing loss of insulin-secreting β-cells in number and function [5]. This progression occurs despite initially effective anti-diabetic therapy, and results in an ongoing need for new therapeutic modalities [6], a situation clearly demonstrated by the United Kingdom Prospective Diabetes Study (UKPDS) [7].
When hypoglycemic pharmacological treatment becomes ineffective in T2D therapy healthcare professionals often find it difficult to decide on the optimal follow-up treatment. The traditional approach is a stepwise increase in oral medication [8]. In recent years, early insulin treatment has been suggested as optimal follow-up therapy because insulin is considered the most potent treatment modality in terms of effectiveness, tolerability, and cost [4, 9]. This last approach has been endorsed by current guidelines and expert consensus statements, such as those published in 2009 by the American Diabetes Association and the European Association for the Study of Diabetes on the management of hyperglycemia in individuals with type 2 diabetes [4]. Although there is agreement on the long-term clinical benefits of insulin, both patients and physicians are often reluctant to commence this type of treatment because of adverse effects, practical concerns, and psychological perceptions [10-13]. Acknowledging the essential role of patient cooperation in successful diabetes management, this study aimed to assess patients' perceptions of different treatment modalities to identify an optimal regimen which achieves good glycemic control and acceptable subjective parameters.
Specifically, our study objectives were:
1. To assess differences in quality of life (QoL) and in patient-perceived difficulties with care with every addition of an oral hypoglycemic agent.
2. To evaluate QoL and perceived difficulties, with either the addition of an oral agent or with the addition of (or total switch to) insulin therapy.
Research design and methods
Study population
The initial sample comprised 988 adult diabetic patients receiving care in 25 primary care clinics (55% of patients) and multi-disciplinary diabetes-specialized clinics (45% of patients) across Israel, who had been surveyed about their diabetes treatment modality-related perception, attitudes, and quality of life [14]. In our analysis, we aimed to evaluate alternative hypoglycemic pharmaceutical approaches for the orally-treated patients. Therefore, we excluded the following:
- Patients treated with insulin 3 times daily or more.
- Patients who began insulin therapy within the first 2 years after diagnosis and were diagnosed before the age of 35.
- Patients treated with diet only.
The final analysis included 714 patients, of whom 520 were being treated exclusively with OHAs and 194 with insulin once or twice daily, with or without oral agents.
Setting
Medical care in Israel is provided by four "preferred provider organizations". Membership is compulsory for all Israeli citizens, which means that the system includes all social classes of the general population. Co-payment medication costs for patients are capped at approximately US$ 80 monthly. The physicians associated with all preferred provider organizations use a computerized medical record exclusively for medical charting. Comprehensive and complete clinical data have been collected in central databases since 1995. Each patient has a unique and permanent identifier which is linked to all clinical and administrative data including prescribed therapies, medications, and laboratory results.
Data collection
As described previously [14], all eligible patients were asked for their written informed consent, and were interviewed (face-to-face) in the clinic by a trained interviewer. The interviews were conducted from March 2007 until March 2010. The survey contained questions about their background characteristics, the status of their diabetes, type of diabetes treatment, health-related QoL, and perception of difficulties associated with diabetes treatment characteristics. Also, patients were asked to report non-pharmaceutical treatments. Recent HbA1c values and other clinical measures were extracted from the patients' computerized medical records. The questionnaire has been described elsewhere [14].
The study protocol was approved by the institutional review board of each of the participating organizations in Israel: Edith Wolfson Medical Center, Hadassah Medical Center, Assuta Medical Center, and three health maintenance organizations, namely Leumit, Meuhedet, and Clalit.
Study variables
Type of hypoglycemic treatment. The participants were grouped into one of the four types of treatment: 1 OHA only, 2 OHA only, 3 OHA only, and insulin once or twice daily with or without OHA. Many physicians continue treatment with metformin when starting insulin treatment for patients with type 2 diabetes.
Glycemic control. HbA1c was assessed as both a continuous and a dichotomous variable. When dichotomized, the cut-off point was determined at 7.5%, i.e. HbA1c ≤ 7.5% (better controlled) vs. HbA1c > 7.5% (poorly controlled). This value was chosen on the basis of current guidelines for glycemic control acknowledging the risk/benefit ratio of tight control among different patient groups [1, 15, 16].
