SUMMARY
Background
Acute lymphoblastic leukemia (ALL) is curable in over 80% of children and adolescents with high-risk features. However, current therapies are associated with symptomatic osteonecrosis that disproportionately affects adolescents, often requires surgery, and is one of the most common causes of short- and long-term morbidity. A strategy is needed to lessen this risk.
Methods
CCG-1961, a multi-cohort randomized cooperative group trial, evaluated components of therapeutic intensification in 2056 eligible, newly diagnosed high-risk patients (white blood cell count ≥50×109/L and/or age ≥10 years). To address osteonecrosis, a novel alternate-week dexamethasone schedule (10 mg/m2/day on days 0-6 and 14-20) was compared to standard continuous dexamethasone (10 mg/m2/day on days 0-20) in randomized regimens with either double or single delayed intensification phases, respectively. Randomization was done based on a randomization schedule generated using permuted blocks within strata. Patients were prospectively monitored clinically for osteonecrosis, with confirmatory imaging of suspected sites. Primary analyses were performed on an intent-to-treat basis and focused on the estimation and comparison of cumulative incidence rates of osteonecrosis both overall and in patient subgroups (age, gender, marrow early response status); final results are herein reported. This study is registered with ClinicalTrials.gov, number NCT00002812.
Findings
Symptomatic osteonecrosis was diagnosed in 143 patients at 377 confirmed skeletal sites, resulting in 139 surgeries. The overall cumulative incidence of osteonecrosis was 7·7% (N=2056) at 5 years, correlating with age at ALL diagnosis (1-9 years 1·0% (N=769), 10-15 years 9·9% (N=1025), ≥16 years 20·0% (N=262), p<0·0001) and gender (≥10 years, female 15·7% (N=525) versus male 9·3% (N=762), p=0·0010). For patients ≥10 years old with a rapid response to induction therapy, the use of alternate-week dexamethasone during delayed intensification phases significantly reduced osteonecrosis incidence compared with continuous dexamethasone (8·7±2·1% (N=420) versus 17·0±2·9% (N=403), p=0·0005), especially those ≥16 years (11·3±5·3% (N=84) versus 37·5±11·1% (N=79), p=0·0003; females 17·2±8·1% (N=32) versus 43·9±14·1% (N=23), p=0·050; males 7·7±5·9% (N=53) versus 34·6±11·6% (N=56), p=0·0014).
Interpretation
Alternate-week dexamethasone during delayed intensification phases effectively reduces osteonecrosis risk in children and adolescents receiving intensified therapy for high-risk ALL.
INTRODUCTION
With current therapy, more than 80% of children and adolescents with acute lymphoblastic leukemia (ALL) will be cured, but osteonecrosis (ON) has become a major cause of acute and long-term morbidity among these patients, particularly adolescents.1-6 The incidence of osteonecrosis among ALL patients ≥10 years old at diagnosis has risen from anecdotal to estimates ranging between 7·4-44·6%.1, 5, 7-11 Osteonecrosis affects weight-bearing joints in 95% of afflicted patients, with operative interventions for symptoms and impaired mobility in over 25%.10
This increasing incidence of osteonecrosis is often attributed to expanding use of dexamethasone in ALL treatment regimens, although increased systemic exposure to methotrexate and asparaginase may also contribute to the pathogenesis.12-14 Compared to prednisone, dexamethasone is 6·5-fold more potent in glucocorticoid effect and more readily penetrates the blood-brain barrier, advantageous factors in treating ALL but deleterious to bone.15, 16 Identifying ways to preserve therapeutic efficacy while limiting osteonecrosis risk is a major challenge in childhood ALL treatment.
