Abstract
Recent data reveal an important role for B cells in the pathogenesis of chronic GVHD (cGVHD). Patients with cGVHD have delayed B-cell reconstitution and elevated BAFF to B-cell ratios compared to patients without cGVHD. The mechanisms promoting and sustaining B-cell activation in this disease, however, remain unknown. As BAFF increases murine B-cell metabolism and survival and maintains autoreactive B-cell clones, we performed ex vivo analyses of peripheral B cells from 51 patients who either had or did not have active cGVHD and were greater than 1 year from the time of allogeneic hematopoietic stem cell transplantation. We found that B cells from patients with active cGVHD were in a heightened metabolic state and were resistant to apoptosis. Exogenous BAFF treatment amplified cell size and survival in B cells from these patients. We found significantly increased signaling through ERK and AKT that associated with decreased levels of proapoptotic Bim, suggesting a mechanistic link between elevated BAFF levels and aberrant B-cell survival. Thus, we identify a role for BAFF in the pathogenesis of cGVHD and define B-cell activation and survival pathways suitable for novel therapeutic development in cGVHD.
Introduction
Chronic GVHD (cGVHD) is a significant cause of nonrelapse mortality in patients after allogeneic hematopoietic stem cell transplant (HSCT). Treatment options remain inadequate because the immune mechanisms underlying the disease are ill defined. Although T lymphocytes have an established role in the pathogenesis of cGVHD,1 murine models and clinical trials implicate an emerging role for B cells in disease pathogenesis.2 In mouse models, depletion of donor B cells in the graft was shown to reduce the incidence of GVHD.3 Subsequently, B cells were found to infiltrate sites of fibrosis in mice with cGVHD, and genetic inhibition of donor B-cell IgG secretion was shown to prevent cGVHD.4 In transplant patients, the presence of antibodies specific for host minor histocompatibility antigens was found to be associated with cGVHD.5,6 In addition, several phase 1/2 trials of B cell–directed therapy for steroid-refractory cGVHD demonstrated clinical efficacy.7–12 Taken together, this work provides compelling evidence for the importance of B cells in cGVHD, but the mechanisms that promote and sustain B-cell involvement in pathogenesis have not been elucidated.
Patients with cGVHD have altered B-cell homeostasis.13–16 B-cell reconstitution is delayed, and plasma B cell–activating factor (BAFF) levels are elevated, resulting in a significantly increased BAFF/B-cell ratio.17 In contrast, cGVHD patients who demonstrate clinical improvement and positive response to treatment have robust recovery of the peripheral naive B-cell pool.13,18,19 These findings are consistent with previous demonstration in murine models that physiologic BAFF/B-cell ratios result in deletion of autoreactive B cells.20 In contrast, when BAFF is in excess, peripheral tolerance is lost and autoreactive B cells survive.21 Whether excess BAFF promotes potentially alloreactive or autoreactive B-cell populations in cGVHD remains unknown.
BAFF increases the survival of both murine and human splenic B cells and has been shown to increase the metabolic state of murine B cells.22–26 The addition of BAFF caused increases in mouse B-cell size, cellular protein content, and alterations in gene transcriptional programs associated with glycolysis and survival.23 B and T cells deprived of physiologic growth factor support lose volume and die unless rescued with exogenous growth factors or the provision of antiapoptotic molecules.27,28 The loss of B-cell volume associated with growth factor deprivation can be overcome by exogenous BAFF.24 Although BAFF signaling in human non-neoplastic B cells remains unexplored, recent studies have elucidated several pathways involved in BAFF-mediated effects on B-cell metabolic activity and survival. Signaling through the AKT pathway has an established role in the maintenance of B-cell growth and survival,29 and BAFF has been shown to activate AKT in murine B cells.23 In addition, BAFF treatment activates extracellular signal-regulated kinase (ERK),30 which directly enhances murine B-cell survival by counteracting the proapoptotic BH3-only protein Bim.30 Bim is crucial for the apoptosis of hematopoietic cells, including B cells,31 and undergoes ubiquitination and degradation by the proteasome after phosphorylation by ERK.32,33 Consequently, autoreactive B cells deficient in Bim are protected from apoptosis through a mechanism involving BAFF.20,34,35
Given these data, we hypothesized that B cells in patients with cGVHD are in a state of constant activation. We aimed to determine whether increased BAFF signaling elevated the metabolic activity of B cells from patients with cGVHD and promoted their survival. Our data show that peripheral B cells purified from patients with cGVHD are in a heightened metabolic state and are resistant to apoptosis. Exogenous BAFF treatment further increased B-cell size and survival. Furthermore, B cells from patients with cGVHD exhibited ongoing signaling through the AKT and ERK pathway, and this was associated with decreased levels of Bim protein. These data suggest a compelling mechanistic link between increased BAFF levels, aberrant survival of B cells, and disease pathogenesis in patients with cGVHD.
