Abstract
Purpose
Genetic research involving human participants can pose challenging questions related to ethical and regulatory standards for research oversight. However, few empirical studies describe how genetic researchers and institutional review board (IRB) professionals conceptualize ethical issues in genetic research or where common ground might exist.
Methods
Parallel online surveys collected information from human genetic researchers (n = 351) and IRB professionals (n = 208) regarding their views about human participant oversight for genetic protocols.
Results
A range of opinions were observed within groups on most issues. In both groups, a minority thought it likely that people would be harmed by participation in genetic research or identified from coded genetic data. A majority of both groups agreed that reconsent should be required for four of the six scenarios presented. Statistically significant differences were observed between groups on some issues, with more genetic researcher respondents trusting the confidentiality of coded data, fewer expecting harms from reidentification, and fewer considering reconsent necessary in certain scenarios.
Conclusions
The range of views observed within and between IRB and genetic researcher groups highlights the complexity and unsettled nature of many ethical issues in genome research. Our findings also identify areas where researcher and IRB views diverge and areas of common ground.
Keywords: comparison, genetics, human genomics, identifiability, institutional review board, reconsent, survey
INTRODUCTION
Ten years after the completion of the Human Genome Project, a combination of technological advances, reduced sequencing costs, and changes in federal funding priorities has moved genomic investigation to the forefront of biomedical research in the United States. Research in this area is increasing dramatically, as are the complexities of the study protocols. In particular, recent developments in science and policy—the acceleration of whole-genome sequencing approaches and the growing emphasis on data sharing within the research community— raise new ethical and social questions regarding informed consent as well as privacy and confidentiality.
Human genetic studies, like all Department of Health and Human Services–funded research involving human participants, fall under the regulatory protections of Title 45, Part 46 of the Code of Federal Regulations.1 Under the law, local institutional review boards (IRBs) are responsible for ensuring that the rights and welfare of human volunteers are protected and that applicable regulatory requirements are met. However, as scholars have pointed out, the existing regulatory system was established in reaction to specific instances of research abuse in the clinical setting and was not designed to provide comprehensive, systematic guidance for observational, population-based studies.2,3 Indeed, some have suggested that existing standards— such as the requirement that the informed consent process provide complete information about the possible risks and harms of participation—may be difficult (if not impossible) to meet in the context of large-scale, longitudinal data repositories and biobanks for which future research questions have yet to be defined.4 In this context, two issues—identifiability and reconsent—pose particular challenges for both scientists and IRBs.
Breach of privacy is generally considered to be the primary risk to participants in population-based genetic studies. Deidentification of study data and biospecimens has long been regarded as a reliable means of protecting study participants’ privacy. However, recent studies have demonstrated that simple deidentification of study data and tissue samples may fail to provide adequate safeguards,5–8 leading to concerns that unauthorized access to genetic data could result in discrimination or stigmatization.9 These concerns appear to persist despite the passage of the Genetic Information Nondiscrimination Act in 2008;10 and some scholars have recently suggested that deidentified data merit more stringent regulatory oversight to avoid harm to study participants.11 New bioinformatics approaches to protect privacy and confidentiality are being developed,12 but experts disagree about whether the unique nature of an individual’s genomic profile entails an irreducible risk of reidentification. The importance of these issues is reflected in the recent Advance Notice of Proposed Rule Making on Revisions to the Common Rule, which includes proposals for uniform data protection standards and written informed consent for all biospecimen collection.13 Available public opinion data suggest that although many potential study participants express concern about privacy, most would still be willing to participate in genetic studies.14–16
Given the current emphasis on sharing existing study data within the scientific community to enhance research efficiencies, questions have also arisen about whether or when investigators should seek study participants’ permission for repurposing such materials (identifiable as well as deidentified) for secondary analyses. In addition, there appears to be some confusion about when reconsent is needed, when a waiver of informed consent can be used, and when a study is exempt because it is not considered human participants research. To date, no policy consensus has been reached about when such “reconsent” is ethically or legally required. Some view autonomy interests of research participants as paramount and advocate obtaining participants’ permission for activities that were not specified in the original consent,4 whereas others argue that the public good of research warrants freer use of deidentified data and specimens.17 Some data on participant preferences suggest that study participants favor reconsent.18 And, although IRBs have the ability to stipulate research exceptions for secondary uses of data in publically accessible repositories such as the database of Genotypes and Phenotypes (dbGaP) based on the terms of the original consent, it is unclear just how broadly such consent language should be interpreted. This matter is further complicated by the fact that data in the dbGaP is deidentified and therefore is not regarded as human participants research.
