Abstract
PURPOSE
To evaluate the patterns of use and the risk of thromboembolic events (TEE) associated with Erythropoietin Stimulating Agents (ESAs) in older patients with metastatic breast cancer receiving chemotherapy.
METHODS
Retrospective study using the SEER-Medicare linked database. Stage IV breast cancer patients diagnosed from 1995–2005, treated with chemotherapy, ≥66 years old, with full coverage of Medicare A and B were included. ICD-9 and HCPCS codes were used to identify the use of ESAs, chemotherapy, and complications of therapy. Analyses included descriptive statistics and logistic regression.
RESULTS
2,266 women were included, 980 (43.3%) received ESAs and 1,286 (56.7%) did not. Patients diagnosed after 1999 or who received treatment with taxanes, anthracyclines or vinorelbine were more likely to receive ESAs. Patients receiving ESAs had higher rates of stroke (18.5% vs 15.1%, p=0.031); DVT (21.3% vs. 14.4%, p<0.001), other/unspecified TEE (19.8% vs 14.7%; p=0.001) and any clot (31.3% vs. 23.4%, p<0.0001). In multivariable analysis, patients receiving ESAs had increased risk for DVT (OR=1.36; 1.05–1.75), and any clot (OR=1.26; 1.02–1.57). A dose-dependent effect was evident for stroke, DVT, other TEE and any clot.
CONCLUSION
In this cohort of patients, the use of ESAs increased the risk of TEEs, with a dose-dependent effect for stroke, DVT, other TEE and any clot. Our data shows that among patients treated with chemotherapy and ESAs for metastatic breast cancer, TEEs are a common event. Therefore caution is recommended when using these agents.
INTRODUCTION
The erythropoiesis-stimulating agents (ESAs), erythropoietin and darbepoetin, have been widely used to increase hemoglobin values and reduce transfusion requirements in cancer patients1–3. Recent clinical trials and various meta-analyses have raised safety concerns, as results suggest that the use of ESAs is associated with adverse outcomes including thromboembolic events (TEEs) and potentially tumor progression and decreased survival4–6.
It is well known that patients with cancer have an increased risk of TEE7; however an even higher incidence of embolic and thrombotic events has been seen in cancer patients treated with ESAs for different malignancies8–11. A recent meta-analysis showed that patients with cancer who received ESAs had a higher risk of TEEs (HR 1.57; 95% CI 1.31–1.87) and mortality (HR 1.10; 95%CI 1.01–1.20) than control patients5. Current ASCO and NCCN guidelines recommend awareness about the adverse outcomes associated with ESAs use, and advise its use exclusively in patients with chemotherapy-induced anemia being treated with non-curative intent12,13.
Despite the evidence, there is not a clear description of the different TEEs that are associated with ESAs use. Also, questions regarding a dose-effect remain unanswered and it is unclear whether increasing doses or longer durations of treatment are associated with worse outcomes. Although ESAs are agents that are widely used to treat cancer patients, to the best of our knowledge, there is no clear description of the patterns of ESAs use in patients with metastatic breast cancer. In this retrospective cohort study, using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, we sought to estimate the use of ESAs and to evaluate the TEEs associated with its use in patients older than 66 years of age, receiving chemotherapy for metastatic breast cancer.
PATIENTS AND METHODS
DATA SOURCE
We used the SEER-Medicare linked database. The SEER program, supported by the U.S. National Cancer Institute (NCI), collects data from tumor registries; during the years included in this study, it covered 14% to 25% of the U.S. population14. The Medicare program is administered by the Centers for Medicare and Medicaid Services and covers 97% of the U.S. population aged 65 and older15. SEER participants are matched with their Medicare records under an agreement between the NCI and Centers for Medicare and Medicaid Services. Of SEER participants who were diagnosed with cancer at age 65 years or older, 94% are matched with their Medicare enrollment records15. Patient demographics, tumor characteristics, and treatment information were extracted from the SEER-Medicare Patient Entitlement and Diagnosis Summary File.
STUDY POPULATION
In the SEER-Medicare database, 268198 men and women were diagnosed with breast cancer between January 1995 and December 2005. We included patients 66 years old who had a diagnosis of stage IV breast cancer (According to the American Joint Committee on Cancer staging system 3rd edition) and were treated with chemotherapy. Patients were required to have Medicare Part A and Part B and not to be members of a Health Maintenance Organization (HMO) for one year prior and after their breast cancer diagnosis, because Medicare claims are not complete for HMO members. Patients who had end stage renal disease (ESRD), prior cancer, or non-carcinoma tumor histology were excluded.
