Abstract
Objective
This study was conducted to evaluate the prevalence of rotavirus disease and to investigate the genotypes of rotavirus strains causing acute gastroenteritis among children aged <5 years old in Marvdasht, Iran.
Methods
One hundred and forty-one children, aged 1 month to 5 years, afflicted with severe diarrhea were enrolled during January 2007 to December 2008. Their stool samples were studied with enzyme immunoassays (EIA) for group A rotaviruses. Rotavirus-positive specimens were genotyped by the Nested RT-PCR using different types of specific primers.
Findings
Out of total collected samples rotavirus infection was detected in 40 (28.37%). Of the rotavirus episodes, 72.91% occurred during the first 2 years of life (P=0.038). The highest prevalence of infection was identified in summer (52.50%) and the lowest in winter (7.50%). The most common clinical features included diarrhea (96.25%), vomiting (82.50%) and fever (45.0%). Mixed genotypes were the predominant G type (60.0%), followed by non-typeable (12.50%), G2 (12.50%), G4 (10.0%) and G1 (5.0%) genotypes. G3/G8 mixed infection is the first of these rotavirus genotypes to be reported in Iran.
Conclusion
Regarding high frequency of rotavirus infection, continuous surveillance is needed to inform diarrhea prevention programs as well as to provide information about the occurrence of new rotavirus strains. This will assist policy makers in decision making on rotavirus vaccine introduction.
Keywords: Rotavirus, Gastroenteritis, Genotyping, Children, Epidemiology
Introduction
Despite the success of the current public health services and hygiene control in the water supply and sanitation, diarrhea remains the second leading cause of death around the world for children under 5 years of age [1]. Recent studies have estimated that diarrheal disease is responsible for 17% of mortality among children under five years of age all over the world [2].
Human rotaviruses are the major etiological agents of acute gastroenteritis in infants and young children worldwide. This virus is associated with 111 million episodes of gastroenteritis, 25 million clinical visits, 2 million hospitalizations and 352,000-592,000 deaths among children aged <5 years worldwide, each year [3]. Virtually all children are infected at least once within the first 5 years of life, with the peak incidence widely quoted as occurring between 6 and 24 months of age [4, 5]. Rotaviruses belong to the Reoviridae family and are composed of three protein layers surrounding 11 segments of double stranded RNA[6]. The rotaviral outer capsid is composed of a protease sensitive protein designated as VP4 and a glycoprotein designated as VP7. The nature of two outer capsid proteins of the virus, VP4 and VP7, elicits the production of neutralizing antibodies to the virus and allows defining the P (for protease sensitive) and G (for glycoprotein) serotypes of the virus [7]. Human rotaviruses are classified into seven serogroups (A-G). Each group is based on its antigenic properties of shared epitopes on the major structural protein, VP6 [8].
Of these, group A rotavirus has been identified as the leading cause of severe diarrhea disease in infants and young children worldwide [9]. The clinical spectrum of rotavirus illness ranges from mild, watery diarrhea of limited duration to severe diarrhea with vomiting and fever that can result in dehydration with shock, electrolyte imbalance, and death [6]. Hospital-based studies conducted in developing and developed countries have revealed that group A rotavirus infection is responsible for 13-50% of all cases of diarrhea in children <5 years old [10–14]. Despite the impact of rotavirus infection in children morbidity and mortality worldwide, very few studies have been performed in Iran. However, several epidemiological studies have shown that rotavirus is a major cause of 27% to 46% of cases of acute diarrhea among infants and children <5 years old in Iran [15–18].
These days, rotavirus vaccines have been developed to reduce the morbidity associated with severe rotavirus diarrhea. Before a rotavirus vaccine program can be implemented, information is needed on the current burden of rotavirus disease and the distribution and frequency of rotavirus strains circulating in different regions of the country. The aim of this study was to monitor the disease burden associated with rotavirus and determine the G genotypes of rotavirus circulating in children aged <5 years in Marvdasht, Iran.