This fluid state of affairs presents challenges for both researchers and IRB members and staff. Anecdotal accounts suggest that tensions and disagreements exist between these groups with respect to the review of genetic research; however, to date, few empirical studies have described how these two constituencies conceptualize the ethical issues involved in the review of genetic research, which issues are of greatest concern, or where common ground might exist. This report presents key findings of a comparative analysis of two national surveys conducted in 2009, one with genetic researchers and one with professionals involved in human participant protections.
MATERIALS AND METHODS
Sample and recruitment
We recruited study participants from the professional societies representing the two primary stakeholder groups. To assess researchers’ views, members of the American Society of Human Genetics (ASHG)19 involved in human genetic research were invited to complete a survey. A parallel survey was offered to members of Public Responsibility in Medicine and Research (PRIM&R)20 involved in the review of human participants proposals. In April 2009, the executive vice president of ASHG and the executive director of PRIM&R sent introductory e-mails to their respective members inviting them to participate in an anonymous, Web-based survey. A reminder e-mail was sent to both groups 2 weeks later, and members were encouraged to forward the invitation to other colleagues who may not have received it. To reach additional IRB professionals, an invitation was also sent to the membership of the Northwest Association for Biomedical Research.
Survey development and data collection
To inform survey development, in-depth individual interviews and focus groups were conducted with 25 human genetic researchers and 31 individuals involved with IRB review. We used the resulting transcripts to identify salient issues for inclusion in the survey and present appropriate answer choices. We also used these data to adapt the survey language to the words informants used to describe the different topics; thus, the survey used terms such as “reconsent” for seeking additional permissions for the use of samples and data and “likelihood of harm” for whether individuals believed a given negative outcome might actually occur. Survey development was also informed by consultation with internal and external content experts, including members and representatives of ASHG and PRIM&R; the tailored design method was used as a general guide in designing the survey tools.21
Each survey contained five main areas: the research study application process, the IRB review process, IRB functions, specific issues in genetic research, and demographic information. The surveys were designed specifically to allow comparisons between the two groups: about two-thirds of the questions on the slightly longer genetic researcher survey overlapped with the survey of IRB professionals. Most of the overlapping questions included Likert rating scales, with a smaller number utilizing dichotomous and categorical response options. Surveys were pretested through 17 cognitive interviews with human genetic researchers and IRB professionals. This process was used to assess clarity and ease of completing the survey.22 Pilot testing of the Web-based software was also conducted to identify any potential technical difficulties. The surveys were hosted on separate URLs at the University of Washington to facilitate data management and tracking. The surveys are available as online supplements, and detailed methods have been published elsewhere.23,24 The interview and survey studies were approved by the University of Washington’s IRB.
Statistical analysis
To facilitate presentation and discussion of the results, the two groups, genomic researchers and IRB professionals, will be referred to as ASHG and PRIM&R, respectively. Responses to all questions presented here were recorded on 5-point Likert scales as described previously23,24 and were first summarized using frequency distributions, separately for each group.