From the initial 268,198 patients with breast cancer diagnosed from 1995 to 2005; 23,885 had history of prior or subsequent malignancies; 843 had unknown month of diagnosis; 85,266 were younger than ≥66 years old at diagnosis; 151,066 had an initial stage other than IV; 2,047 did not have full coverage of both Medicare A and B or were members of an HMO, 16 had non carcinoma histology and 45 had ESRD. From the remaining 5030 patients, 2764 did not receive chemotherapy. A total of 2266 patients were included in this study.
DATA EXTRACTION AND DEFINITIONS
To identify patients who received ESAs, we looked for related codes in the Medicare claims at any time after diagnosis. The following HCPCS codes were used for defining ESAs regimens: Q0136, J0885, Q0137, J0880, J0881 and C1774. For this analysis, a single dose of erythropoietin alfa was defined as 40,000 units and was considered equivalent to a dose of darbepoietin alfa of 200mcg12. To identify patients that received chemotherapy we search for the following codes: ICD-9-CM procedure code 9925 for a hospital inpatient or outpatient facility claim of chemotherapy; HCPCS codes J8510, J8520, J8521, J5530 through J8999, and J9000 through J9999, excluding J9202, J9209, J9212 through J9214, J9217, J9218 in physician, outpatient or DME files. Revenue center codes 0331, 0332 and 0335 for an outpatient claim of chemotherapy; and the ICD-9-CM V codes V58.1, V66.2, V67.2 in inpatient, outpatient, physician or DME files were also used.
In order to look for toxicities associated with ESAs, TEEs were identified using the following ICD-09 diagnosis codes in inpatient, DME, physician and outpatient files: Diagnosis code 410 was identified as Myocardial infarction (MI) 410; 433–436, 438 as stroke; code 415.1× was identified as pulmonary embolism (PE); codes 451, 453.1, 453.2, and 453.4 were identified as Deep Vein Thrombosis (DVT); and codes 452, 453, 453.0, 453.3, 453.5, 453.6, 453.7, 453.8, and 453.9 were identified as other/unclassified TEEs. The other/unclassified category includes codes for various conditions such as portal vein thrombosis and Budd-Chiari syndrome, renal vein thrombosis, thrombosis of veins excluding the pulmonary, cerebral and coronary veins and embolism or thrombosis of unspecified veins, among others. A comorbidity score was calculated using Klabunde’s adaptation of the Charlson comorbidity index from the SAS macro provided by NCI16–19. The comorbidities included in the score are myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, diabetes (with and without end-organ damage), chronic pulmonary disease, connective tissue disease, ulcer disease, liver disease, renal disease, hemiplegia, and AIDS.
Demographic data was obtained from the SEER-Medicare Patient Entitlement and Diagnosis Summary File. For the census tract variables of education and poverty level, quartiles were calculated in increasing order. Data from the 2000 census were supplemented with 1990 data if 2000 data were missing. Patients were followed up from diagnosis date until loss of Medicare coverage, enrollment in HMO, or death.
STATISTICAL ANALYSIS
Demographic and tumor characteristics between patients receiving or not receiving ESAs were compared using the χ2 test, or Wilcoxon’s test as indicated. To determine the factors associated with ESAs use, a multivariable logistic regression model was used to calculate the odds ratio (OR) of receiving ESAs. The variables included in the multivariable logistic regression model included the type of chemotherapy that was used (taxanes, anthracyclines, vinorelbine), age, gender, race, marital status, education level, poverty level, year of diagnosis, geographical location, tumor grade, estrogen receptor status, and comorbidities. The different TEE categories evaluated were MI, stroke, PE, DVT, other/unclassified TEE and any clot. Any clot was defined as at least one event in any of the other previously mentioned categories. For each TEE category, the proportion of patients experiencing the outcome was calculated according to ESAs group. In order to avoid bias, we excluded the patients that had the outcome under study the year prior to their breast cancer diagnosis. For each of these outcome variables, we performed a univariable analysis with ESAs in the model. Then for the multivariable logistic regression analysis, we adjusted ESAs by age, race, education level, poverty level, year of diagnosis, geographical region, estrogen receptor status, comorbidities and transfusion. Results are expressed in Odds Ratios (OR) with 95% Wald Confidence Intervals (CIs).
In order to evaluate for a potential dose-effect, a similar multivariable analysis was performed using ESAs as a categorical variable based on the number of doses received per patient (>0–5, 5–16, >16), the reference value was no use of ESAs. When a change in the estimate by dose was seen, a trend was evaluated by introducing the ESAs dose category as a numerical variable and testing the significance of the model’s linearity in the same multivariable model. All computer programming and statistical analyses were performed with the SAS system (Cary, NC), and all tests were two sided.
According to the National Cancer Institute regulations, and in order to preserve confidentiality, in Table 1, the categories that included less than 11 patients were omitted (educational level unknown, poverty level unknown); however such variables were still included in our analysis.