Subjects and Methods
Sampling: This cross sectional descriptive study was done on 141 stool specimens from children aged <5 years who were hospitalized for acute gastroenteritis in Motahary Hospital, Marvdasht, Iran, during January 2007 to December 2008. The fecal specimens were transported to the infectious disease unit laboratory and stored at -80°C until use for the detection of group A rotaviruses. Information regarding age, sex, type of nutrition (breast-fed and/or bottle-fed), hospitalization and clinical symptoms such as diarrhea, vomiting, fever and convulsion, were recorded for each child. According to WHO's recommendation, all children presenting with gastroenteritis were classified in specific age groups (e.g. 0–2, 3–5, 6–8, 9–11, 12–17, 18–23, 24–35, 36–47 and 48–59 months) so that age-specific incidence rates of hospitalization could be calculated[19].
Rotavirus detection: The specimens were tested by a solid-phase sandwich-type enzyme immunoassay method (Rotavirus Ag ELISA, DRG, Germany). OD values above the cut off value (0.15+OD of the negative control) were considered positive for rotavirus antigen.
Viral genome extraction: dsRNA was extracted from stool specimens by using RNX-Plus kit (CinnaGen, Tehran, Iran), according to the manufacturers protocol.
Reverse transcription-polymerase chain reaction: Briefly, 5 µl of dsRNA was added with mix of DMSO, 5X RT buffer, dNTPs, primers Beg9, End9, DW, denatured at 97°C for 5 min, then followed by addition of RT enzyme and RNase inhibitor to a final volume of 20 µl. The RT-PCR reaction was carried out for 60 min at 42°C to produce the complementary (cDNA) used for PCR amplification rotavirus.
Nested multiplex PCR for G genotyping: Briefly, 10µl of viral cDNA was added to a mix containing MgCl2, dNTPs, 10X PCR buffer, Taq DNA polymerase and the forward primer Beg9 and the reverse primer End9 to a final volume of 50 µl. The thermocycler program was carried out at 94°C for 1 min, followed by 30 cycles at 42°C for 2 min, 72°C for 2 min and a final extension at 72°C for 5 min. 5 µl of the resulting amplicons of 1062 bp were then used as a template in the second round of PCR. The multiplex reaction mix also included each of the G-type-specific primers, aBT1 (G1), aCT2 (G2), aET3 (G3), aDT4 (G4), aAT8 (G8) and aFT9 (G9), provided by World Health Organization [20]. Cycling was done with 20 cycles of the same cycling profile of the first reaction. All PCR products were analyzed by electrophoresis in 1.2% agarose gel that contained ethidium bromide (0.5µg/ml) and visualized under UV illumination.
Statistical analysis: Data were statistically analyzed by SPSS version 16 (SPSS Inc., Chicago, IL, USA). Chi-square, ANOVA and Binomial tests were used to determine the significance of difference observed between two different groups of patients. P values <0.05 were considered statistically significant.
Ethical issues: The study was approved by Ethical and Research Committee of the Shiraz University of Medical Sciences, Iran. A verbal consent was taken from either parent of the enrolled child prior to the interview and collection of stool samples.
Findings
Serology of rotavirus infection: A total of 141 stool samples were collected from children aged <5 years. All children had diarrhea for a period of 1–5 days before hospitalization. Rotavirus was confirmed in 40 of 141 (28.37%) stool specimens analyzed for the presence of group A rotavirus antigen by EIA.