Methods
Patient characteristics
Patient samples were collected after written informed consent. The Institutional Review Board at the University of North Carolina Chapel Hill or the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center approved all studies. This study was conducted in accordance with the Declaration of Helsinki. Blood was obtained from 32 patients at the North Carolina Cancer Center and 19 patients at Dana-Farber/Harvard Cancer Center. Clinical characteristics of the 51 patients are included in Table 1. All patients were > 12 months from time of allogeneic HSCT, not receiving high-dose steroids (≥ 0.5 mg/kg per day or ≥ 50 mg/day) and had never received rituximab (α-CD20 mAb). cGVHD status at time of sample collection, not necessarily on first diagnosis, was determined according to documented clinical examination and laboratory testing (in accordance with the Seattle Criteria and National Institutes of Health cGVHD consensus criteria) and confirmed by a physician blinded to our experimental data. Sixteen patients were classified as having active cGVHD at the time of specimen collection. Thirty-five patients were classified as not having cGVHD at the time of specimen collection. Patients without cGVHD at the date of collection included patients who may have previously had cGVHD (20 patients, 57%) in addition to patients who never developed cGVHD (15 patients, 43%). Importantly, there were no significant differences in clinical characteristics or immune suppressive treatment between patients with resolved disease and patients who never developed disease (prednisone: P = .10; mycophenolate mofetil: P = .50; tacrolimus: P = .62; rapamycin: P = 1.00; antithymocyte globulin: 0.19; alemtuzumab: P = 1.00; and data not shown). Median time of analysis after transplantation for patients with cGVHD (64 months) was significantly earlier than patients without cGVHD (89 months, P = .02). Patients with cGVHD were also treated with significantly increased low-dose prednisone (P = .01) and mycophenolate mofetil (P = .001) on the date of collection compared with patients without cGVHD. All other clinical characteristics, including age, sex, number and type of transplant, immunosuppressive treatment, history of acute GVHD, and underlying hematologic malignancy, were similar in patients with active cGVHD and those without cGVHD.
Table 1.
Clinical characteristics of 51 patients after allogeneic HSCT
| Characteristic | −cGVHD (N = 35) | +cGVHD (N = 16) | P |
|---|---|---|---|
| No. of transplants (%) | |||
| 1 transplant | 31 (89) | 12 (75) | .24 |
| 2 transplants | 4 (11) | 4 (25) | |
| Median age, y (range) | 48 (21-70) | 49 (31-64) | .86 |
| Sex, no. (%) of females | 13 (37) | 8 (50) | .54 |
| Median time after transplant, mo (range) | 89 (36-396) | 64 (32-145) | .02 |
| Conditioning regimen (%) | |||
| Myeloblative | 20 (57) | 7 (56) | .55 |
| Nonmyeloblative | 15 (43) | 9 (44) | |
| Source of graft (%) | |||
| Peripheral blood | 29 (83) | 15 (94) | .41 |
| Bone marrow | 6 (17) | 1 (6) | |
| HLA matching (%) | |||
| Matched, unrelated | 14 (40) | 7 (44) | .85 |
| Matched, related | 16 (46) | 6 (38) | |
| Mismatched | 5 (14) | 3 (19) | |
| Immunosuppressive treatment (%) | |||
| None | 14 (40) | 2 (13) | .06 |
| Prednisone ≤ 50 mg (4-50 mg)/day | 8 (23) | 10 (63) | .01 |
| MMF | 2 (6) | 3 (19) | .31 |
| Tacrolimus | 4 (11) | 9 (56) | .001 |
| Rapamycin | 1 (3) | 1 (6) | .53 |
| ATG | 14 (40) | 9 (56) | .37 |
| Alemtuzumab | 1 (3) | 0 (0) | 1.00 |
| aGVHD grade 1-4 (%) | 16 (46) | 9 (56) | .56 |
| Disease (%) | |||
| AML/AML from MDS | 9 (26) | 9 (56) | .26 |
| ALL | 1 (3) | 0 (0) | |
| CML | 6 (17) | 0 (0) | |
| CLL | 2 (6) | 1 (6) | |
| MDS | 6 (17) | 2 (13) | |
| NHL | 6 (17) | 2 (13) | |
| MM | 1 (3) | 0 (0) | |
| AA | 1 (3) | 2 (13) | |
| Other | 3 (9) | 0 (0) |
MMF indicates mycophenolate mofetil; ATG, antithymocyte globulin; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphoblastic leukemia; NHL, non-Hodgkin leukemia; MM, multiple myeloma; and AA, aplastic anemia.
PBMC collection and B-cell purification
Patient blood was collected into EDTA-containing tubes. PBMCs were isolated under sterile conditions using a Ficoll gradient (Ficoll-Paque PLUS; GE Healthcare). PBMCs were viably frozen (10% DMSO) or B cells were immediately isolated for experimental analysis as noted. Unless indicated, B cells were purified by positive selection with immunomagnetic beads according to the manufacturer's instructions (Miltenyi Biotec; CD19 microbeads) to ≥ 95% purity as measured by CD20 expression.