Table 1.
Patient and tumor characteristics according to erythropoiesis stimulating agent (ESA) treatment status
| Variable | Strata | No ESAs 1286 | ESAs 980 | P* |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age group | 66–70 | 359 (27.9) | 338 (34.5) | <.0001 |
| 71–75 | 374 (29.2) | 282 (28.8) | ||
| 76–80 | 306 (24) | 233 (23.8) | ||
| >80 | 243 (19) | 127 (12.8) | ||
| Gender | Female | 1272 (98.9) | 968 (98.8) | 0.764 |
| Male | 14 (1.1) | 12 (1.2) | ||
| Race | White | 1057 (82.2) | 800 (81.6) | 0.928 |
| Black | 138 (10.7) | 110 (11.3) | ||
| Other | 91 (7.1) | 70 (7.1) | ||
| Education§ | Highest quartile | 306 (23.8) | 263 (26.8) | 0.327 |
| High quartile | 322 (25.0) | 242 (24.7) | ||
| Low quartile | 323 (25.1) | 244 (24.9) | ||
| Lowest quartile | 335 (26.0) | 231 (23.6) | ||
| Poverty§ | Highest quartile | 306 (23.8) | 261 (26.6) | 0.072 |
| High quartile | 307 (23.9) | 261 (26.6) | ||
| Low quartile | 335 (26.0) | 230 (23.5) | ||
| Lowest quartile | 338 (26.0) | 228 (23.3) | ||
| Year of diagnosis | 1995–1999 | 465 (36.2) | 155 (15.8) | <.0001 |
| 2000–2002 | 422 (32.8) | 393 (40.1) | ||
| 2003–2005 | 399 (31.0) | 432 (44.1) | ||
| Region | Connecticut | 126 (9.8) | 80(8.2) | <.0001 |
| Detroit | 153 (11.9) | 93 (9.5) | ||
| California+Hawaii | 337 (26.2) | 286 (29.2) | ||
| Iowa | 135 (10.5) | 60 (6.1) | ||
| New Mexico | 34 (2.6) | 15 (1.5) | ||
| Seattle | 85 (6.6) | 53 (5.4) | ||
| Utah | 41 (3.2) | 18 (1.8) | ||
| Atlanta+Rural Georgia | 60 (4.7) | 53 (5.6) | ||
| Kentucky | 76 (5.9) | 49 (5.0) | ||
| Louisiana | 55 (4.3) | 76 (7.8) | ||
| New Jersey | 184 (14.3) | 195 (19.9) | ||
| Tumor grade | 1 | 67 (5.2) | 48 (4.9) | 0.736 |
| 2 | 343 (26.7) | 253 (25.8) | ||
| 3 | 486 (37.8) | 393 (40.1) | ||
| unknown | 390 (30.3) | 286 (29.2) | ||
| ER status | Positive | 644 (50.1) | 497(50.7) | 0.009 |
| Negative | 285 (22.2) | 259 (26.4) | ||
| unknown | 357 (27.8) | 224 (22.9) | ||
| Charlson score | 0 | 990 (77.0) | 742 (75.7) | 0.776 |
| 1 | 194 (15.1) | 155 (15.8) | ||
| 2+ | 103 (7.9) | 83 (8.5) | ||
| Anthracycline | No | 946 (73.6) | 592 (60.4) | <.0001 |
| Yes | 340 (26.4) | 388 (39.9) | ||
| Taxane | No | 865 (67.3) | 356 (36.3) | <.0001 |
| Yes | 421 (32.7) | 624 (63.7) | ||
| Vinorelbine | No | 1204 (93.6) | 714 (72.9) | <.0001 |
| Yes | 82 (6.4) | 266 (27.1) | ||
| Blood transfusion | No | 1087 (84.5) | 727 (74.2) | <.0001 |
| Yes | 199 (15.5) | 253 (25.8) | ||
P* is chi-square p value
Education and poverty levels are categorized by census tract.
RESULTS
Our final cohort included 2,266 patients, 980 (43.3%) of them received ESAs and 1,286 (56.7%) did not; median time of follow up was 37 months. Patient demographics and tumor characteristics are listed in Table-1. The median time from diagnosis to first dose of chemotherapy was 3 months. Amongst patients receiving ESAs, median time to first ESAs dose was 5 months, and the median number of doses administered was 9 (standard deviation 18.3).
