Abstract
Homopolymers of β-lactams can be grown by surface-initiated polymerization. These surface-linked β-peptides are living polymers with the potential to be utilized as tunable, protease-resistant interfaces in multiphase structural composites where the characteristics of the interface influence bulk properties.
Biopolymers play many roles, including formation of the structural components of organisms. They can be the structure (cell walls1), can guide structure formation (diatom frustule2) or can be a minor but critical component of a composite. This last case is examplified by substances such as seashell nacre where polypeptides present on surfaces of aragonite platelets comprise less than 5% of the composite by weight, and yet contribute to an increase in toughness by several orders of magnitude over the toughness of aragonite alone.3 Polypeptides can also perform as biocompatible4,5 or antibiotic surfaces.6,7 Polypeptides are, however, readily degraded in vivo by unbiquitous proteolytic enzymes.
β-Peptides, homologs of the naturally occuring α-peptides, are polymers of β-amino acids that incorporate an additional carbon between the amine and the carbonyl group of the monomers. β-Peptides form all of the secondary structures found with the α-peptides, yet are highly resistant to proteolytic enzymes.8,9 We report here the base-catalyzed growth of β-peptide homopolymers on glass and silica particles, by surface-initiated polymerization of readily available β-lactams.
Surface-attached polymers can be formed by linking a pre-formed polymer ("grafting to") or by growing a polypeptide from a polymerization initiating group secured to the surface ("grafting from"). This latter method, surface-initiated polymerization, is a powerful process for creating a densely coated surface.10–12 In the case of β-peptide homopolymers, the "grafting to" method cannot be used because they undergo self-association to give insoluble oligomers, making grafting impossible. The self association of β-peptides has been a long standing obstacle to the study of these polymers.13,14 Use of surface-initiated polymerzation has the potential to circumvent this solubility issue.
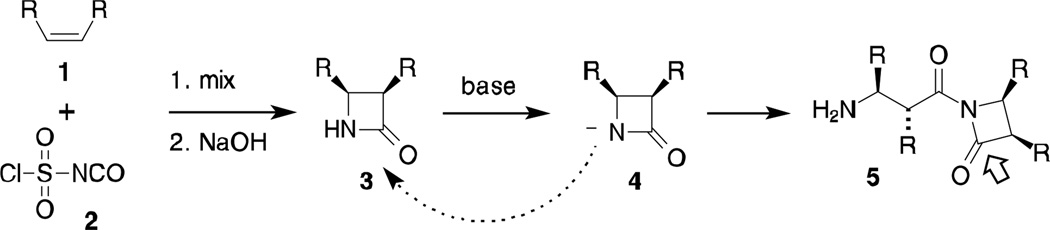
Polymerization of β-lactams 3 directly yields β-peptides without the need for additional activation of the monomer and without byproducts. β-Lactams are readily prepared by cycloaddition of chlorosulfonyl isocyanate 2 with alkenes, Scheme 1.15 Under basic conditions, attack of deprotonated lactam 4 on another monomer yields N-acylated β-lactam 5. Subsequent attack of 4 on activated β-lactam 5 (arrow) leads to β-peptide formation. Growth of β-peptides under basic catalysis occurs almost exclusively from N-acylated β-lactams such as 5. Gellman16 and others14,17 have demonstrated that an added N-activated β-lactam can act as an efficient initiating group, leading to low polydispersity. Based on this precedent, we have prepared an activated β-lactam initiating group 10 to study surface-initiated polymerization of β-lactam monomers, Scheme 2.
Scheme 1.
β-Lactam formation and base-catalyzed polymerization.