Health-related quality of life. Overall QoL was assessed using the visual analogue scale (VAS) technique [17]. The scale indicates the current health status and ranges between zero (worst imaginable state of health) and 100 (best imaginable state of health). Diabetes-specific QoL was measured by the Diabetes Quality of Life Brief Clinical Inventory (DQOL-BCI) [18]. The DQOL-BCI is a calculated score which ranges from 1 (best diabetes-specific QoL) to 5 (worst diabetes-specific QoL). For the sake of clarity, the diabetes-specific QoL values were inverted, so that for all QoL variables higher scores indicate a better quality of life.
Patient-perceived difficulties in diabetes treatment (PDDT). Patient perception of 12 different diabetes treatment characteristics was assessed using the PDDT scale which scores the characteristics on a scale ranging from 1 (very difficult) to 5 (easy) [14]. These characteristics were:
1. Adherence to self-monitoring of glucose schedule.
2. Frequency of self-monitoring of glucose.
3. Adherence to medication administration schedule.
4. Frequency of medication administration.
5. Multiple number of medications.
6. Synchronization between meals and medications.
7. Dependence on the medications.
8. Pain associated with treatment.
9. Diet restrictions.
10. Self-care.
11. Coordination between multiple healthcare providers.
12. Cost of treatment.
Each attribute was treated independently of the others, as the areas of difficulty do not necessarily correlate.
Data analysis
Descriptive statistics were applied to describe sociodemographic details, background information, and the diabetes-related health status of the respondents. Trend analysis was performed using the general linear model procedure. Continuous parameters were compared using the two-sample t-test. An analysis of variance (ANOVA) was performed after adjustments for potential confounders. This test had a power of 90% to detect a difference in mean scores of 0.50 units for each of the PDDT attributes. Categorical parameters were compared using the chi-square and Fisher's exact test. Multivariate regression analysis was performed to explore the contribution of each of the PDDT attributes to health-related QoL and to glycemic control.
All statistical analysis was performed using the SAS software version 9.1 (SAS Institute, Inc, Cary, NC, USA). The tests were two-tailed with a 5% significance level.
Results
Data on sociodemographic and clinical characteristics of the participants and type of treatment are summarized in Table 1.
Table 1. Characteristics of the study participants.
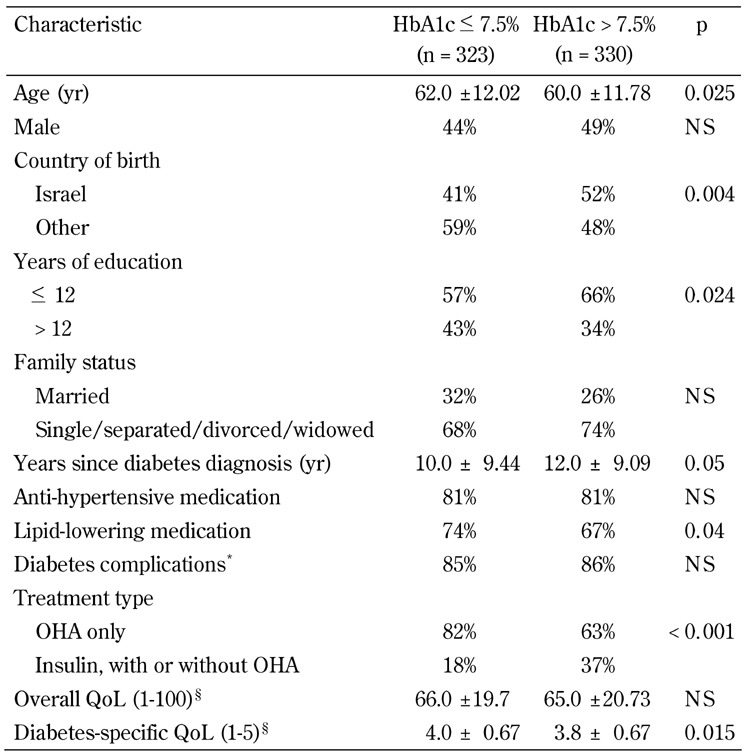
Legend: Data are mean ± SD, or percentages. Missing values were excluded from calculations of percentages and means. OHA - oral hypoglycemic agent, QoL - quality of life, NS - not significant. * One complication or more, as indicated by the patient out of 23 options, including micro- and macrovascular complications. § Range of overall QoL scale was 1-100 and range of diabetes-specific QoL scale was 1-5. Higher scores are better.