Differences in frequency of responses between ASHG and PRIM&R were tested using ordinal logistic regression, which allows for multiple categories of the outcome variable and adjustment for potential confounders. The response categories (dependent variable) were ordered and coded as follows: strongly agree/very likely responses as 0, agree/likely as 1, neutral as 2, disagree/unlikely responses as 3, and strongly disagree/ very unlikely as 4. With ordinal logistic regression, several cumulative logits are modeled using all possible cut points of the dependent variable, but a single summary odds ratio (OR) and 95% confidence interval (CI) describing the relationship between the independent and dependent variable is obtained. Results were similar when the outcomes were collapsed into three categories (strongly agree/very likely and agree/likely, neutral, disagree/unlikely and strongly disagree/very unlikely) (data not shown). All models include adjustment for gender and years of work experience (≤5 or >5 years), as adjustment for these variables had a modest effect on the measures of association. Additional adjustment for those performing the opposite role (i.e., engaging in research for IRB professionals and serving on an IRB for genetic researchers) did not alter the results (data not shown). Stata v.9.2 was used for all analyses.25 A P value ≤0.05 was considered statistically significant for all tests. Finally, sample sizes varied by question because participants were allowed to skip any question they did not wish to answer.
RESULTS
A total of 580 online surveys were completed and were available for analysis, representing 372 individuals from ASHG and 208 from PRIM&R. Of the 372 ASHG respondents, 21 had never conducted human genetics research and were excluded from all analyses. Demographic characteristics of the remaining 559 eligible individuals are shown in Table 1 (demographic characteristics were similar for ineligible and eligible ASHG respondents (data not shown)). Both samples contained a higher percentage of women than men, with more women represented in the PRIM&R sample. Both groups included a larger proportion of individuals with more than 5 years of working experience in their area, and both samples contained individuals with experience in the opposite area.
Table 1.
Characteristics of study sample
| ASHG | PRIM&R | Total | |
|---|---|---|---|
| N = 351 | N = 208 | N = 559 | |
| Gender | |||
| Female | 182 (52%) | 158 (76%) | 340 (61%) |
| Male | 161 (46%) | 46 (22%) | 207 (37%) |
| Missing | 8 (2%) | 4 (2%) | 12 (2%) |
| Years workinga | |||
| ≤5 years | 61 (17%) | 80 (38%) | 141 (25%) |
| >5 years | 289 (82%) | 118 (57%) | 407 (73%) |
| Missing | 1 (0%) | 10 (5%) | 11 (2%) |
| Opposite service | |||
| Yes | 94 (27%) | 91 (44%) | 185 (33%) |
| No | 253 (72%) | 110 (53%) | 363 (65%) |
| Missing | 4 (1%) | 7 (3%) | 11 (2%) |
ASHG, American Society of Human Genetics; PRIM&R, Public Responsibility in Medicine and Research.
Percentages do not add to 100% due to rounding.
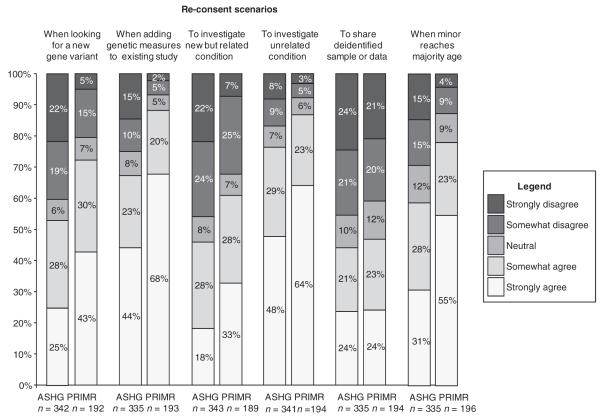
Reconsent scenarios
For each of six scenarios in which reconsent might be warranted, the survey asked respondents to indicate their level of agreement/disagreement with a statement about the ethical necessity of reconsent (Figure 1). The ASHG and PRIM&R groups had similar overall responses, with a majority of both groups, between 53% and 87%, agreeing that reconsent should occur when researchers want to look for a new gene variant or add genetic measures to an existing study, when researchers want to investigate an unrelated condition, and when a minor reaches majority age (Figure 1). However, there was a range of opinions within each group regarding conditions under which study participants should be approached for reconsent. In addition, ASHG respondents tended to be less likely than PRIM&R respondents to endorse reconsent. These differences are reflected in the results from the ordinal logistic regression models (Table 2), which show significant differences (P < 0.01) in the distribution of responses between the two groups for five of the six reconsent scenarios presented (see Figure 1): for example, 41% of ASHG respondents considered reconsent unnecessary if an investigator wished to study a genetic variant not included in the original consent, as compared with 20% of PRIM&R respondents (OR = 2.06; 95% CI = 1.49, 2.85). Similar differences were seen for reconsent when adding a genetic measure to a study that did not originally include such measures; reconsent investigating a new, but related condition or an unrelated condition; or when consent was originally given by parents on behalf of a child who is no longer a minor. For one scenario, which described researchers sharing deidentified samples or data with another investigator at an outside institution, both groups were about evenly divided in their opinions, with similar numbers of respondents in agreement with the statement as disagreeing with it, and the difference was not statistically significant (OR = 1.15; 95% CI = 0.82, 1.60).