When evaluating the factors associated with ESAs use, we observed that the patients who received anthracyclines (OR 1.85; 95%CI 1.48–2.31), taxanes (OR 2.74; 95%CI 2.25–3.35) and vinorelbine (OR 4.51; 95%CI 3.37–6.04), were more likely to receive ESAs than patients who were not treated with such antineoplastic agents. Other factors associated with the use of ESAs were year of diagnosis (2000–2002 vs. 1995–1999 OR 3.06; 95%CI 2.32–4.04 and 2003–2005 vs. 1995–1999 OR 3.76; 95% CI 2.83–5.00), and some geographical locations. The complete multivariable analysis is shown in Table-2.
Table 2.
Multivariable analysis of factors predicting erythropoiesis stimulating agents (ESAs) use.
| Effect | OR | 95% CI |
|
|---|---|---|---|
| LowerCI | UpperCI | ||
| Age 66–70 (Reference) | |||
| 71–75 | 0.92 | 0.72 | 1.18 |
| 76–80 | 1.00 | 0.77 | 1.31 |
| >80 | 0.88 | 0.65 | 1.20 |
| Race Black vs White | 1.05 | 0.74 | 1.49 |
| Race Other vs White | 1.0 | 0.67 | 1.48 |
| Year of cancer diagnosis 1995–1999 (Reference) | |||
| 2000–2002 | 3.06 | 2.32 | 4.04 |
| 2003–2005 | 3.76 | 2.83 | 5.00 |
| Region California+Hawaii (Reference) | |||
| Atlanta+Rural Georgia | 1.37 | 0.87 | 2.18 |
| Connecticut | 0.67 | 0.45 | 0.99 |
| Detroit | 0.67 | 0.46 | 0.97 |
| Iowa | 0.57 | 0.38 | 0.86 |
| Kentucky | 0.56 | 0.35 | 0.90 |
| Louisiana | 1.52 | 0.97 | 2.38 |
| New Jersey | 0.86 | 0.63 | 1.17 |
| New Mexico | 0.41 | 0.20 | 0.83 |
| Seattle | 0.78 | 0.50 | 1.23 |
| Utah | 0.55 | 0.28 | 1.07 |
| Tumor grade 1 (Reference) | |||
| Tumor grade 2 | 0.96 | 0.61 | 1.50 |
| Tumor grade 3 | 0.96 | 0.62 | 1.49 |
| Tumor grade unknown | 1.15 | 0.74 | 1.80 |
| Estrogen Receptor Negative (Reference) | |||
| Estrogen Receptor Positive | 0.88 | 0.68 | 1.12 |
| Estrogen Receptor Unknown | 0.72 | 0.56 | 0.91 |
| Charlson comorbidity score 0 (Reference) | |||
| Charlson comorbidity score 1 | 1.04 | 0.80 | 1.36 |
| Charlson comorbidity score >=2 | 1.21 | 0.85 | 1.71 |
| Anthracycline treatment | 1.85 | 1.48 | 2.31 |
| Taxane treatment | 2.74 | 2.25 | 3.35 |
| Vinorelbine treatment | 4.51 | 3.37 | 6.04 |
| Blood transfusion | 1.93 | 1.52 | 2.44 |
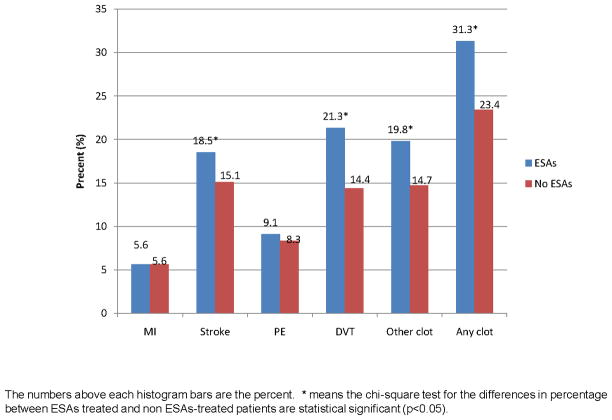
The rates of TEEs among patients who received and did not receive ESAs are shown in Figure-1. Higher crude rates of stroke (18.5% vs. 15.1%, p=0.031), DVT (21.3% vs. 14.4%, p<0.0001), other/unspecified TEE (19.8% vs. 14.7, p=0.001) and any clot (31.3% vs. 23.4%, p<0.0001) were seen in patients who received ESAs. No difference in the rate of MI (p=0.989) or PE (p=0.523) was observed. The crude and adjusted estimates for the analysis of ESAs use on TEEs are shown in Table-3. After adjusting for possible confounders, we observed that patients receiving ESAs had an increased risk of developing a DVT (OR 1.36; 95%CI 1.05–1.75) and any clot (OR 1.26; 95%CI 1.02–1.57) compared with patients that did not received ESAs. A trend for increased risk was seen for stroke (OR 1.24; 95%CI 0.96–1.60) and other/unspecified TEE (OR 1.11; 95% CI 0.86–1.44), but statistical significance was not achieved.