Purification of B-cell subsets
Large-volume leukapheresis products were obtained from 3 patients with cGVHD. The PBMCs were isolated, as described in “PBMC collection and B-cell purification,” and viably frozen. Total B cells were initially purified by negative selection using the human B-cell isolation kit II (Miltenyi Biotec) to ≥ 98% purity as measured by CD19 expression according to the manufacturer's instructions. Subsequently, CD27+ and CD27− subsets were purified by positive selection (Miltenyi Biotec; CD27 microbeads). Transitional B cells (IgD+ CD38Hi CD27−) and pregerminal center B cells (IgD+ CD38Hi CD27+) were sorted by flow cytometry to ≥ 98% purity (BD FACSAria Special Order Cell-Sorting System).
Protein content and flow cytometric analysis of cell size
The cellular protein content of purified B cells was quantified using the BCA Protein Assay (Pierce, Thermo Scientific). B-cell size was determined by flow cytometric analysis of the median forward scatter. Viably frozen PBMCs were thawed, B cells immunomagnetically separated, and allowed to rest for 24 hours before analysis. This rest period did not result in differential viability between patient samples (data not shown) and was found to be necessary to reduce background signaling after the separation procedure (data not shown).23 Cells were acquired on a MACSQuant Analyzer using MACSQuantify software (Miltenyi Biotec) and analyzed using FlowJo Version 8.7.1 (TreeStar). Individual experiments to analyze cell size were performed on a single day to ensure that voltage settings were exactly the same to allow for accurate comparison.
Detection of apoptosis
B cells were freshly isolated on the date of sample collection by negative selection using immunomagnetic beads (Miltenyi Biotec, human B cell isolation kit II). Cells were plated (5 × 105 cells/mL) in RPMI 1640 complete medium (RPMI 1640 supplemented with 10% FBS, l-glutamine, sodium pyruvate, penicillin/streptomycin, and HEPES) at 37°C in a CO2 incubator for 24 or 48 hours. Annexin V and propidium iodide staining was carried out according to the manufacturer's instructions (FITC Annexin V Apoptosis Detection Kit; BD Biosciences PharMingen). Forward/side scatter characteristics were used to define the viable B cells for analysis of apoptosis as previously described.36
In vitro BAFF treatment
B cells were plated (5 × 105 cells/mL) in RPMI 1640 complete media and stimulated with 2.5 ng/mL human recombinant BAFF produced in modified human 293 cells (Genway Biotech). Previous dose titrations of BAFF (2.5, 25, and 50 ng/mL) confirmed that this was an appropriate dose (data not shown). Cells were incubated for 24 or 48 hours at 37°C in a CO2 incubator and harvested for analysis of cell size (see “Detection of apoptosis”) or cell survival. The characteristics of forward and side scatter on flow cytometry were used to determine the frequency of live cells. The increased incidence of live cells in response to stimulation with BAFF, compared with complete media alone, was calculated: percent increase is equal to the difference in treatment divided by complete media.
Immunoblot analysis
B cells were lysed in buffer containing 50mM Tris, pH 7.5, 150mM NaCl, 1mM EDTA, 1% NP-40, 1mM NA3O4V, and protease inhibitor cocktail (Roche Diagnostics). Lysates were separated by SDS-PAGE (NuPage 4%-12% Bis Tris Gel; Invitrogen) and transferred onto a PVDF membrane (iBlot Gel Transfer Device; Invitrogen). Membranes were incubated with the following anti–human antibodies: rabbit pAKT (S473), rabbit pERK1/2 (Thr202/204), mouse AKT, mouse ERK1/2 (all from Cell Signaling) or Bim (Stratagene) and β-actin (Stratagene). For the detection of phosphorylated proteins, membranes were developed with anti–rabbit HRP (Cell Signaling) and the ECL Plus Western Blotting Detection System (GE Healthcare) using the Typhoon Scanner 9400. The peroxidase activity was inactivated by incubation in 30% H2O2, and the membrane was reprobed for the control protein. For detection of Bim, the membrane was stripped, reprobed for β-actin, and developed on film. Signal quantification was performed on raw images using ImageJ Version 1.44o software (National Institutes of Health).
Statistical methods
For analysis of 1 continuous variable between 2 groups, an unpaired, 2-tailed Student t test was used. If the assumption of equal variances was violated, the Satterthwaite method was used. For analysis of categorical variables, the Fisher exact test was used. All tests were considered statistically significant if P < .05.