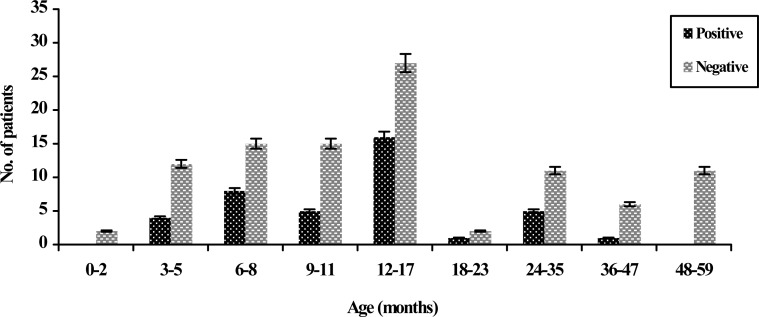
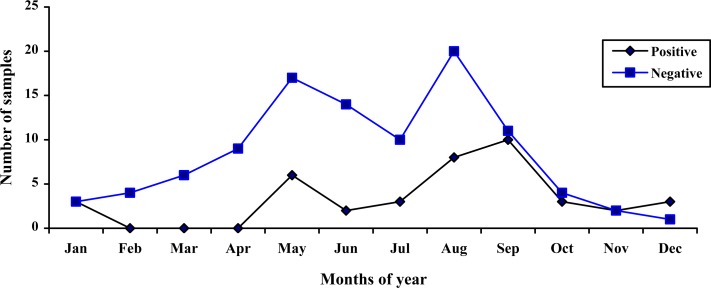
Rotavirus and demographic data: Rotavirus was detected more often (16.31%) in boys than in girls (12.05%). There was no statistically significant difference in the sex distribution between these populations (P=0.4). All the children with gastroenteritis were aged 1 to 59 months (Fig. 1). The age group analysis of rotavirus-positive cases revealed that the highest infection rate was among children less than 24 months of age with 72.91% prevalence. Children between 12-17 months of age were the most affected (40.0%), while those within the age bracket of 0-2 and 48-59 months were the least affected, because no rotavirus infection was detected (P=0.04). The study of clinical manifestations in rotavirus gastroenteritis cases showed that most children with infection had diarrhea (96.25%), vomiting (82.50%) and fever (45.0%). Convulsion was not identified in children with rotavirus infection (P=0.08). According to the season distribution, it was observed that rotavirus infection was detected throughout the year but the relative frequency of rotavirus gastroenteritis was highest in summer. The seasonal distribution of rotavirus infection was as follows: 20.0% in spring, 52.50% in summer, 20.0% in autumn, and 7.50% in winter. A significant relationship was also found between rotavirus infection and seasonal distribution (P=0.02) (Table 1). The highest rate of detection of rotavirus gastroenteritis was found in September (25.0%) and the lowest in the months February to April, in which no infection was detected (P=0.04) (Fig. 2).
Fig. 1.
Age distribution of rotavirus gastroenteritis in children
Table 1.
Distribution of G genotypes in different seasons
| Genotypes Season | G1 | G2 | G3 | G4 | G8 | G9 | NTA* | Mix | Total |
|---|---|---|---|---|---|---|---|---|---|
| Spring | 1 (2.5%) | 0 | - | - | - | - | 1 (2.5%) | 6 (15%) | 8 (20%) |
| Summer | 1 (2.5%) | 4 (10%) | - | 2 (5%) | - | - | 2 (5%) | 12 (30%) | 21 (52.5%) |
| Autumn | - | 1 (2.5%) | - | - | - | - | 1 (2.5%) | 6 (15%) | 8 (20%) |
| Winter | - | - | - | 2 (5%) | - | - | 1 (2.5%) | - | 3 (7.5%) |
| Total | 2 (5%) | 2 (12.5%) | 0 | 4 (10%) | 0 | 0 | 5 (12.5%) | 24 (60%) | 40 (100%) |
NTA: Non-typeable genotypes
Fig. 2.