Trend in values of QoL measures and patient-perceived difficulties with care. Among patients treated exclusively with OHAs, there was no significant difference in the value of diabetes-specific QoL when these were assessed according to the number of oral hypoglycemic medications (from 1 to 3 oral agents). The results remained non-significant among patients with HbA1c > 7.5 and among better controlled patients with HbA1c ≤ 7.5. However, in both groups, the overall patient-perceived QoL was found to deteriorate with each additional medication, although the p for trend did not reach significance level.
When a trend in the level of the patient-perceived difficulty was assessed for each of the diabetes treatment attributes, a significant decrease in perception of difficulties was found as the number of OHAs increased for two treatment characteristics only, and among the poorer controlled patients only. The characteristics in question were: adherence to self-glucose monitoring schedule and diet restrictions. Both were perceived to be easier with each additional medication.
Comparison of QoL measures and patient-perceived difficulties between patients poorly controlled on a certain OHA regimen and patients better controlled on an alternative regimen with an increased number of OHAs or insulin. Better controlled patients under the 2-OHA regimen perceived fewer difficulties regarding all PDDT attributes than poorly controlled patients on a single OHA treatment (Table 2). Whereas, patients in the insulin-based group reported a significantly lower overall QoL, and perceived the attribute "pain associated with treatment" as significantly more difficult, than poorly controlled 1-OHA treated patients.
Table 2. Comparison of quality of life and patient-perceived difficulties between patients poorly controlled on 1-OHA therapy and patients better controlled on 2-OHA or insulin-based therapy.
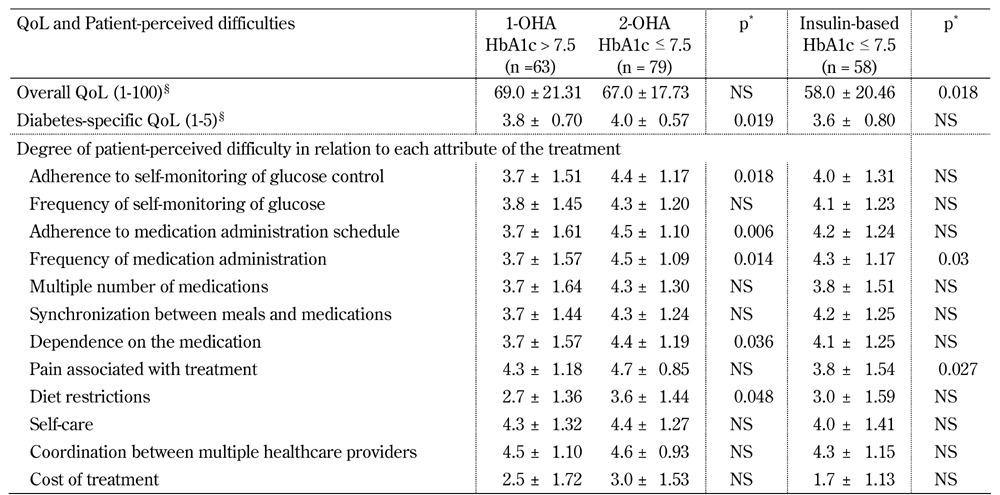
Legend: Data are mean ± SD. Missing values were excluded from calculations of means. QoL - quality of life. * Adjusted for age and diabetes duration. § Range of overall QoL scale was 1-100 and range of diabetes-specific QoL scale was 1-5. Higher scores are better.