Figure 1.
Thromboembolic events (TEEs) among patients who received and did not receive ESAs.
Table 3.
Univariable and Multivariable Analyses¥ of different thromboembolic events (TEEs) according to erythropoiesis stimulating agents (ESAs) use.
| Event | ESAs treatment | Univariable | Multivariable | ESAs dose | Event | Univariable | Multivariable | P_trend | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes (%) | No (%) | OR (95% CI) | OR (95% CI) | Yes | No | OR (95% CI) | OR (95% CI) | |||
| MI | ||||||||||
| No | 925 (94.4) | 1214 (94.4) | Reference | Reference | 0 | 72 (56.7) | 1214 (56.8) | Reference | Reference | 0.905 |
| Yes | 55 (5.6) | 72 (5.6) | 1.00 (0.70,1.44) | 0.99 (0.65,1.52) | 1–5 | 20 (15.7) | 335 (15.7) | 1.01 (0.60,1.68) | 1.01 (0.59,1.75) | |
| 5–16 | 15 (11.8) | 308 (14.4) | 0.82 (0.46,1.45) | 0.87 (0.47,1.61) | ||||||
| > 16 | 20 (15.7) | 282 (13.2) | 1.20 (0.72,2.00) | 1.11 (0.61,2.02) | ||||||
| Stroke | ||||||||||
| No | 799 (81.5) | 1092 (84.9) | Reference | Reference | 0 | 194 (51.7) | 1092 (57.7) | Reference | Reference | 0.014 |
| Yes | 181 (18.5) | 194 (15.1) | 1.28 (1.02,1.59) | 1.24 (0.96,1.60) | 1–5 | 55 (14.7) | 300 (15.9) | 1.03 (0.75,1.43) | 1.04 (0.74,1.46) | |
| 5–16 | 60 (16.0) | 263 (13.9) | 1.28 (0.93,1.77) | 1.29 (0.91,1.83) | ||||||
| > 16 | 66 (17.6) | 236 (12.5) | 1.57 (1.15,2.15) | 1.54 (1.08,2.21) | ||||||
| PE | ||||||||||
| No | 891 (90.9) | 1179 (91.7) | Reference | Reference | 0 | 107 (54.6) | 1179 (57.0) | Reference | Reference | 0.659 |
| Yes | 89 (9.1) | 107 (8.3) | 1.10 (0.82,1.48) | 1.00 (0.71,1.40) | 1–5 | 29 (14.8) | 326 (15.7) | 0.98 (0.64,1.50) | 0.91 (0.58,1.42) | |
| 5–16 | 29 (14.8) | 294 (14.2) | 1.09 (0.71,1.67) | 0.99 (0.62,1.58) | ||||||
| > 16 | 31 (15.8) | 271 (13.1) | 1.26 (0.83,1.92) | 1.15 (0.71,1.86) | ||||||
| DVT | ||||||||||
| No | 771 (78.7) | 1101 (85.6) | Reference | Reference | 0 | 185 (47.0) | 1101 (58.8) | Reference | Reference | 0.000 |
| Yes | 209 (21.3) | 185 (14.4) | 1.61 (1.30,2.01) | 1.36 (1.05,1.75) | 1–5 | 51 (12.9) | 304 (16.2) | 1.00 (0.71,1.40) | 0.90 (0.63,1.29) | |
| 5–16 | 72 (18.3) | 251 (13.4) | 1.71 (1.26,2.32) | 1.51 (1.08,2.12) | ||||||
| > 16 | 86 (21.8) | 216 (11.5) | 2.37 (1.77,3.18) | 2.01 (1.43,2.84) | ||||||
| Other clot | ||||||||||
| No | 786 (80.2) | 1097 (85.3) | Reference | Reference | 0 | 189 (49.3) | 1097 (58.3) | Reference | Reference | 0.010 |
| Yes | 194 (19.8) | 189 (14.7) | 1.43 (1.15,1.79) | 1.11 (0.86,1.44) | 1–5 | 46 (12.0) | 309 (16.4) | 0.86 (0.61,1.22) | 0.75 (0.52,1.08) | |
| 5–16 | 68 (17.8) | 255 (13.5) | 1.55 (1.14,2.11) | 1.26 (0.89,1.77) | ||||||
| > 16 | 80 (20.9) | 222 (11.8) | 2.09 (1.55,2.82) | 1.56 (1.10,2.21) | ||||||
| Any clot | ||||||||||
| No | 673 (68.7) | 985 (76.6) | Reference | Reference | 0 | 301 (49.5) | 985 (59.4) | Reference | Reference | 0.000 |
| Yes | 307 (31.3) | 301 (23.4) | 1.49 (1.24,1.80) | 1.26 (1.02,1.57) | 1–5 | 80 (13.2) | 275 (16.6) | 0.95 (0.72,1.26) | 0.86 (0.64,1.16) | |
| 5–16 | 107 (17.6) | 216 (13.0) | 1.62 (1.24,2.11) | 1.44 (1.07,1.92) | ||||||
| > 16 | 120 (19.7) | 182 (11.0) | 2.16 (1.66,2.81) | 1.82 (1.34,2.47) | ||||||
Multivariable analysis was adjusted for age, race, education, poverty level, year of cancer diagnosis, region, tumor grade, estrogen receptor status, Charlson comorbidity, treatment of anthracycline, taxane, vinorelbine and blood transfusion.