Monthly distribution of human rotaviruses
Rotavirus genotyping: Genotyping was performed on 40 rotavirus positive stool samples by using Nested RT-PCR. The most common circulating genotypes in the population under surveillance were mixed genotypes, being identified in 24 strains out of 40 samples (60%), followed by non-typeable (12.50%), G2 (12.50%), G4 (10.0%) and G1 (5.0%). The genotypes G3, G8 and G9 were not detected individually. But G3 and G8 genotypes were observed in mixed infections. The most prevalent mixed genotypes in children with rotavirus infection are shown in Table 2. (P=0.001). Of these, G3/G8 mixed genotype of rotavirus is reported for the first time in Iran. The most frequent detected genotypes were mixed genotypes in both females (58.82%) and males (60.87%). No statistically significant differences between the genotype distribution and gender were observed (P=0.6). The most prevalent rotavirus genotype reported was mixed infection in spring (15.0%), summer (30.0%), autumn (15.0%) and G4 in winter (5.0%) seasons. Statistically significant differences were found for the distribution of the genotypes and seasons (P=0.03) (Table 1).
Table 2.
Distribution of rotavirus mixed genotypes
| Genotypes | G1 | G2 | G3 | G4 | G8 |
|---|---|---|---|---|---|
| G1 | 2 (5%) | - | - | 1 (2.5%) | 11 (27.5%) |
| G2 | - | 5 (12.5%) | - | 3 (7.5%) | 6 (15%) |
| G3 | - | - | - | 1 (2.5%) | 2 (5%) |
| G4 | 1 (2.5%) | 3 (7.5%) | 1 (2.5%) | 4 (10%) | - |
| G8 | 11 (27.5%) | 6 (15.%) | 2 (5.%) | - | - |
Discussion
In this study, we describe the results of a survey in Marvdasht, Iran to determine the importance and epidemiological features of rotavirus infections in a specific geographic area (Marvdasht and surrounding rural area) and a target population (children under 5 years old). We detected rotaviruses in the specimens of 28.37% of patients with acute diarrhea. This result is comparable to the disease burden of rotavirus seen in previous investigations in Iran and various other countries, being associated with 13 to 50% of all cases of gastroenteritis [10–12, 15–17]. In the present study, the prevalence of rotavirus in the first 24 months of life was significantly higher than that in older age groups. This age distribution is comparable to previous reports in the other parts of the world [5, 12, 13, 15, 21]. The high burden of rotavirus infection in young children highlights the need for vaccine to offer optimal protection against severe rotavirus disease in children aged younger than 2 years. Epidemiological studies in different regions of the world have indicated that in temperate climates, rotavirus diarrhea occurs predominantly during the cooler months and rarely in the summer months [15, 21–23]. However, seasonal patterns in tropical climates have shown rates of rotavirus infection throughout the year with seasonal trends that are less well defined [5, 13, 23, 24]. Our data demonstrated that there was a significant correlation between the seasonal distribution and rotavirus-positive cases. During the study's surveillance period, rotavirus gastroenteritis occurred throughout the year, with more cases occurring in the summer months and a seasonal peak observed in the months of July and August. This is distinct from seasonal rotavirus diarrhea in tropical countries. This result may have been due to the collection of more samples in the summer. However, extensive epidemiological studies over longer periods of time should be encouraged to determine more accurately the seasonal pattern of rotavirus infection in Marvdasht, Iran. During this study, diarrhea was the symptom most commonly reported in association with rotavirus diarrhea, followed by vomiting and fever. These findings are similar to those results observed in Iran and other countries [11, 21, 25–27]. Currently, analysis of rotavirus strains/genotypes is the key to evaluating the suitability of mass vaccination of children around the world. The observation of G and P distribution and diversity contribute to a better understanding of rotaviruses in circulation and help to characterize the various antigenic shifts that could reduce vaccine efficiency. In the present study, the most frequent circulating genotypes in the population under surveillance were mixed genotypes. The proportion of mixed infections (60.0%) detected among children with acute gastroenteritis, was