Figure 1.
distribution of responses regarding reconsent under different scenarios: American society of Human Genetics (AsGH) and Public Responsibility in medicine and Research (PRim&R).
Table 2.
Ordinal regression results comparing strength of agreement with the question between the ASHG and PRIM&R samples
| Type | Question | OR | 95% CI | P value |
|---|---|---|---|---|
| Reconsent | I want to look for a genetic variant or gene other than the one mentioned in the original consent form. |
2.06 | 1.49, 2.85 | <0.001 |
| I want to investigate a different, but related, condition or clinical manifestation. | 1.83 | 1.32, 2.54 | <0.001 | |
| I want to investigate an unrelated condition or clinical manifestation. | 1.61 | 1.12, 2.32 | 0.011 | |
| I want to add genetic measures to a study that did not originally include them. | 2.58 | 1.77, 3.76 | <0.001 | |
| I want to share the participant’s deidentified sample or data (without a linkage file) with an investigator at another institution. |
1.15 | 0.82, 1.60 | 0.414 | |
| The original consent was given by a minor subject’s parents and the subject is now old enough to decide for him or herself. |
2.45 | 1.70, 3.52 | <0.001 | |
| Identifiability and possible harms |
How likely is it that a research participant would be personally identified in a study involving coded genetic data? |
1.78 | 1.27, 2.48 | 0.001 |
| How likely is it that a research participant would be harmed as a result of identification from coded genetic data? |
3.82 | 2.65, 5.50 | <0.001 | |
| How likely is it that a federal agency (e.g., Homeland Security) or other law- enforcement agency might compel investigators to disclose information about genetic research participants? |
2.02 | 1.37, 2.97 | <0.001 |
All models included gender and years of work experience (≤5 or >5) as covariates. An OR >1 indicates that ASHG respondents were more likely than PRIM&R respondents to disagree with the statement.
ASHG, American Society of Human Genetics; CI, confidence interval; OR, odds ratio; PRIM&R, Public Responsibility in Medicine and Research.