P_trend is testing for upward or downward trend in the odds ratios as the total doses of ESAs increases
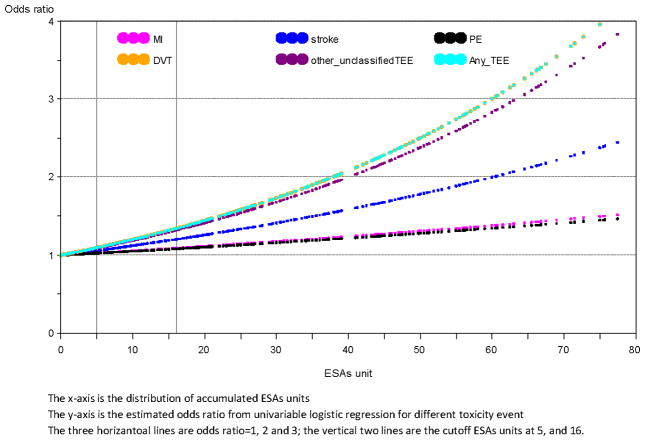
In order to explore whether a dose-effect was associated with the development of TEEs, among patients that received ESAS we categorized the number of doses as follows: >0–5, ≥5–16 and ≥16. When such categories were entered in the multivariable model, we observed an association between higher number of doses and an increased risk of stroke (p for trend= 0.014), DVT (p for trend <0.0001), other/unclassified TEE (p=0.010) and any clot (p for trend <0.0001). In Figure-2 we show the plot of the estimated OD to develop a TEEs according to different ESAS doses.
Figure 2.
Plot of estimated odds ratios for developing thromboembolic events according to different ESAs dose distribution.
DISCUSSION
Our study shows that, in patients older than 66 years old with metastatic breast cancer receiving chemotherapy, the use of ESAs increases the risk of TEEs, specifically DVTs and any clot. The magnitude of the increase was notable. Patients treated with ESAs had an absolute increase in the rates of clots of almost 10% compared to patients not treated with ESAs. A dose-dependent effect for stroke, DVT, other/unspecified TEE and any clot was seen, such that the risk was increased for patients who received more than 5 doses of ESAs, and the risk was even higher in those receiving more than 16 doses, there was no apparent increase in risk for patients who only received 1–4 doses.
Our results are consistent with previously published data. In a recent meta-analysis that included 51 phase III clinical trials (n=8172)5, the risk of TEE (HR 1.57; 95%CI 1.31–1.8). This estimate is higher than what we report, but it is important to mention that in this meta-analysis, patients with different malignancies were included and also that treatment and target hemoglobin varied amongst trials, as also did the TEE definitions. Aapro et al20, performed a meta-analysis that included 12 trials and a total of 2297 patients. The analysis was stratified for a target hemoglobin of ≤11g/dL, corresponding to current European Organization for Research and Treatment of Cancer guidelines21. An increased frequency of TEEs was seen with epoetin-beta vs control (7 vs. 4%); however, TEEs-related mortality was similar in both groups20. In our analysis, the absolute increase in risk of TEEs was more pronounced, possibly because our population was older and our study cohort was not limited to patients on clinical trials, who presumably would be healthier.
Hershman et al22, very recently reported data on an observational study using the SEER-Medicare database. They included patients diagnosed with diffuse B-cell lymphoma, colon, breast and lung cancer in different stages, receiving chemotherapy. They observed that the use of ESAs was associated with more recent diagnosis, younger age, comorbidities, female sex and metastatic or recurrent cancer. From the 14024 patients with breast cancer, 27.4% received ESAS and 12% had an episode of thrombosis. In the analysis of the complete cohort they observed higher rates of thrombosis in the patients that received ESAS (HR: 1.93; 95%CI 1.79–2.07)22. The risk estimates that we found are in same direction but of less magnitude; maybe associated with our more homogeneous patient population.