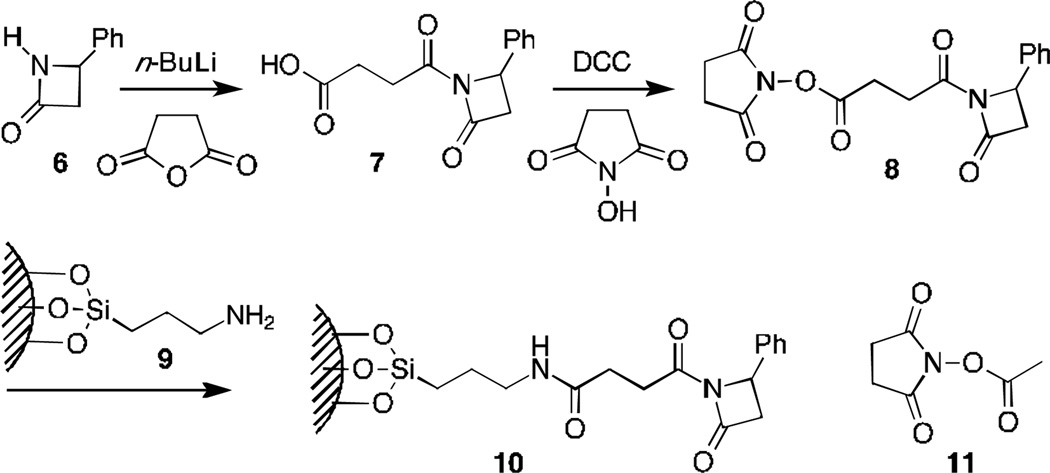
Scheme 2.
Preparation of surface-linked polymerization initiating group.
Treating β-lactam 618 with n-butyllithium followed by succinic anhydride gave acid 7. Condensation of 7 with N-hydroxy succinimide then gave bifunctional 8. While reaction at either terminus would provide a polymerization intiation site,16 when 8 was treated with a primary amine the hydroxysuccinimide was cleanly displaced. Treatment of aminopropylsilane coated glass particles 9 (surface area 10 m2/g, amine coverage19 7.8 µmol/g) led to polymerization substrate 10. Reanalysis of surface amine density indicated that >90% had reacted.
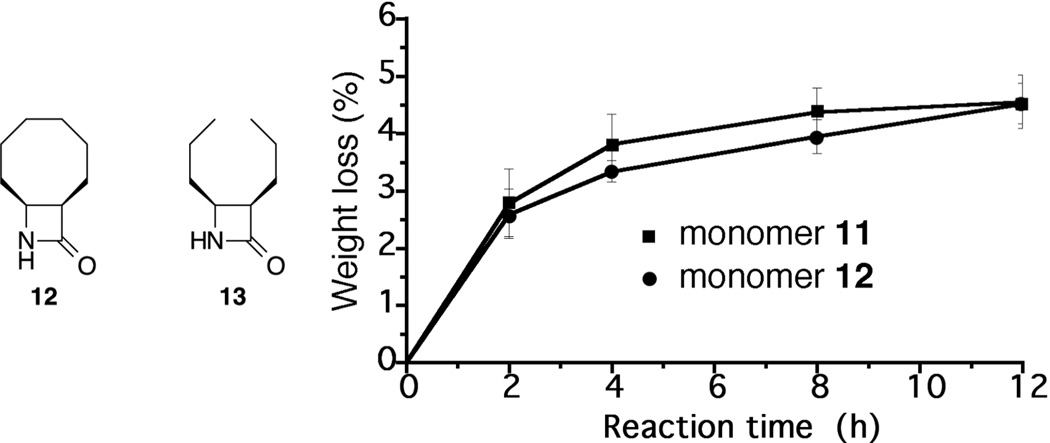
Two β-lactams were selected for initial studies, 12 and 13, Figure 1. Cyclooctane-fused 12 was found by Gellman to give cyclooctane β-peptides that retained solubility, presumably because of limited intermolecular associations. The closely related cis-3,4-di-n-propyl azetidinone 13 lacks the conformational constraints of the fused cyclooctane ring and its cis stereochemistry was expected to yield n-octane β-peptide oligomers with conformations suitable for β-sheet interactions.20,21
Figure 1.
Monomers and polymerization weight gain (TGA) with reaction time.
Suspensions of initiator-linked glass particles 10, combined with β-lactam 12 or 13 (100 equiv, 0.2 M in DMF) at 0 °C, were treated with lithium hexamethyldisilazide (15 equiv, 1 M in THF) and then quenched after a predetermined time with sat. NH4Cl. The treated glass was washed three times each with THF, water, acetone, chloroform and acetone, and then dried overnight at ambient temperature under vacuum. Reactions run in triplicate for 2, 4, 8 and 12 h were analyzed by TGA and the results are shown in Figure 2. Extensive washing of the product was undertaken to ensure that only covalently attached polymer was retained. Polymers of 12 are known to have good solubility properties,16,22 while 13 would be expected to have limited solubility. Nevertheless, the average weight gain with reaction time increased similarly for both monomers.