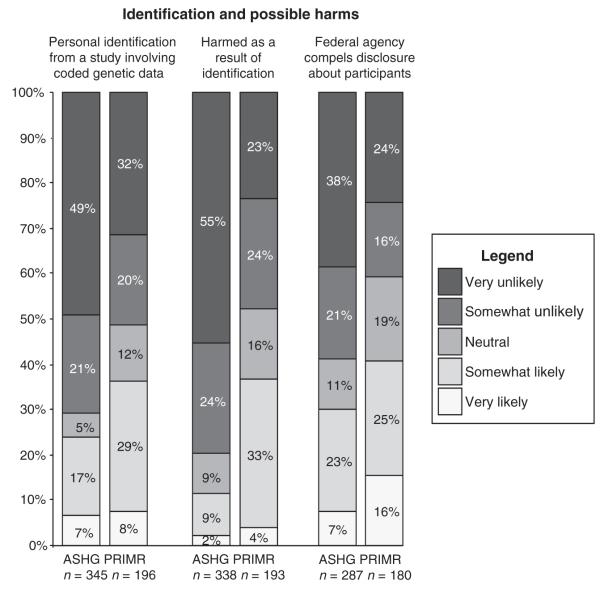
Identifiability and possible harms
Within both groups, however, there was a range of opinions regarding the likelihood of an individual research participant being reidentified from coded data, the likelihood that harm would occur if an individual were to be reidentified, and the likelihood of a federal agency compelling a researcher to disclose study data (Figure 2). The PRIM&R group had a higher percentage of people responding “neutral” to all three questions than did the ASHG group. The distribution of responses between the groups was statistically significantly different (P < 0.001) for all three items after adjusting for gender and years of work experience (Table 2). Based on results from the ordinal logistic regression models, ASHG respondents considered reidentification and compelled disclosure less likely than did PRIM&R respondents (OR = 1.78; 95% CI = 1.27, 2.48); they also considered it less likely that a federal agency would compel investigators to disclose information about study participants (OR = 2.02; 95% CI = 1.37, 2.97), or that harm to participants would occur as a result of reidentification from coded data (OR = 3.82; 95% CI = 2.65, 5.50).
Figure 2.
distribution of responses regarding research participant identification and likelihood of related harms: American society of Human Genetics (AsGH) and Public Responsibility in medicine and Research (PRim&R).
DISCUSSION
Both groups displayed a range of opinions on most questions. These observations suggest that several issues remain unresolved within each group regarding reidentification and its associated risk for participants and the circumstances under which reconsent is necessary for new uses of a research sample. Nevertheless, our results also confirm differences between genetic researchers and IRBs,23 in that we could discern differences in the distribution of opinions between the groups.
Our findings indicate that the circumstances under which reconsent should be obtained are an unsettled issue, with strong differences of opinion within both researcher and IRB professional groups. There are also differences between the groups in the distribution of opinions, with the PRIM&R group generally more likely to support reconsent than the ASHG group. Some investigators may assume that research volunteers in large-scale genetic studies are “information altruists”26 who want their information to be used for the greatest possible good—a view that has, in fact, been expressed by some study participants.15,16,18 This view, however, cannot be assumed for all research participants. The Belmont Principles,27 which provide the foundation of research regulations, include the principle of “respect for persons.” Informed consent is perhaps the most important way in which that principle is implemented in current practice, although some have recently argued in favor of more comprehensive strategies for researchers to engage with participants, communities, and the general public.28 Relevant to this issue, the US Department of Health & Human Services Advanced Notice of Proposed Rule Making on the Common Rule proposes that reconsent be required when a sample is submitted to repository.13
With respect to reidentification indicate, a minority of both groups thought that personal identification was likely or would harm participants if they were reidentified from coded data. As with the data on reconsent, the overall distributions of responses between the groups were significantly different. In general, ASHG respondents were more skeptical (unlikely) in their responses, whereas PRIM&R respondents were more neutral. The differences in the distribution of responses between the two groups on these issues may be due, in part, to the respective roles and responsibilities of scientists (i.e., advancing research) and IRB professionals (i.e., overseeing human participant protections and compliance with regulatory requirements). Investigators may be more likely to minimize potential risks when they seem unlikely or appear to have a low probability of occurring. Furthermore, as researchers manage their own human genetic studies, they may be influenced by their own experience with implementing specific protocols to ensure privacy protections for their participants. Conversely, the de facto risk-management function served by many IRBs within their institutions may engender the opposite outlook by placing more emphasis on the “not likely but possible” risk of harm to participants.
There may also be differences in how the two groups think about the mechanics of reidentification: although it is technically possible to match a deidentified genomic profile with a labeled sample and thereby reidentify an individual study participant, researchers may consider this scenario less realistic and logistically more challenging than do IRB professionals. To complicate matters, policies are evolving on deidentification as a means to protect study participants from potential harms.13 There are also practical considerations about what deidentification means to different stakeholders and what deidentification means when genetic information is involved. For example, some individuals may see no practical difference between fully anonymized data and data that are coded and retain links to identifiers. These differences in opinion result in different risk assessment about potential privacy risks to individuals29 and groups.11 Although one study has assessed patients’ views on identifiability,30 documentation is lacking about whether actual harms to participants have occurred as a result of reidentification. Information from study participants about the level of risk they are comfortable with may help to inform the debate on this particular issue. This issue may also take on a different perspective if the uniform data protections proposed by the US Department of Health & Human Services13 are passed.