We did not observe an increased risk of MI in patients receiving ESAS. This association has not been consistently reported in cancer patients; however, it has been reported in the renal literature23,24. In ours study, the absolute number of patients with a stroke was higher among patients receiving ESAs. In the multivariable model there was a non significant trend for increase risk of stroke in patients treated with ESAS and we observed a significant trend in the number of doses and the risk of developing stroke. This observation has not been reported in the oncological literature, however higher rates of fatal and non-fatal stroke have been seen in diabetic patients with ESRD receiving darbepoietin (HR 1.92; 95%CI 1.38–2.68)25. In approximately 30% of patients with ESRD treated with ESAS hypertension develops or worsens23, and this phenomenon has been linked to a higher risk of vascular events23,26. Further studies are warranted to define the cardiovascular and cerebrovascular events associated with ESAS use in cancer patients.
It has been suggested that hemoglobin level may relate to the risk of TEEs, with greater risk associated with higher hemoglobin levels27. Some of the trials where an excess of TEEs was reported, targeted hemoglobin levels higher than those recommended by current ESAs labeling, or enrolled patients who were not anemic at baseline. In the BEST study2, patients with metastatic breast cancer treated with chemotherapy, were randomized to receive erythropoietin or not. Similar rates of TEE were seen between groups (16% vs. 14%). The trial was stopped because of increased mortality in the group treated with erythropoietin; a subsequent chart review revealed an increase number of TEE-associated deaths in those who received ESAs (14 vs. 4). Non anemic patients were targeted to hemoglobin levels of 12–14g/dL, the target was maintained for 59% of patient-weeks in the treatment arm, compared with 45% of patient-weeks in the placebo group (p<0.001)2. In a similar group of patients, the BRAVE trial8 evaluated whether erythropoietin use improved survival in patients with metastatic breast cancer. A total of 463 patients treated with anthracyline- and/or taxanes based chemotherapy were randomized to receive 30,000 units of erythropoietin beta or control once weekly for 24 weeks. After 18 months, no differences in overall survival (p=0.522) or progression-free survival (p=0.448) were seen; however patients that treated with ESAs experienced more TEEs than controls (13% vs. 6%; p=0.012), with no difference in the rate of serious TEEs (4% vs 3%)8. In a different patient population, Henke et al28, in an attempt to sensitize head and neck tumors to radiation therapy, randomized patients to receive erythropoietin or placebo targeting a hemoglobin level of 12–14 g/dL in women and 13–15 g/dL in men. Vascular events defined as hypertension, hemorrhage, DVT and PE were seen in 11% of patients in the treatment arm and in only 5% of the patients randomized to placebo. The number of patients who died from cardiac disorders was higher in the treatment arm (n=10 vs. 5). Unfortunately, in the SEER-Medicare dataset, no information is available on patient hemoglobin, so it was not possible to evaluate the impact of hemoglobin levels.
In our study we found that patients that received more than 5 doses of ESAs had a higher risk of developing a stroke, a DVT. Other/unspecified TEE and any clot. Contrary to our results, Rosenzweig et al10, found no relationship with the number of doses or the mean number of weeks a patient received erythropoietin. This trial was stopped early because 28.5% of the patients in the treatment arm developed a TEE, compared with no event at all in the control arm.
Recently, Khorana et al29, identified an association between red blood cell transfusions and an increased risk of TEEs. In a large retrospective cohort study in patients with cancer, they observed that transfusions increased the risk of venous thromboembolism (OR 1.53; 95%CI 1.46–1.61). In order to avoid that our risk estimates were confounded by this association, we included transfusion in our regression model.
To the best of our knowledge, this is the largest study including exclusively patients with metastatic breast cancer receiving chemotherapy, in which an assessment of the patterns of ESAs use and the relationship between ESAs and different TEEs is made. One of the strengths of this study is that it involves a large, population-based cohort of older patients, therefore it includes patients that may be excluded from randomized clinical trials, possibly reflecting real clinical practice. It is important to mention that in our cohort of patients we observed a high incidence of TEEs. We believe that this study includes a high risk group, independently of whether patients received ESAs or not. Old age, comorbidities, and that all of the patients had metastatic disease and were receiving chemotherapy likely contributed to the high incidence of TEEs observed. Additionally, we defined our outcomes by the presence of at least one diagnosis code for that specific TEE, which could have resulted in an over diagnosis of the outcomes. However, if misclassification occurred, it would have been non-differential between our study groups, thus, not likely to cause any change in our risk estimates.