relatively high compared to that reported for children from Iran (2.6%) [15], Albania (2.0%) [28], Denmark (12%) [29] and Indonesia (23.0%) [30]. High mixed infection with different rotavirus strains may reflect frequent contamination of water resources with rotavirus strains that facilitate generation of novel rotavirus strains through a re-assortment process. The second most common genotype was the non-typeable genotypes, present in 12.50% of the evaluated samples. Analysis of rotavirus genotypes has reported the prevalence of non-typeable genotypes in children with acute diarrhea in other regions of Iran, as well as in other countries [15, 16, 28–31]. The non-typeable rotavirus strains for the G genotype could be related to the presence of novel strains, the failure of the genotyping due to the presence of the other genes not investigated in this survey; for example, rare genotypes such as G5, G6, G11, and failure in RT-PCR technique [20]. Numerous epidemiologic studies have shown the G2 genotype as one of the most prevalent rotavirus genotypes worldwide [11, 24, 28, 32]. In this study, serotype G2 strains were identified in five children with severe diarrhea, and represented 12.50% of the total strains identified. In recent years G4 strain has been detected at relatively high frequency from South Korea[25], to Italy[28], to Brazil[31] and some regions of Iran[15, 33]. However, G4 was detected as the fourth genotype only in 10.0% of all children with rotavirus gastroenteritis. Molecular epidemio-logical studies globally have indicated that G1 is the most common circulating genotype [5, 14, 16, 30]. However the G1 genotype was observed only in 5.0% of all children with rotavirus gastroenteritis. In the current study, neither the G3 nor the G8 genotypes were detected individually. These findings are distinct from those results observed in Sierra Leone[11] and China[34], where these G types have been identified as the most common genotypes in children. Recently, G9 has appeared as the common genotype in Albania[28], Cuba[35], but in our study it was not identified. The emergence of G9 as an important genotype in developing and industrialized countries necessitates the inclusion of G9 in future rotavirus vaccines. One limitation of the present study is the possibility that study population may not be representative of the disease burden of rotavirus among all 0–5-year-old Iranian children. This was because we identified the prevalence of rotavirus gastroenteritis from only one city in Iran and the period of surveillance was limited to a period of 12 months. Therefore, in order to have a comprehensive picture of strain distribution in the country, it is necessary to continue strain characterization in other regions of Iran. Another possible limitation is that true rotavirus prevalence can be higher than estimated here (28.37%), because in this study only hospitalized children with moderate to severe diarrhea have been evaluated and the proportion of rotavirus infections among children with only home care or outpatient visits have not been estimated. Results of this study provide strong support to the concept that rotavirus vaccination of infants may have a major impact in reducing rotavirus gastroenteritis morbidity as well as pressure on healthcare services concerning acute diarrhea among young children in Iran.
Conclusion
Frequencies of mixed Genotypes are indicating severe infection because of low sanitation and transmission of recombinant strains transferred from animals to humans. The noticeable frequency of non-typeable genotypes indicates the necessity of using another primer for characterization of unusual genotypes. Diagnosis of the high prevalence of group A rotavirus infection in hospitalized patients with diarrhea and genotyping of single and co-infection of circulating rotavirus isolates, provide useful data for designing future related research needed to formulate new effective vaccines, especially for infants less than 5 years old.
Acknowledgment
The authors wish to express their thanks and appreciation to the Islamic Azad University, Jahrom Branch, for financial and executive support of this Project. We acknowledge the cooperation of the Ethical and Research Committee of the Shiraz University of Medical Sciences and the personal contributions of Mehdi Kargar and Dr. Ramin Yaghobi in this study.