Results
B cells from patients with cGVHD are in a heightened metabolic state
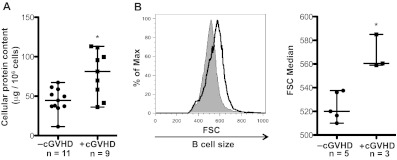
Although BAFF has been shown to increase murine B-cell metabolism,23 the functional effects of elevated BAFF levels on B-cell metabolism in cGVHD patients remain unknown. The metabolic activity of B cells can be measured by the amount of cellular protein and the size of the cell.23,24,22 We analyzed cell protein content and size of B cells from patients with and without cGVHD. B-cell protein content was analyzed in 20 patients and was significantly increased in patients with cGVHD compared with patients without cGVHD (Figure 1A). B cells from patients with cGVHD were also significantly increased in size ex vivo compared to B cells from patients without cGVHD after a 24-hour rest period (Figure 1B). Although the increase in protein content and cell size may correlate with disease onset and severity, it is difficult to ascertain in this study because of limited sample numbers.
Figure 1.
B cells from patients with cGVHD have increased protein content and cell size. (A) B-cell protein content per million cells in peripheral B cells isolated from patients without active cGVHD (−cGVHD, n = 11) and with active cGVHD (+cGVHD, n = 9). Data are median ± range from 5 independent experiments. *P = .004 (unpaired 2-tailed t test). (B) B-cell size measured by forward scatter (FSC) in peripheral B cells from patients without cGVHD (−cGVHD) and with cGVHD (+cGVHD) after 24 hours of incubation. Left: Histograms of median FSC of a patient without active cGVHD (shaded area, thin line) and with active cGVHD (open area, bold line). Right: Median of FSC quantified in patients without cGVHD (−cGVHD, n = 5) and with cGVHD (+cGVHD, n = 3). Data are median ± range from 2 independent experiments. *P = .004 (unpaired 2-tailed t test).
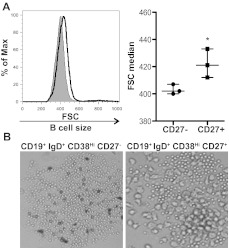
Using IgG production as a surrogate for B-cell activation, we previously found that the CD27+ B-cell subset was activated in patients with cGVHD.14 These cells constitutively produced IgG ex vivo, something that does not occur in ex vivo isolated CD27+ B cells from allogeneic HCST patients without cGVHD or healthy donors.14 We examined whether activated CD27+ B cells had increased size compared to the CD27− B-cell subset in patients with cGVHD. Analysis of these purified subsets requires large-volume leukapheresis samples, therefore limiting our patient number to the 3 patients with cGVHD who consented to collection. We found that CD27+ B cells were significantly increased in size compared to CD27− B cells (Figure 2A). This CD27+ B-cell population can be further subdivided by the expression of IgD and CD38.26,36 Of these, the pregerminal center (GC) B cells (CD19+ IgD+ CD38Hi CD27+) have been shown to be numerically increased in patients with cGVHD.14 We examined pregerminal B cells and their CD27− transitional B-cell counterpart (CD19+ IgD+ CD38Hi CD27−) by light microscopy and found that cells within the CD27+ pre-GC population were indeed enlarged (Figure 2B). Therefore, although the total B-cell population is in a heightened metabolic state, unmanipulated CD27+ B cells and CD27+ subsets are enlarged, suggesting that these cells are in vivo activated in patients with cGVHD.
Figure 2.
CD27+ B cells in patients with cGVHD are enlarged compared to CD27− B cells. (A) Cell size analyzed by forward scatter (FSC) of CD27− and CD27+ B-cell subsets from patients with cGVHD incubated in complete media for 24 hours. Left: Histograms show CD27− B cells (shaded area, thin line) and CD27+ (open area, bold line) from a patient with active cGVHD. Right: Median of FSC quantified in CD27− and CD27+ B cells from patients with active cGVHD. Data are median ± range from a single experiment; n = 3. *P = .042 (unpaired 2-tailed t test). (B) Imaging by light microscopy of IgD+ CD38Hi CD27− B cells and IgD+ CD38Hi CD27+ B cells from a patient with cGVHD incubated for 24 hours. Original magnification ×40.
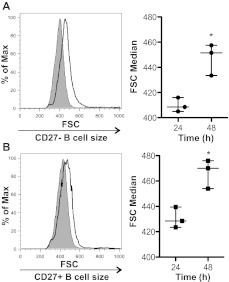
Exogenous BAFF further increases B-cell size
In cGVHD patients in vivo, B cells are bathed in increased levels of BAFF for a prolonged period of time as these patients have elevated BAFF to B-cell ratios.14 Previous data in murine models demonstrate that BAFF increases the cell size of splenic B cells22,23 and prevents the normal ex vivo loss of cell volume that naturally occurs in B cells.24 To test whether BAFF could amplify B-cell size in patients with cGVHD, we added exogenous BAFF to B cells from patients with active disease. Two pilot experiments (data not shown) demonstrated that addition of BAFF to both the CD27+ and CD27− B-cell subsets from 2 patients with active disease resulted in an increase in cell size. Therefore, we examined the effect of exogenous BAFF treatment on CD27+ and CD27− purified subsets from 3 patients with active cGVHD. We found that incubation with BAFF significantly increased the cell size of both the CD27− B-cell subset (Figure 3A) and the activated CD27+ B-cell subset (Figure 3B). These data suggest that BAFF in patients with cGVHD may be responsible for amplifying their metabolic state, as measured by cell size.