When poorly controlled patients from the 2-OHA group were compared with better controlled patients from the 3-OHA group (Table 3), no significant differences were observed for both QoL measures and treatment attributes, except for "pain associated with treatment" which was perceived to be easier by the better controlled patients on the 3-OHA regimen. However, compared to the insulin-based alternative (Table 3), the latter patients reported lower QoL and higher perception of difficulties regarding pain and cost associated with treatment. These differences were significant.
Table 3. Comparison of quality of life and patient-perceived difficulties between patients poorly controlled on 2-OHA therapy and patients better controlled on 3-OHA or insulin-based therapy.
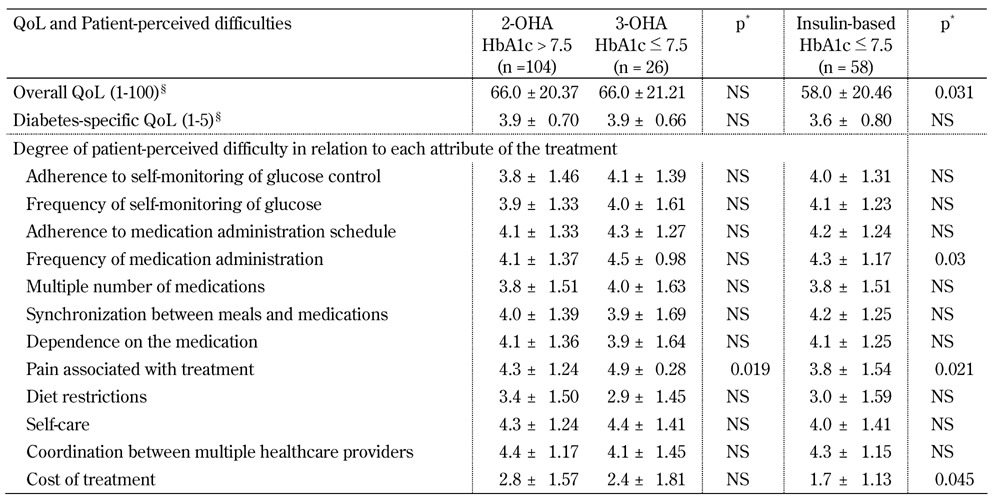
Legend: Data are mean ± SD. Missing values were excluded from calculations of means. QoL - quality of life. * Adjusted for age and diabetes duration. § Range of overall QoL scale was 1-100 and range of diabetes-specific QoL scale was 1-5. Higher scores are better.
When QoL and patient-perceived difficulties were compared between patients poorly controlled on 3 OHAs and patients better controlled on an insulin-based treatment, we found two significant differences. Treatment attribute "frequency of medication administration" was perceived to be easier among patients treated with insulin. Whereas, "pain associated with treatment" was perceived to be more difficult among patients treated with insulin (Table 4). All analyses were adjusted for age and diabetes duration of the participant.
Table 4. Comparison of quality of life and patient-perceived difficulties between patients poorly controlled on 3-OHA therapy and patients better controlled on insulin-based therapy.