Conflict of Interest
None
References
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Boerma T, Fat DM. Global and regional causes of death. Br Med Bull. 2009;92(1):7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 3.Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–72. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark HF, Marcello AE, Lawley D, et al. Unexpectedly high burden of rotavirus gastroenteritis in very young infants. BMC Pediatr. 2010;10:40. doi: 10.1186/1471-2431-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mast TC, Chen PY, Lu KC, et al. Epidemiology and Economic burden of Rotavirus gastroenteritis in hospitals and pediatric clinics in Taiwan, 2005-2006. Vaccine. 2010;28(17):3008–13. doi: 10.1016/j.vaccine.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Estes MK, Kapikian AZ. Rotaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Lippincott Publishers; 2007. pp. 1917–74. [Google Scholar]
- 7.Hoshino Y, Kapikian AZ. Classification of rotavirus VP4 and VP7 serotypes. Arch Virol Suppl. 1996;12:99–111. doi: 10.1007/978-3-7091-6553-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Kobayashi N, Nagashima S, et al. Molecular characterization of the VP1, VP2, VP4, VP6, NSP1 and NSP2 genes of bovine group B rotaviruses: identification of a novel VP4 genotype. Arch Virol. 2010;155(2):159–67. doi: 10.1007/s00705-009-0555-x. [DOI] [PubMed] [Google Scholar]
- 9.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed S, Luthful Kabir A, Rahman A, et al. Severity of rotavirus diarrhea in children: one year experience in a children hospital of Bangladesh. Iranian J Pediatr. 2009;19(2):108–16. [Google Scholar]
- 11.Jere KC, Sawyerr T, Seheri LM, et al. A first report on the characterization of rotavirus strains in Sierra Leone. J Med Virol. 2011;83(3):540–50. doi: 10.1002/jmv.21999. [DOI] [PubMed] [Google Scholar]
- 12.Junaid SA, Umeh C, Olabode AO, et al. Incidence of rotavirus infection in children with gastroenteritis attending Jos university teaching hospital, Nigeria. Virol J. 2011;8:233. doi: 10.1186/1743-422X-8-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linhares AC, Stupka JA, Ciapponi A, et al. Burden and typing of rotavirus group A in Latin America and the Caribbean: systematic review and meta-analysis. Rev Med Virol. 2011;21(2):89–109. doi: 10.1002/rmv.682. [DOI] [PubMed] [Google Scholar]
- 14.Lorrot M, Bon F, El Hajje MJ, et al. Epidemiology and clinical features of gastroenteritis in hospitalized children: prospective survey during a 2-year period in a Parisian hospital, France. Eur J Clin Microbiol Infect Dis. 2011;30(3):361–8. doi: 10.1007/s10096-010-1094-9. [DOI] [PubMed] [Google Scholar]
- 15.Kargar M, Akbarizadeh AR. Prevalence and molecular genotyping of group A rotaviruses in Iranian children. Indian J Virol. 2011 doi: 10.1007/s13337-012-0070-7. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kargar M, Najafi A, Zandi K. Genotypic distribution of rotavirus strains causing severe gastroenteritis in children under 5 years old in Borazjan, Iran. African J Microbiol Res. 2011;5(19):2936–41. [Google Scholar]
- 17.Sadeghian A, Hamedi A, Sadeghian M, Sadeghian H. Incidence of rotavirus diarrhea in children under 6 years referred to the pediatric emergency and clinic of Ghaem Hospital, Mashhad, Iran. Acta Medica Iranica. 2010;48(4):263–5. [PubMed] [Google Scholar]
- 18.Zaraei-Mahmoodabadi B, Kargar M, Tabatabaei H, et al. Determination of annual incidence, age specific incidence rate and risk of rotavirus gastroenteritis among children in Iran. Iranian J Virol. 2009;3(1):39–42. [Google Scholar]
- 19.World Health Organization, Department of vaccines and Biological. Generic protocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children. 2002. Available at: http://whqlibdoc.who.int/hq/2002/WHO_V&B_02.15.pdf. Access date: Oct, 2010. CH 1211 Geneva 27, Switzerland.
- 20.World Health Organization, Department of Immunization, Vaccines and Biological. Manual of rotavirus detection and characterization methods. 2008. Available at: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.17_eng.pdf. Access date: Oct, 2010. CH-1211 Geneva 27, Switzerland.