Figure 3.
BAFF increases the cellular size of CD27− and CD27+ B cells from patients with cGVHD. (A) CD27− B cells or (B) CD27+ B cells incubated with BAFF for 24 or 48 hours and cell size analyzed by forward scatter (FSC). Left: Histograms represent (A) CD27− or (B) CD27+ B cells from a patient with cGVHD treated with BAFF for 24 hours (shaded area, thin line) and 48 hours (open area, bold line). Right: Median FSC quantified at 24 and 48 hours after BAFF treatment in (A) CD27− and (B) CD27+ B cells from patients with cGVHD. (A) Data are median ± range from a single experiment; n = 3. *P = .009 (unpaired 2-tailed t test). (B) Data are median ± range from a single experiment; n = 3. *P = .011 (unpaired 2-tailed t test).
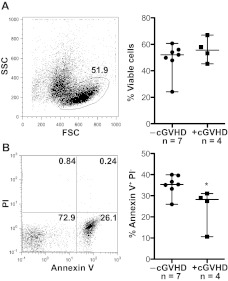
B-cell apoptosis is decreased in patients with cGVHD
Because the metabolic state of B cells and their resistance to apoptosis are inextricably linked,29,37 we directly tested whether B cells from cGVHD patients were resistant to apoptosis. Immediately after peripheral blood collection, B cells were isolated and incubated without the presence of additional growth factors. The forward/side scatter characteristics were used to define the viable B-cell population, which was analyzed for apoptosis by expression of annexin V and propidium iodide. There were no differences in the frequency of viable cells or apoptotic cells at 24 hours between patients with cGVHD and without cGVHD (data not shown). Thus, no difference in viability or apoptosis at this time point impacted accuracy of cell size measurements (Figure 1B). At 48 hours, there was also no statistically significant difference in the frequency of viable cells between patient phenotypes (Figure 4A). B cells from patients with cGVHD, however, have a significantly decreased frequency of apoptotic cells at 48 hours ex vivo compared to patients without cGVHD (Figure 4B). These data taken together suggest that B cells in patients with cGVHD are activated and resistant to apoptosis.
Figure 4.
Patients with cGVHD have decreased B-cell apoptosis. Frequency of apoptotic cells measured by annexin V and propidium iodide (PI) staining in peripheral B cells from patients without cGVHD. (A) Left: Representative dot plot of a patient with cGVHD at 48 hours. The frequency of viable cells defined by the forward (FSC) and side scatter (SSC) characteristics, 51.9%. Right: Frequency of viable cells quantified at 48 hours in patients without cGVHD (−cGVHD) and with cGVHD (+cGVHD). Data are median ± range. −cGVHD, n = 7. +cGVHD, n = 4. *P = .36 (unpaired 2-tailed t test). (B) Left: Representative dot plot of the same patient as in panel A showing the frequency of apoptotic cells defined as annexin V+ PI− cells (lower right quadrant, 26.1%). Right: The frequency of apoptotic cells quantified at 48 hours in patients without cGVHD (−cGVHD) and with cGVHD (+cGVHD). Data are median ± range. −cGVHD, n = 7. +cGVHD, n = 4. *P = .032 (unpaired 2-tailed t test).
BAFF promotes survival of CD27− B cells
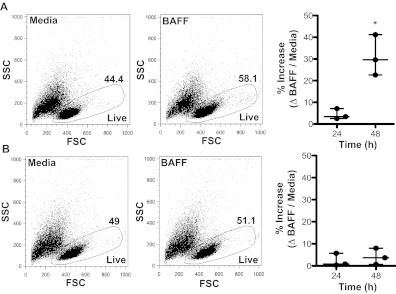
BAFF is essential for the survival of B cells and is associated with a concordant reduction in apoptosis.17,24 Overexpression of BAFF in murine models drives B-cell hyperplasia, whereas neutralization results in B-cell death.25 In humans, BAFF promotes the survival of total splenic B cells and the memory (CD20+ CD27+) B-cell subset.26 To test whether the increased plasma BAFF levels in patients with cGVHD prolonged B-cell survival, we isolated CD27− and CD27+ B-cell subsets from patients with disease and incubated them with BAFF. There was not a substantial survival effect of BAFF on either B-cell subpopulation at 24 hours (CD27− B cells: 0.8%; CD27+ B cells: 3.4%). Notably, at 48 hours, BAFF significantly increased the percent of live CD27− B cells from patients with cGVHD (by 29.6%; Figure 5A), but not the percent of live CD27+ B cells (Figure 5B). These data suggest increased levels of BAFF prolong the survival of CD27− B cells in patients with cGVHD.