A limitation of our study is that the SEER-Medicare data does not allow for assessment of the extent of the disease, the severity of outcomes, or for an analysis that takes into account the patient’s hemoglobin level, which as previously mentioned, has been related with adverse outcomes. It is possible that factors such as a large volume of disease or genetic predisposition may impact TEE incidence, but we were not able to adjust for such factors. One will expect however, that these potential confounders were present in similar proportion in patients that received and did not receive ESAs. It is possible that patients receiving ESAs were not captured in Medicare claims; however, because of the expense of the medication and the exclusion of patients with secondary insurance, we believe is unlikely this occurred in a significant number of patients. A minor limitation of our study is that our cohort exclusively includes patients aged 66 and older, the results, thus, may not be applicable to a population of younger, and in general, healthier patients. Additional studies are needed to prospectively confirm these findings and assess the complications and toxicities associated with the use of ESAs in younger women with metastatic breast cancer. It is important to mention that observational studies cannot establish causality. There is data from randomized trials proving the association between the use of ESAs and TEEs, however our study cannot rule out that the reason for ESAs administration also placed patients at higher risk of developing a TEE.
In summary, we have shown that in this cohort of patients, the use of ESAs increases the risk of certain TEEs. Our data shows that among patients treated with chemotherapy and ESAs for metastatic breast cancer, TEEs are a common event. Therefore, ESAs should be used for the minimum necessary time in order to reduce the risk of complications.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding Source: This work was supported by NIH K07-CA109064 (Giordano).
Footnotes
Prior Presentations: Presented at the 2009 Annual Meeting of the American Society of Clinical Oncology. (May 29th- June 2nd, Orlando, Fl)
Disclaimers: The authors have no potential conflicts of interest.
References
- 1.Steinbrook R. Erythropoietin, the FDA, and oncology. N Engl J Med. 2007;356:2448–51. doi: 10.1056/NEJMp078100. [DOI] [PubMed] [Google Scholar]
- 2.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–72. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 3.Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94:1211–20. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 4.Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N Engl J Med. 2007;356:2445–8. doi: 10.1056/NEJMp078101. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–24. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 6.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–42. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 7.Seddighzadeh A, Shetty R, Goldhaber SZ. Venous thromboembolism in patients with active cancer. Thromb Haemost. 2007;98:656–61. [PubMed] [Google Scholar]
- 8.Aapro M, Leonard RC, Barnadas A, et al. Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol. 2008;26:592–8. doi: 10.1200/JCO.2007.11.5378. [DOI] [PubMed] [Google Scholar]
- 9.Pirker R, Ramlau RA, Schuette W, et al. Safety and efficacy of darbepoetin alpha in previously untreated extensive-stage small-cell lung cancer treated with platinum plus etoposide. J Clin Oncol. 2008;26:2342–9. doi: 10.1200/JCO.2007.15.0748. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig MQ, Bender CM, Lucke JP, et al. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J Pain Symptom Manage. 2004;27:185–90. doi: 10.1016/j.jpainsymman.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26:1040–50. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 12.NCCN Clinical Practice Guidelines in Oncology. Cancer-and Chemotherapy-Induced Anemia., V.3.2009
- 13.Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26:132–49. doi: 10.1200/JCO.2007.14.3396. [DOI] [PubMed] [Google Scholar]
- 14.Hellman S. Stopping metastases at their source. N Engl J Med. 1997;337:996–7. doi: 10.1056/NEJM199710023371408. [DOI] [PubMed] [Google Scholar]
- 15.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–6. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–90. [DOI] [PubMed] [Google Scholar]
- 20.Aapro M, Scherhag A, Burger HU. Effect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patients. Br J Cancer. 2008;99:14–22. doi: 10.1038/sj.bjc.6604408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43:258–70. doi: 10.1016/j.ejca.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Hershman D, Buono D, Malin J, et al. Patterns of use and risks associated with Erythopoiesis-Stimulating agents among Medicare patients with cancer. J Natl Cancer Inst. 2009;101:101–9. doi: 10.1093/jnci/djp387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maschio G. Erythropoietin and systemic hypertension. Nephrol Dial Transplant. 1995;10 (Suppl 2):74–9. doi: 10.1093/ndt/10.supp2.74. [DOI] [PubMed] [Google Scholar]
- 24.Smith KJ, Bleyer AJ, Little WC, et al. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538–48. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer MA, Burdmann EA, Chen CY, et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 26.Roger SD, Fluck RJ, McMahon AC, et al. Recombinant erythropoietin increases blood pressure in experimental hypertension and uraemia without change in vascular cytosolic calcium. Nephron. 1996;73:212–8. doi: 10.1159/000189043. [DOI] [PubMed] [Google Scholar]
- 27.Dicato M. Venous thromboembolic events and erythropoiesis-stimulating agents: an update. Oncologist. 2008;13 (Suppl 3):11–5. doi: 10.1634/theoncologist.13-S3-11. [DOI] [PubMed] [Google Scholar]
- 28.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–60. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 29.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]