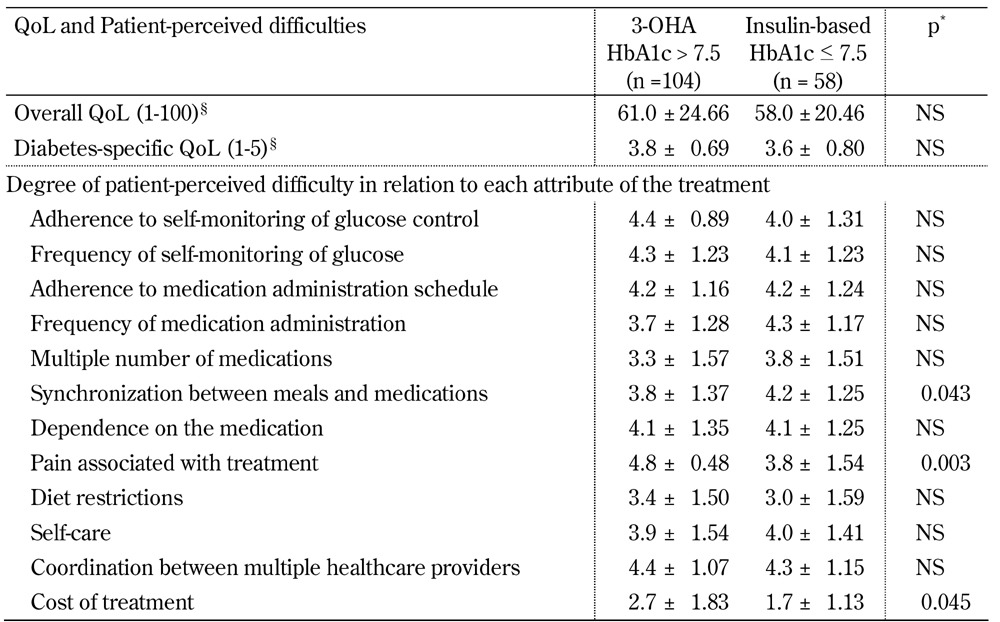
Legend: Data are mean ± SD. Missing values were excluded from calculations of means. QoL - quality of life. * Adjusted for age and diabetes duration. § Range of overall QoL scale was 1-100 and range of diabetes-specific QoL scale was 1-5. Higher scores are better.
PDDT predictors of glycemic control and QoL. Multivariate analysis revealed that the following 5 out of the 12 PDDT attributes made significant contributions to diabetes-specific QoL:
- Self-care (p < 0.0001).
- Costs of treatment (p = 0.0007).
- Pain associated with treatment (p = 0.0025).
- Diet restrictions (p = 0.0033).
- Coordination between multiple health care providers (p = 0.0402).
Overall model R-square was 52%.
When linear regression analysis was applied to examine the contribution of the 12 PTTD attributes to overall QoL, three showed a significant contribution:
- Self-care (p < 0.0001).
- Costs of treatment (p = 0.0009).
- Adherence to self-monitoring of glucose schedule (p = 0.027).
The overall model R-square was 32%.
Logistic regression analysis, using an HbA1c cutoff point of 7.5%, showed that the following four attributes were significant:
- Coordination between multiple healthcare providers (p = 0.0082).
- Synchronization between meals and medications (p = 0.0322).
- Frequency of medication administration (p = 0.0354).
- Adherence to medication administration schedule (p = 0.0487).
All models were adjusted for disease duration, primary physician in diabetes care (either general practitioner or diabetes specialist), diabetes complications, comorbidities, and number of self-glucose monitoring tests per week. The sex of the participant was not found to be associated with glycemic control or with QoL, and was therefore not included in the analysis.
Discussion
Our main finding was that the addition of an oral agent to an OHA-based treatment regimen was not associated with more perceived difficulties with care or with altered QoL. Whereas, the insulin-based regimen is associated with decreased QoL (mainly overall QoL). Also, in our evaluation of the stepwise oral medication strategy, patients perceived several diabetes treatment attributes to be easier when assessed for the more burdensome OHA regimen. These differences were apparent with regards to self-care tasks such as adherence to medication administration schedule and synchronization between meals and medications. The reasons for this result may have been that patients with a longer diabetes duration found routine management of their disease easier, and were thus empowered to make the necessary changes. It is also possible that diabetic patients experience better QoL once they are within their recommended HbA1c target range as the objective and subjective state of the disease may exhibit bi-directional influence [19].
In contrast to these results, when the perception of the poorly controlled patients on each one of the OHA regimens was tested against the perception of insulin-treated patients, QoL measures were found to be lower and patient-perceived treatment difficulties higher (although not always reaching statistical significance). Patients already taking three oral medications were the only exception. They did not report any QoL deterioration or more difficulties with care relating to an addition of insulin, except for "pain", which is consistently perceived to be more difficult with insulin therapy. Thus, patients with a longer disease duration and a lower QoL perception, and who failed to achieve treatment goals on three oral agents, may ultimately improve QoL by switching to insulin.