Figure 5.
BAFF promotes survival of CD27− B cells from patients with cGVHD. Frequency of live cells with or without BAFF measured by FSC and SSC in (A) CD27− or (B) CD27+ B cells from patients with cGVHD. (A) Left panel: Dot plots show the frequency of live CD27− B cells from a patient with cGVHD treated with media (44.4%) or BAFF (58.1%) for 48 hours. Right: Percent increase in live CD27− B cells in response to BAFF quantified at 24 and 48 hours. Data are median ± range from a single experiment; n = 3. *P = .009 (unpaired 2-tailed t test). (B) Left panel: Dot blots show the frequency of live CD27+ B cells from a patient with cGVHD treated with media (49.0%) or BAFF (51.1%) for 48 hours. Right: Percent increase in live CD27+ B cells in response to BAFF quantified at 24 and 48 hours. Data are median ± range from a single experiment; n = 3. Not significant, P = .53 (unpaired 2-tailed t test).
Signaling associated with metabolic activity and survival is increased in B cells from patients with cGVHD
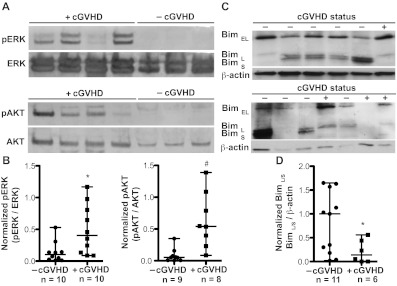
In murine B cells, BAFF activates AKT by phosphorylation, resulting in an increase in metabolic activity and protein synthesis.23,24 In addition, BAFF controls murine B-cell survival by triggering the ERK pathway, resulting in ubiquitination and degradation of the proapoptotic molecule Bim.30 To begin to determine the molecular mechanisms underlying BAFF-mediated modulation of metabolic activity and apoptosis in B cells from patients with cGVHD, we tested whether the ERK and AKT pathways were activated in B cells freshly isolated from patients with cGVHD. Indeed, ERK phosphorylation (at Thr202 and 204) and AKT phosphorylation (at S473) were uniquely present in B cells from patients with cGVHD (Figure 6A). The expression of phosphorylated ERK and AKT, normalized to their nonphosphorylated counterparts, was significantly increased in B cells from patients with cGVHD compared to patients without disease (Figure 6B). Because BAFF-driven activation of ERK and subsequent decrease in Bim blocks B-cell apoptosis,30 we analyzed the expression of the 3 known isoforms of Bim (BimEL, BimL, and BimS) in patients with cGVHD. Although all 3 isoforms induce apoptosis, BimS appears to be the most potent.38 As shown in Figure 6C, B cells from patients with cGVHD have decreased expression of BimL and BimS compared to patients without disease. No differential expression of the most prominent Bim isoform found in murine B cells, BimEL,38,39 was found. The decrease in total BimL and BimS was collectively quantified due to the close proximity in molecular weight of these isoforms: BimL, 21 kDa; BimS, 19 kDa. We found that B cells from patients with active cGVHD have significantly decreased expression of BimL/S, normalized to β-actin, compared to patients without cGVHD (Figure 6D). The decrease in Bim coupled with the increase in AKT and ERK activation suggest that BAFF mediates B-cell activation and survival in cGVHD patients.
Figure 6.
Patients with cGVHD have increased B-cell signaling associated with metabolic activation and survival. (A) Phosphorylation of ERK (pERK at Thr 202 and Thr 204) and AKT (pAKT at S473) measured by immunoblot analysis of peripheral B cells from patients with cGVHD (+cGVHD) and without cGVHD (−cGVHD). Expression was controlled by reprobing with the respective nonphosphospecific protein. (B) Normalized B-cell expression of phosphorylated ERK (left) and phosphorylated AKT (right) in patients without cGVHD (−cGVHD) and with cGVHD (+cGVHD). Data are median ± range, pooled from 5 independent experiments. −cGVHD, n = 9 or 10. +cGVHD, n = 8-10. *P = .021 (unpaired 2-tailed t test with Satterthwaite correction). #P = .009 (unpaired 2-tailed t test with Satterthwaite correction). (C) Isoforms of Bim (Bim EL, Bim L, and Bim S) analyzed by immunoblot analysis of peripheral B cells from patients without cGVHD (−cGVHD) and with cGVHD (+cGVHD). Expression was controlled by reprobing for β-actin. (D) Normalized B-cell expression of the long and short isoforms of Bim in patients without cGVHD (−cGVHD) and with cGVHD (+cGVHD). Data are median ± range from 3 independent experiments. −cGVHD, n = 11. +cGVHD, n = 6. *P = .017 (unpaired 2-tailed t test with Satterthwaite correction).