- 21.Bonkoungou IJ, Sanou I, Bon F, et al. Epidemiology of rotavirus infection among young children with acute diarrhoea in Burkina Faso. BMC Pediatr. 2010;10:94. doi: 10.1186/1471-2431-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68(2):171–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Levy K, Hubbard AE, Eisenberg JNS. Seasonality of rotavirus disease in the tropics: a systematic review, meta-analysis. Int J Epidemiol. 2009;38(6):1487–96. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatte VS, Gentsch JR, Chitambar SD. Characterization of group A rotavirus infections in adolescents and adults from Pune, India: 1993-1996 and 2004-2007. J Med Virol. 2010;82(3):519–27. doi: 10.1002/jmv.21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim SY, Jung YC, Le VP, et al. Genetic variation of G4P[6] rotaviruses: evidence for novel strains circulating between the hospital and community. J Med Virol. 2010;82(4):700–6. doi: 10.1002/jmv.21698. [DOI] [PubMed] [Google Scholar]
- 26.Kargar M, Akbarzadeh AR, Yaghobi R. Epidemiological features of rotaviral, bacterial, and parasitic infections among hospitalized children in Jahrom 2006-2007. J Qazvin Univ Med Sci. 2011;14(4):34–41. [In Persian] [Google Scholar]
- 27.Kargar M, Najafi A, Zandi K, Barazesh A. Frequency and demographic study of Rotavirus acute gastroenteritis in hospitalized children of Borazjan city during 2008-2009. J Shaheed Sadoughi Univ Med Sci. 2011;19(1):94–103. [In Persian] [Google Scholar]
- 28.Annarita P, Grassi T, Donia D, et al. Detection and molecular characterization of human rotaviruses isolated in Italy and Albania. J Med Virol. 2010;82(3):510–18. doi: 10.1002/jmv.21700. [DOI] [PubMed] [Google Scholar]
- 29.Fischer TK, Eugen-Olsen J, Pedersen AG, et al. Characterization of rotavirus strains in a Danish population: High frequency of mixed infections and diversity within the VP4 gene of P[8] strains. J Clin Microbiol. 2005;43(3):1099–104. doi: 10.1128/JCM.43.3.1099-1104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radji M, Putman SH, Malik A, et al. Molecular characterization of human group A rotavirus from stool samples in young children with diarrhea in Indonesia. Southeast Asian J Trop Med Public Health. 2010;41(2):341–6. [PubMed] [Google Scholar]
- 31.Santos JS, Alfieri AF, Leite JPG, et al. Molecular epidemiology of the human group A rotavirus in the Paraná State, Brazil. Braz Arch Biol Technol. 2008;51(2):287–94. [Google Scholar]
- 32.Finamore E, Vitiello M, Kampanaraki A, et al. G2 as an emerging rotavirus strain in pediatric gastroenteritis in southern Italy. Infection. 2011;39(2):113–9. doi: 10.1007/s15010-011-0102-z. [DOI] [PubMed] [Google Scholar]
- 33.Esteghamati A, Gouya M, Keshtkar A, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in Iran. J Infect Dis. 2009;200(Suppl 1):S244–7. doi: 10.1086/605050. [DOI] [PubMed] [Google Scholar]
- 34.Wang YH, Kobayashi N, Zhou X, et al. Phylogenetic analysis of rotaviruses with predominant G3 and emerging G9 genotypes from adults and children in Wuhan, China. J Med Virol. 2009;81(2):382–9. doi: 10.1002/jmv.21387. [DOI] [PubMed] [Google Scholar]
- 35.Ribas MA, Nagashima S, Calzado A, et al. Emergence of G9 as a predominant genotype of human rotaviruses in Cuba. J Med Virol. 2011;83(4):738–44. doi: 10.1002/jmv.22020. [DOI] [PubMed] [Google Scholar]