There are several limitations of this study. As discussed previously, there are issues related to the generalizabilty of these findings to the broader communities of biomedical research, other genetic researchers, and IRB professionals.23,24 Specifically, although it is difficult to calculate an exact figure, response rates to the surveys were low (~8% for both). Thus, it is possible that the results do not fully represent the range of opinions within the two groups, and a larger sampling may in fact show no differences between the groups. Alternatively, it is also possible that with a larger sample size, additional differences could be detected. In addition, our study did not assess views of research participants, which would have added a valuable perspective. Future studies to assess research participants’, or potential research participants’, expectations about the use of their samples and deidentified data, understanding about protections and benefits, and level of risk they are willing to take would generate helpful information for researchers, IRB professionals, and the policy-making community.
In closing, these findings identify unresolved issues in IRB review of genetics research involving human participants and provide a starting point for discussions on the best ways to provide research oversight and participant protections. Our study also suggests that empiric data can help to identify the most problematic issues and define different perspectives. Deliberations involving all stakeholders, including groups representing research participants, funders, and policy makers, as well as researchers and IRB professionals, can help to develop consensus.
ACKNOWLEDGMENTS
This investigation involved multiple collaborators including the University of Washington Center for Genomics and Healthcare Equality, Case Western Reserve University Center for Genetics Research Ethics and Law, Public Responsibility in Medicine and Research (PRIM&R), the American Society of Human Genetics (ASHG), and the Genetic Research Review in Genetics Project (GRRIP) investigators. Additional GRRIP project investigators include (in alphabetical order) Joann Boughman, Lynn Dressler, William L. Freeman, Nancy Gerson, Eric Juengst, Patricia Marshall, P. Pearl O’Rourke, Roselle Ponsaran, and Nancy Press. Support for this research comes from National Human Genome Research Institute grants P50 HG003374, P50 HG003390, and U01 HG004609. The investigators thank members of PRIM&R and ASHG for their participation in this study. The authors also thank Jia Yin Wan for her assistance in organizing the data.
Footnotes
DISCLOSURE The authors declare no conflict of interest.
REFERENCES
- 1.Code of Federal Regulations CFR. 2005;45:46. [Google Scholar]
- 2.Jonsen AR. The Birth of Bioethics. Oxford University Press; New York: 1998. [Google Scholar]
- 3.National Institutes of Health [Accessed 6 October 2010];Human Subjects’ Protections. http://www. bioethics.nih.gov/research/protection.shtml.
- 4.Greely HT. The uneasy ethical and legal underpinnings of large-scale genomic biobanks. Annu Rev Genomics Hum Genet. 2007;8:343–364. doi: 10.1146/annurev.genom.7.080505.115721. [DOI] [PubMed] [Google Scholar]
- 5.Homer N, Szelinger S, Redman M, et al. Resolving individuals contributing trace amounts of DNA to highly complex mixtures using high-density SNP genotyping microarrays. PLoS Genet. 2008;4:e1000167. doi: 10.1371/journal.pgen.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Z, Owen AB, Altman RB. Genetics. Genomic research and human subject privacy. Science. 2004;305:183. doi: 10.1126/science.1095019. [DOI] [PubMed] [Google Scholar]
- 7.Malin B, Sweeney L. How (not) to protect genomic data privacy in a distributed network: using trail re-identification to evaluate and design anonymity protection systems. J Biomed Inform. 2004;37:179–192. doi: 10.1016/j.jbi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 8.McGuire AL, Gibbs RA. Genetics. No longer de-identified. Science. 2006;312:370–371. doi: 10.1126/science.1125339. [DOI] [PubMed] [Google Scholar]
- 9.Caulfield T, McGuire AL, Cho M, et al. Research ethics recommendations for whole-genome research: consensus statement. PLoS Biol. 2008;6:e73. doi: 10.1371/journal.pbio.0060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genetic Information Nondiscrimination Act, Public Law 110-233. Stat. 122:881. (final passage 21 May 2008) [Google Scholar]
- 11.Rothstein MA. Is deidentification sufficient to protect health privacy in research? Am J Bioeth. 2010;10:3–11. doi: 10.1080/15265161.2010.494215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed 28 October 2011];HHS ANPRM for Revision to Common Rule. Information Related to Advanced Notice of Proposed Rulemaking (ANPRM) for Revisions to the Common Rule. http://www.hhs.gov/ohrp/humansubjects/anprm2011page.html.