Unlike our findings, a previous study on QoL and insulin showed a greater improvement in the insulin glargine arm with respect to the adjusted OHA arm [14]. However, in their study, Houlden et al. focused on treatment satisfaction (such as perceived frequency of hyper- and hypoglycemia) and the QoL instrument was different from ours; they used a questionnaire which measured the impact of diabetes on QoL in an individualized and indirect manner. In this context, it is important to note that the various QoL assessment tools available today offer a broad range of conceptions and measurement approaches [20, 21], suggesting that each tool may yield a different result.
In our study, a systematically significant difference in perception of difficulties was evident for "pain associated with treatment". This particular treatment attribute was directly related to the way in which insulin was administrated and to the need for multiple self-glucose tests resulting from insulin therapy. The observed increase in patient-perceived difficulties related to insulin therapy is supported by the work of others which specifically identified the "pain issue" as a barrier to the introduction of insulin therapy [15]. Nevertheless, we can learn from the study by Hermanns et al. [22] that this negative attitude against insulin therapy is often resolved when patients become more experienced with this treatment, which involves their learning of new skills required to handle it. This may require increased awareness on the part of the treating physicians both regarding the above outcome and the need to communicate this outcome with conviction to patients starting insulin therapy [13].
Finally, our assessment of the contributions of the various treatment characteristics to quality of life generated models which accounted for 52% (diabetes-specific QoL) and 32% (overall QoL) in the variance of these measures. It is not surprising that the diabetes-specific parameter was better explained, probably owing to the fact that disease-specific assessments offer greater sensitivity than generic ones. This was reflected by the attributes that were identified as significant contributors to diabetes-specific QoL. These were distinctive characteristics of care in diabetes, including "pain associated with treatment", "diet restrictions", and "coordination between multiple healthcare providers". Interestingly, we found that the cost of treatment significantly impacted both QoL measures, especially in view of the fact that co-payment of medication costs in Israel is reduced for patients receiving social security benefits who have the option to pay only half the relevant amount. With regard to the multivariate analysis of factors contributing to the patients' HbA1c level, we found that three out of the four significant attributes which predicted poorer glycemic control were those related to medication regimens ("frequency of medication administration", "adherence to medication administration schedule", and "synchronization between meals and medications").
This study has certain limitations. Firstly, since the research design was cross-sectional, associations were found and comparisons made on the assumption that the patients were representative of their respective groups. The latter can be safely assumed as patients were recruited in an unbiased manner. Secondly, the sample population described had poorer treatment process indicators than the general diabetes population in the country [23] (HbA1c < 7%: 32% and 49%, respectively; and blood pressure lower than 130 mm/Hg systolic and 80 mm/Hg diastolic: 47% and 67%, respectively). This may have been reflect the longer duration of diabetes in our sample. The reason for the difference was that half our sample was recruited from secondary care treatment centers. However, we believe that our findings are general since the patients were assumed to represent the average patient in their pre-defined group, as mentioned above. Thirdly, although we were unable to assess whether non-pharmaceutical interventions were properly exhausted before changing drug therapy, a bias of this kind would not be expected to be differential. Finally, it should be noted that a few patients with maturity onset diabetes of the young (MODY) may have been included in the analysis because the type of diabetes was not determined independently and relied on the generic diagnosis. However, this was unlikely to have affected the results.
Conclusions
Ideally, improved metabolic control through the patient's compliance with the treatment protocol should not adversely effect the patient's well-being. A better understanding of the trade-off between different treatment strategies, QoL, and patient-perceived difficulties is needed to optimize the treatment based on the patient profile.
We conclude that, from the patient's point of view, it may be in his interest to exhaust the oral medication strategy first before switching to an insulin-based diabetes therapy. When physicians change pharmaceutical therapy they may consider addressing diabetes-specific treatment attributes, in particular pain, and reducing the use of glucometers to the minimum necessary.
Disclosure: The authors report no conflict of interests.