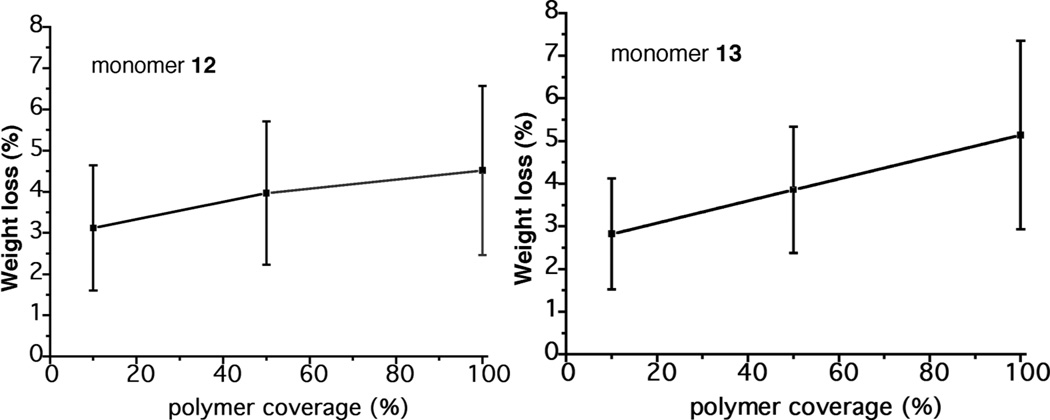
Figure 2.
Weight gain as a function of surface coverage.
Treatment of the glass particles with the initiator linking unit 8 diluted by succinimidyl acetate 11 (8/11 1:1 and 1:9) gave 50% and 10% initiator coverage, respectively.10 Polymerization of monomers 12 and 13 was then conducted with the resulting glass using 8 h reaction times, under conditions identical to those described above. The results are shown in Figure 2. For these reactions, run in triplicate, polymerization of monomer 12 gave an average of 5.2% weight gain for the silica particles without dilution of the initiator. At 50% and 10% coverage the weight gains were 4.8% and 4.5%, respectively. The change in weight gains for monomer 13 at 100%, 50% and 10% coverage were 5.1%, 3.9% and 2.8%, respectively. In each case, the same quantities of monomer and base were used, e.g., 200 equiv and 1000 equiv of monomer relative to initiator for 50% and 10% initiator coverage, respectively. The nonlinear polymer weight gains with decreasing initiator coverage may be due to an increase in the length of the polymer chains with an increase in the monomer/initiator ratio. This would be consistent with the work of Gellman, et al. in their solution studies of monomer 12 polymerization, where diminishing quantities of initiator gave a corresponding increase in polymer length.16,17
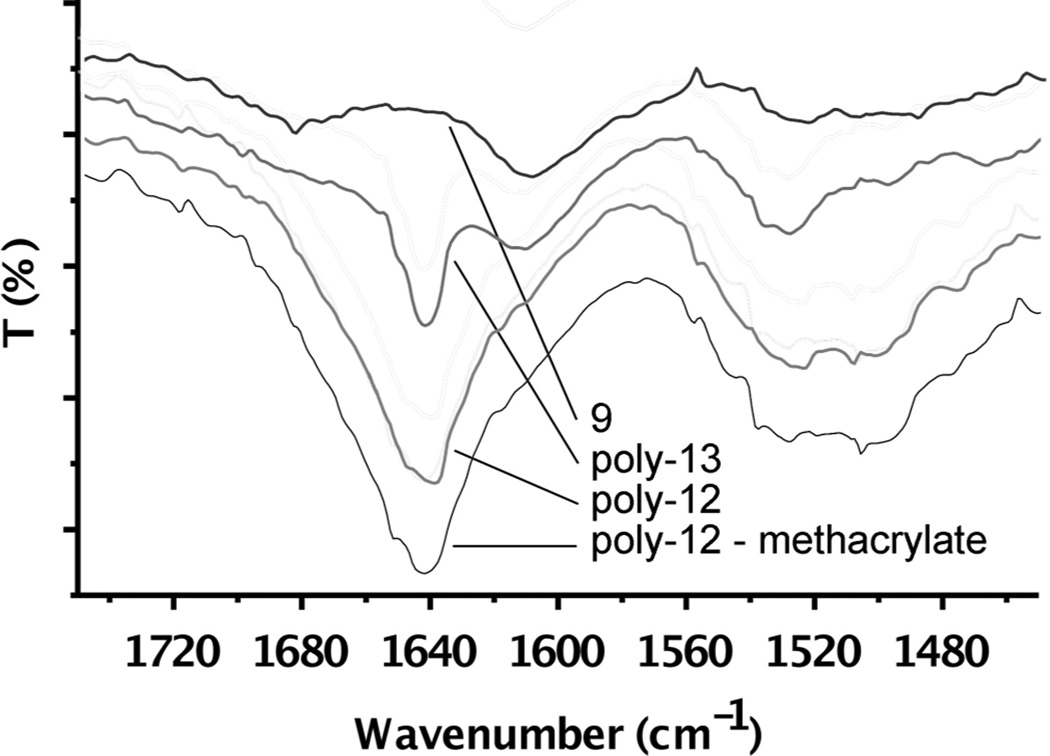
The carbonyl region of four infrared spectra are shown in Figure 3 using identically treated silica (vide infra). Little absorption is seen for the aminosilylated silica 9. After attachment of the initiator and polymerization of monomers 12 and 13, significant absorptions are seen for characteristic amide I (ca. 1640 cm−1) and amide II (1520-30 cm−1). Little change was noted after derivatization with methacrylate. This is unsurprising as the amides far outnumber the esters and have a larger extinction coefficient.23