Discussion
The role played by B cells in the pathogenesis of cGVHD is not completely understood. Our findings indicate that excess BAFF leads to increased metabolic activity and survival of B cells in patients with cGVHD and suggest aberrant responsiveness to BAFF. Not only is there functional and biochemical evidence of in vivo BAFF signaling in B cells from patients with cGVHD, but such cells also remain responsive to BAFF supplementation in vitro.
Patients with cGVHD have elevated BAFF/B-cell ratios14 coupled with abnormal B-cell homeostasis.13–16 The present study, the first to show that BAFF alters metabolic activity and survival of B cells from patients with cGVHD, provides data that are consistent with murine models showing that BAFF can control both metabolism and apoptosis in B cells in other diseases.23,30 We found both increased total protein content and increased size of B cells and CD27+ B-cell subsets (Figures 1 and 2). Size heterogeneity within the CD27+ pre-GC population was also seen (Figure 2B), potentially related to nonsynchronous cell cycle states or to the presence of additional subpopulations within the pre-GC subset. BAFF mediates transcription and translation of genes required for cell cycle progression in murine B cells, including phosphorylation of the key marker for entry into S phase, Rb.23 Given such, it seems probable that cells within the CD27+ pre-GC population were at different stages of the cell cycle when visualized and, therefore, displayed heterogeneity in regards to cell size. In addition, there are subpopulations of pre-GC B cells as defined by the expression of one of the BAFF receptors, TACI.14 These intermediate and high subpopulations of TACI expression may have variable responses to a potential BAFF-driven increase in cell size.
Our finding of differential effect of exogenous BAFF treatment on survival of CD27− B cells (increased) and CD27+ B cells (not increased) from patients with cGVHD is especially interesting in light of the finding by others that the CD27− B-cell subset is expanded in cGVHD patients.15 Human CD27− CD21Lo CD19+ B cells found in patients with rheumatoid arthritis and common variable immunodeficiency (CVID) are autoreactive and anergic cells.40 Whether the CD27− B cells driven to survive by BAFF in patients with cGVHD are autoreactive and/or anergic remains to be determined. On the other hand, exogenous BAFF did not alter in vitro survival of CD27+ B cells from patients with cGVHD. One potential explanation is a reduced dependence on BAFF for survival, something that is seen with murine memory B cells in vivo.41 It is possible that BAFF-mediated in vivo activation leads to a reduced dependence on growth factor mediated (ie, BAFF) survival ex vivo. Taken together, these data suggest that BAFF: (1) elevates the metabolic activity of both CD27− and CD27+ B-cell subsets in patients with cGVHD; and (2) is an important survival factor for the CD27− B-cell subset.
BAFF-induced signaling cascades associated with murine B-cell metabolic activity and survival are triggered in patients with cGVHD. AKT activation is directly associated with metabolic state of murine B cells,23 whereas ERK activation results in degradation of proapoptotic Bim and rescue of B cells from B-cell receptor-induced death.30 To our knowledge, this is the first study that examines BAFF-mediated B-cell signaling in patients with cGVHD. We find that B cells from cGVHD patients have significantly increased activation of AKT and ERK and significantly decreased expression of Bim protein (Figure 6). Activation of these pathways in murine B cells occurs by both B-cell receptor engagement and BAFF stimulation. There is no BAFF-specific B-cell signaling pathway identified to date; thus, our study cannot directly address whether BAFF is responsible for the activation of AKT and ERK.
Excess BAFF results in survival of autoreactive B cells in murine models.20,21 A key mechanism allowing such survival is loss of Bim protein.34,35 Decreased expression of Bim is found in murine B cells in response to BAFF signaling and, importantly, not in response to B-cell receptor activation or engagement. It is therefore possible that the activation of these pathways in B cells from patients with cGVHD occurs directly because of the elevated BAFF/B-cell ratio. Our data corroborate a similar finding in cGVHD patients: loss of Bim protein in B cells from patients with cGVHD (Figure 6D) results in the survival of potentially autoreactive B cells. These data are interesting in the context of regulatory T cells (Tregs) as they can inhibit proliferation and induce apoptosis of autoreactive murine B cells.42 Patients with cGVHD have a lower frequency of Tregs,43,44 in part the result of increased susceptibility to Fas-mediated apoptosis.45 The alterations in Treg and B-cell homeostasis in patients with cGVHD, coupled with our current study, suggest that further studies on T-/B-cell interactions in the pathophysiology of cGVHD are necessary.
BAFF is capable of amplifying B-cell responses to antigen.23,46 Whether the increased BAFF and altered B-cell signaling in patients with cGVHD amplifies the response to the numerous alloantigens and autoantigens present in the post-HSCT setting warrants further study. Taken together, our data suggest a mechanistic link between elevated BAFF levels, improved metabolic activity and survival of B cells, and disease pathogenesis in patients with cGVHD. In addition, our data suggest novel therapeutic targets in human cGVHD.