Acknowledgments
This work was performed in partial fulfillment of the requirements for a Ph.D. degree by Ms. Orly Tamir, Sackler Faculty of Medicine, Tel Aviv University, Israel. The authors would like to thank Dr. Shmuel S. Schwartzenberg, Rabin Medical Center, Israel, for his critical comments on the manuscript. The study was supported by a research grant from the Israel National Institute for Health Policy Research (grant number 05-67). The supporting institute had no involvement in the study design, collection, analysis, and interpretation of the data, writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- 1.American Diabetes Association. Standards of medical care in diabetes - 2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pera PI. Living with diabetes: quality of care and quality of life. Patient Prefer Adherence. 2011;5:65–72. doi: 10.2147/PPA.S16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med. 2002;113(Suppl 6A):3S–11S. doi: 10.1016/s0002-9343(02)01276-7. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Goldstein BJ, Vinik AI, O'Neill MC, Porter LE, Heise MA, Kravitz B, Dirani RG, Freed MI. Effect of early addition of rosiglitazone to sulphonylurea therapy in older type 2 diabetes patients (>60 years): the Rosiglitazone Early vs. SULphonylurea Titration (RESULT) study. Diabetes Obes Metab. 2006;8(1):49–57. doi: 10.1111/j.1463-1326.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 7.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 8.Houlden R, Ross S, Harris S, Yale JF, Sauriol L, Gerstein HC. Treatment satisfaction and quality of life using an early insulinization strategy with insulin glargine compared to an adjusted oral therapy in the management of Type 2 diabetes: the Canadian INSIGHT Study. Diabetes Res Clin Pract. 2007;78(2):254–258. doi: 10.1016/j.diabres.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347(17):1342–1349. doi: 10.1056/NEJMcp021106. [DOI] [PubMed] [Google Scholar]
- 10.Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28(10):2543–2545. doi: 10.2337/diacare.28.10.2543. [DOI] [PubMed] [Google Scholar]
- 11.Casciano R, Malangone E, Ramachandran A, Gagliardino JJ. A quantitative assessment of patient barriers to insulin. Int J Clin Pract. 2011;65(4):408–414. doi: 10.1111/j.1742-1241.2010.02590.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62(6):860–868. doi: 10.1111/j.1742-1241.2008.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naker S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: family physicians' misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21(4):220–226. doi: 10.1016/j.jdiacomp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Tamir O, Wainstein J, Abadi-Korek I, Horowitz E, Shemer J. The patient-perceived difficulty in diabetes treatment (PDDT) scale identifies barriers to care. Diabetes Metab Res Rev. 2012 doi: 10.1002/dmrr.1300. In press. [DOI] [PubMed] [Google Scholar]
- 15.Pozzilli P, Leslie RD, Chan J, De Fronzo R, Monnier L, Raz I, Del Prato S. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician's personalized approach. Diabetes Metab Res Rev. 2010;26(4):239–244. doi: 10.1002/dmrr.1092. [DOI] [PubMed] [Google Scholar]
- 16.Montori VM, Fernandez-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med. 2009;150(11):803–808. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
- 17.Brazier J, Deverill M, Green C, Harper R, Booth A. A review of the use of health status measures in economic evaluation. Health Technol Assessment. 1999;3(9):1–164. [PubMed] [Google Scholar]
- 18.Burroughs TE, Deskin R, Waterman BM, Gilin D, McGill J. Development and validation of the Diabetes Quality of Life Brief Clinical Inventory. Diabetes Spectrum. 2004;17:41–49. [Google Scholar]
- 19.Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med. 1996;334(13):835–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 20.Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome - a review of quality of life measurement in adults with diabetes. Diabet Med. 2009;26(4):315–327. doi: 10.1111/j.1464-5491.2009.02682.x. [DOI] [PubMed] [Google Scholar]
- 21.Watkins K, Connell CM. Measurement of health-related QOL in diabetes mellitus. Pharmacoeconomics. 2004;22:1109–1126. doi: 10.2165/00019053-200422170-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hermanns N, Mahr M, Kulzer B, Skovlund SE, Haak T. Barriers towards insulin therapy in type 2 diabetic patients: results of an observational longitudinal study. Health Qual Life Outcomes. 2010;8:113–118. doi: 10.1186/1477-7525-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. http://www.health.gov.il. Israel Ministry of Health; 2008. Apr, Quality indicators for community health care in Israel. Report to the public: 2005-2007. [Google Scholar]