Figure 3.
IR spectra of aminosilylated silica (9) and after polymerization of 12 and 13.
It is notable that the carbonyl absorbances for poly-13 are much less broad than poly-12. This is consistent with poly-13 having extensive hydrogen bonding interactions, whereas poly-12 would not.16
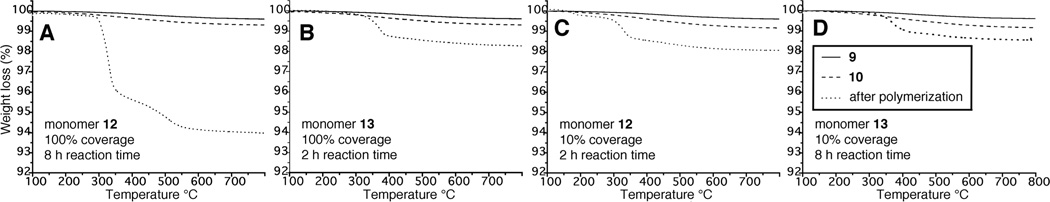
The reaction parameters were surveyed using commercial aminopropyl silane coated silica (surface area 4.5 m2/g, amine coverage 6.5 µmol/g) and a fractional factorial experimental design, Figure 4. In these four experiments, TGA of the commercial aminopropyl silane coated silica 9 found ca. 0.3% organic material. A small increase in the volatiles was observed after derivatization of the surface amino groups with 8 and 8/11 mixtures. With cyclooctane monomer 12, 100% initiating group coverage and 8 h polymerization time, Graph A, an additional 5.4% surface organics resulted from base-catalyzed polymerization. When the initiator coverage was reduced to 10% and the reaction time was reduced to 2 h, Graph C, the added cyclooctane polymer was lowered to 1.1%. Switching to monomer 13, with 100% initiator coverage and 2 h reaction time, Graph B, the additional polymer was 1% of the silica. Returning to 8 h reaction time, with 10% initiator coverage, Graph D, polymerization of monomer 13 gave 0.7% polymer weight.
Figure 4.
TGA analysis of four experiments surveying monomers 12 and 13, reaction time and surface density of the β-peptide.