Acknowledgments
The authors thank the patients and their families, Drs Robert Soiffer and Joseph Antin at the Dana-Farber Cancer Institute for providing infrastructure for sample collection, and Drs Yuri Fedoriw and Christopher Karp for critical review of the manuscript.
This work was supported by the National Marrow Donor Program through the Amy Strelzer Manasevit Scholars Program funded by the Be The Match Foundation and the National Marrow Donor Program in addition to the National Institutes of Health (grants K08HL107756 and CA142106).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.L.A. designed the study, performed the research, analyzed and interpreted the data, and wrote the paper; M.S.F. performed cell isolations, provided technical assistance, and edited the paper; J.W. performed cell isolations and staining for the analysis of apoptosis; P.A.R. provided vital patient phenotypes, clinical information, and helpful discussions; N.S.B. performed cell isolations and immunoblotting for Bim; A.M.D. calculated and/or confirmed statistical analysis; T.H., A.S., P.A., J.C., D.A.G., R.I., A.E., T.C.S., K.R., C.C., J.R., J.S., and A.S.B., collected and provided critical patient samples, clinical information, and helpful discussions; and S.S. conceived of the study, supervised study design and interpretation, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Sarantopoulos, Division of Hematology/Oncology, UNC Lineberger Comprehensive Cancer Center, 2nd Floor, Rm 21-247, CB# 7295, Chapel Hill, NC 27599; e-mail: stefanie_sarantopoulos@med.unc.edu.
References
- 1.Anderson BE, Taylor PA, McNiff JM, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood. 2008;111(10):5242–5251. doi: 10.1182/blood-2007-09-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–4927. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 3.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16(2):289–295. [PubMed] [Google Scholar]
- 4.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103(1):353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Lee JW, Jung CW, et al. Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study. Haematologica. 2010;95(11):1935–1942. doi: 10.3324/haematol.2010.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9(8):505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(3):273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 12.Mohty M, Marchetti N, El-Cheikh J, Faucher C, Furst S, Blaise D. Rituximab as salvage therapy for refractory chronic GVHD. Bone Marrow Transplant. 2008;41(10):909–911. doi: 10.1038/bmt.2008.12. [DOI] [PubMed] [Google Scholar]
- 13.Sarantopoulos S, Stevenson KE, Kim HT, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease (cGVHD). Blood. 2011;117(7):2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greinix HT, Pohlreich D, Kouba M, et al. Elevated numbers of immature/transitional CD21- B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(2):208–219. doi: 10.1016/j.bbmt.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Kuzmina Z, Greinix HT, Weigl R, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117(7):2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 17.Rolink AG, Melchers F. BAFFled B cells survive and thrive: roles of BAFF in B-cell development. Curr Opin Immunol. 2002;14(2):266–275. doi: 10.1016/s0952-7915(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 18.Fedoriw Y, Samulski TD, Deal AM, et al. Bone marrow B cell precursor number after allogeneic stem cell transplantation and GVHD development. Biol Blood Marrow Transplant. 2012;18(6):968–973. doi: 10.1016/j.bbmt.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dorp S, Pietersma F, Wolfl M, et al. Rituximab treatment before reduced-intensity conditioning transplantation associates with a decreased incidence of extensive chronic GVHD. Biol Blood Marrow Transplant. 2009;15(6):671–678. doi: 10.1016/j.bbmt.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20(4):441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 21.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Otipoby KL, Sasaki Y, Schmidt-Supprian M, et al. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci U S A. 2008;105(34):12435–12438. doi: 10.1073/pnas.0805460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203(11):2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodland RT, Fox CJ, Schmidt MR, et al. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111(2):750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 26.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112(2):286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167(12):6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 28.Tumang JR, Owyang A, Andjelic S, et al. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur J Immunol. 1998;28(12):4299–4312. doi: 10.1002/(SICI)1521-4141(199812)28:12<4299::AID-IMMU4299>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol Metab. 2002;13(2):75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 30.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202(10):1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 32.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278(21):18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 33.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279(10):8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 34.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198(7):1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med. 2006;203(3):731–741. doi: 10.1084/jem.20051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105(11):4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammerman PS, Fox CJ, Thompson CB. Beginnings of a signal-transduction pathway for bioenergetic control of cell survival. Trends Biochem Sci. 2004;29(11):586–592. doi: 10.1016/j.tibs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor L, Strasser A, O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene. 2008;27(Suppl 1):S128–S136. doi: 10.1038/onc.2009.50. [DOI] [PubMed] [Google Scholar]
- 40.Isnardi I, Ng YS, Menard L, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115(24):5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benson MJ, Dillon SR, Castigli E, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180(6):3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting edge: CD25+ regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181(7):4447–4451. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
- 43.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 45.Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hase H, Kanno Y, Kojima M, et al. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood. 2004;103(6):2257–2265. doi: 10.1182/blood-2003-08-2694. [DOI] [PubMed] [Google Scholar]