- 14.Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson KL. Public opinion about the importance of privacy in biobank research. Am J Hum Genet. 2009;85:643–654. doi: 10.1016/j.ajhg.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemke AA, Wolf WA, Hebert-Beirne J, Smith ME. Public and biobank participant attitudes toward genetic research participation and data sharing. Public Health Genomics. 2010;13:368–377. doi: 10.1159/000276767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinidad SB, Fullerton SM, Bares JM, Jarvik GP, Larson EB, Burke W. Genomic research and wide data sharing: views of prospective participants. Genet Med. 2010;12:486–495. doi: 10.1097/GIM.0b013e3181e38f9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bathe OF, McGuire AL. The ethical use of existing samples for genome research. Genet Med. 2009;11:712–715. doi: 10.1097/GIM.0b013e3181b2e168. [DOI] [PubMed] [Google Scholar]
- 18.Ludman EJ, Fullerton SM, Spangler L, et al. Glad you asked: participants’ opinions of re-consent for dbGap data submission. J Empir Res Hum Res Ethics. 2010;5:9–16. doi: 10.1525/jer.2010.5.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American Society of Human Genetics http://www.ashg.org/
- 20.Public Responsibility in Medicine and Research. http://www.primr.org/
- 21.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. Wiley; Hoboken, NJ: 2007. [Google Scholar]
- 22.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. SAGE; London: 2005. [Google Scholar]
- 23.Edwards KL, Lemke AA, Trinidad SB, et al. Attitudes toward genetic research review: results from a survey of human genetics researchers. Public Health Genomics. 2011;14:337–345. doi: 10.1159/000324931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemke AA, Trinidad SB, Edwards KL, Starks H, Wiesner GL, GRRIP Consortium Attitudes toward genetic research review: results from a national survey of professionals involved in human subjects protection. J Empir Res Hum Res Ethics. 2010;5:83–91. doi: 10.1525/jer.2010.5.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stata Statistical Software: Release 9.2 [computer program] Stata Corporation; College Station, TX: 2001. [Google Scholar]
- 26.Kohane IS, Altman RB. Health-information altruists–a potentially critical resource. N Engl J Med. 2005;353:2074–2077. doi: 10.1056/NEJMsb051220. [DOI] [PubMed] [Google Scholar]
- 27.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Department of Health, Education, and Welfare; Washington, DC: 1979. [PubMed] [Google Scholar]
- 28.Trinidad SB, Fullerton SM, Ludman EJ, Jarvik GP, Larson EB, Burke W. Research ethics. Research practice and participant preferences: the growing gulf. Science. 2011;331:287–288. doi: 10.1126/science.1199000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loukides G, Gkoulalas-Divanis A, Malin B. Anonymization of electronic medical records for validating genome-wide association studies. Proc Natl Acad Sci USA. 2010;107:7898–7903. doi: 10.1073/pnas.0911686107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hull SC, Sharp RR, Botkin JR, et al. Patients’ views on identifiability of samples and informed consent for genetic research. Am J Bioeth. 2008;8:62–70. doi: 10.1080/15265160802478404. [DOI] [PMC free article] [PubMed] [Google Scholar]