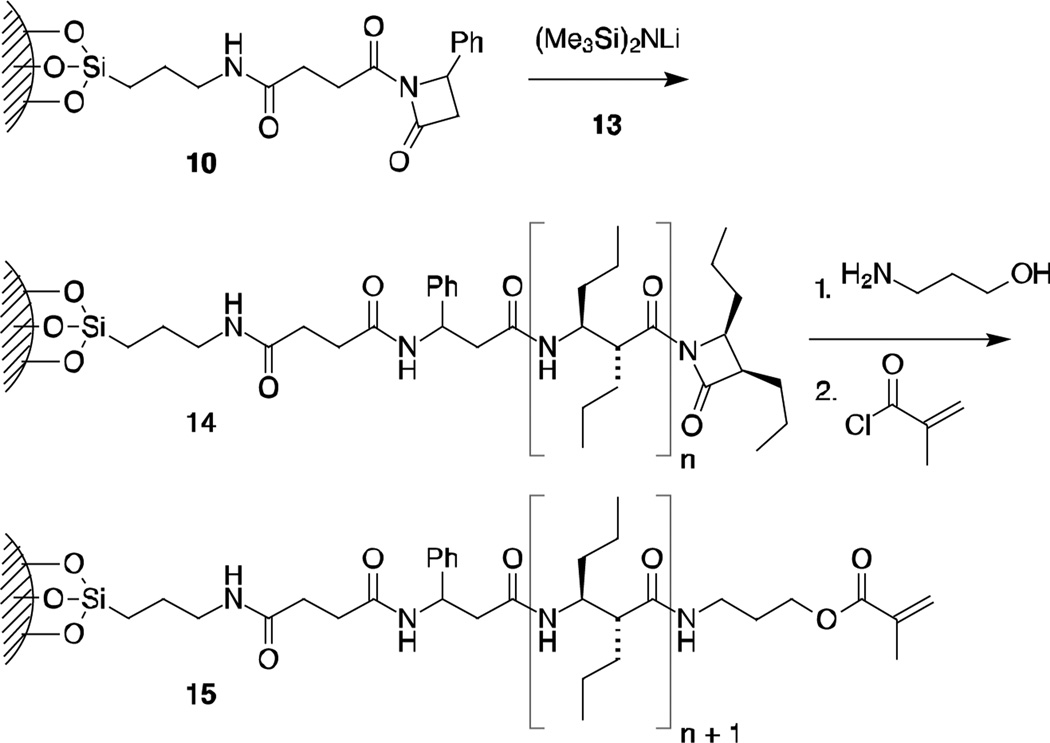
The polymerization reaction is depicted in Scheme 3. A suspension of particles coated with initiator (10) is treated with a large excess of monomer 12 or 13 and a catalytic amount of strong base (lithium hexamethyldisilazide). In the case of 13, attack of the β-lactam monomer anion on the activated β-lactam in 10 leads to ring opening and generation of a new activated lactam terminus. A second monomer anion will attack the terminal carbonyl and generate 14 (n = 1).‡ Termination of the polymerization by depletion of the monomer or by quenching the process with mild acid will leave a reactive terminal β-lactam 14.
Scheme 3.
Elaboration of the living polymers.
A variety of nucleophiles can be used to open the β-lactam in 14. Scheme 3 illustrates the use of 3-aminopropanol. Reaction of the nucleophilic amine followed by capping of the resulting terminal alcohol with methacryloyl chloride leads to an acrylate-terminated β-peptide. Treatment of the silica-β-peptide particles in this way leads to additional weight as determined by TGA, of about 0.3%. The incorporation of these silica–β-peptide–methacrylate particles into composites and the resulting properties of these composites will be reported elsewhere.
β-Peptides are outstanding surrogates for naturally occuring α-peptides, forming secondary and tertiary structures that effectively mimic the natural products but are also stable towards proteolytic enzymes. We have found that homopolymers of β-lactams can be grown from surfaces functionalized with N-acylated β-lactam initiating groups. It appears that utilizing this "grafted from" technology can overcome the propensity of β-peptides to precipitate as insoluble matter.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant DE 019885. We are grateful to Esstech and HPF The Mineral Engineers Division of Quarzwerke for donation of materials.
Footnotes
Electronic Supplementary Information (ESI) available: Preparation and spectra of 8 and representative procedures. See DOI: 10.1039/b000000x/
In this example, lactam 13 and its polymer are depicted as a single enantiomer for simplicity. While it is very likely that the details of polymerization and the properties of the polymer derived from the racemic monomer will be different from that derived from a single enantiomer, the ability of the polymer produced from 3,4-cis disubstituted β-lactams such as 13 to adopt a β-sheet compatible conformation should be similar in both cases. The use of racemic α-amino acids to prepare β-sheets has been addressed theoretically24 and experimentally.25
Notes and references
- 1.Buchanan BB, Gruissem W, Jones RL. Biochemistry & Molecular Biology of Plants. Wiley: Somerset, NJ; 2002. [Google Scholar]
- 2.Nassif N, Livage J. Chem. Soc. Rev. 2011;40:849. doi: 10.1039/c0cs00122h. [DOI] [PubMed] [Google Scholar]
- 3.Meyers M, Lin A, Seki Y, Chen P-Y, Kad B, Bodde S. JOM-J. Min. Met. Mat. S. 2006;58:35. [Google Scholar]
- 4.Ceylan H, Tekinay AB, Guler MO. Biomaterials. 2011;32:8797. doi: 10.1016/j.biomaterials.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Hnilova M, So CR, Oren EE, Wilson BR, Kacar T, Tamerler C, Sarikaya M. Soft. Matter. 2012;8:4327. [Google Scholar]
- 6.Arvidsson PI, Frackenpohl J, Ryder NS, Liechty B, Petersen F, Zimmermann H, Camenisch GP, Woessner R, Seebach D. ChemBioChem. 2001;2:771. doi: 10.1002/1439-7633(20011001)2:10<771::aid-cbic771>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Porter EA, Weisblum B, Gellman SH. J. Am. Chem. Soc. 2002;124:7324. doi: 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]
- 8.Seebach D, Beck AK, Bierbaum DJ. Chemistry. &. Biodiversity. 2004;1:1111. doi: 10.1002/cbdv.200490087. [DOI] [PubMed] [Google Scholar]
- 9.Cheng RP, Gellman SH, DeGrado WF. Chem. Rev. 2001;101:3219. doi: 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]
- 10.Tsujii Y, Ohno K, Yamamoto S, Goto A, Fukuda T. Adv. Polym. Sci. 2006;197:1. [Google Scholar]
- 11.Advincula R. Adv. Polym. Sci. 2006;197:107. [Google Scholar]
- 12.Buchmeiser MR. Adv. Polym. Sci. 2006;197:137. [Google Scholar]
- 13.Graf R, Lohaus G, Borner K, Schmidt E, Bestian H. Angew. Chem. Int. Ed. 1962;1:481. [Google Scholar]
- 14.Hashimoto K. Prog. Poly. Sci. 2000;25:1411. [Google Scholar]
- 15.Rasmussen JK, Hassner A. Chem. Rev. 1976;76:389. [Google Scholar]
- 16.Zhang J, Kissounko DA, Lee SE, Gellman SH, Stahl SS. J. Am. Chem. Soc. 2009;131:1589. doi: 10.1021/ja8069192. [DOI] [PubMed] [Google Scholar]
- 17.Sebenda J, Hauer J. Polym. Bull. 1981;5:529. [Google Scholar]
- 18.Huang H, Iwasawa N, Mukaiyama T. Chemistry. Letters. 1984;13:1465. [Google Scholar]
- 19.Gaur RK, Gupta KC. Anal. Biochem. 1989;180:253. doi: 10.1016/0003-2697(89)90426-0. [DOI] [PubMed] [Google Scholar]
- 20.Seebach D, Hook DF, Glättli A. Pept. Sci. 2006;84:23. doi: 10.1002/bip.20391. [DOI] [PubMed] [Google Scholar]
- 21.Langenhan JM, Gellman SH. Org. Lett. 2004;6:937. doi: 10.1021/ol0364430. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Gellman SH, Stahl SS. Macromolecules. 2010;43:5618. [Google Scholar]
- 23.Flett MSC. Spectrochim. Acta. 1962;18:1537. [Google Scholar]
- 24.Pauling L, Corey RB. Proc. Natl. Acad. Sci. USA. 1953;39:253. doi: 10.1073/pnas.39.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein I, Eliash R, Bolbach G, Weissbuch I, Lahav M. Angew. Chem. Int. Ed. Engl. 2007;46:3710. doi: 10.1002/anie.200605040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.