The Children’s Oncology Group (COG) first identified a high risk of osteonecrosis in the CCG-1882 trial that tested augmented versus standard therapy for patients with a slow early response to initial therapy. Augmented therapy produced superior event-free and overall survival rates, but both regimens were associated with unacceptably high osteonecrosis rates in patients ≥10 years old at diagnosis and showed a trend toward a higher rate with augmented therapy (23·2±4·8 versus 16·4±4·3% at 3 years, p=0·27).10, 17 Since the augmented regimen included two, rather than one, delayed intensification and interim maintenance phases, it was hypothesized that the additional dexamethasone exposure and perhaps other components of therapy contributed to osteonecrosis risk.
Based on the findings of CCG-1882, the successor CCG-1961 trial was designed to evaluate as specific aims whether components of augmented therapy would benefit high-risk ALL patients with a rapid early response to therapy, and whether dexamethasone dose modification would lower osteonecrosis risk. The CCG-1961 trial employed a randomized 2×2 factorial design. This trial showed that intensified post-induction therapy was superior to standard intensity therapy, and one versus two interim maintenance and delayed intensification phases produced equivalent results.18 In an effort to decrease osteonecrosis risk, all patients enrolled in this trial who were randomized to regimens with two interim maintenance and delayed intensification phases received dexamethasone on an experimental alternate-week basis (days 0-6 and 14-20) during each delayed intensification phase, while patients randomized to regimens with single interim maintenance and delayed intensification phases received dexamethasone in the standard continuous fashion (days 0-20). We report the results of this prospective osteonecrosis risk comparison, representing the largest study to date and the first intervention identified to decrease its risk.
METHODS
Patients and Treatment Protocol
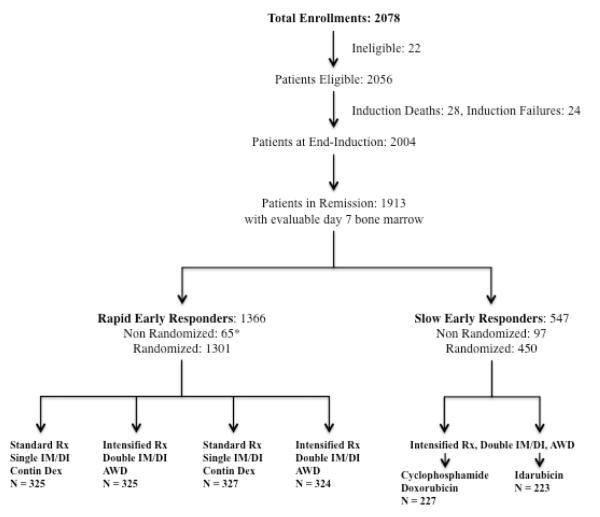
CCG-1961 enrolled children and adolescents 1-21 years of age with newly diagnosed high-risk ALL (initial white blood cell count ≥50×109/L and/or age ≥10 years) between 16 September 1996 and 1 May 2002. Eligibility criteria, baseline patient characteristics, and therapy details have been published (see also Figure 1, Table 1, Supplemental Tables 1 & 2).18 All patients received a common prednisone-based four-drug induction that included 28 days of prednisone 60 mg/m2/day without taper. Early response to therapy was determined by bone marrow aspiration on day 7 of induction therapy. Patients with a rapid early response had ≤25% blasts at days 7 and attained a complete remission (<5% blasts) at day 28. Following completion of induction, these patients were randomized in a 2×2 factorial design that resulted in assignment to one of four post-induction regimens (Table 2, Supplemental Table 3). Patients with a slow early response had >25% blasts at day 7 and were randomized separately to one of two post-induction regimens consisting of the same intensified therapy backbone with two interim maintenance and delayed intensification phases, and during each delayed intensification phase either doxorubicin 25 mg/m2 on days 0, 7, and 14 (standard) or idarubicin 10 mg/m2 on days 0 and 1 in combination with cyclophosphamide 1000 mg/m2 on day 2 (experimental). Baseline patient characteristics between the randomized rapid early reponse patients were similar (Table 1). 18 Duration of treatment was approximately 27 months for females and 39 months for males. Total corticosteroid therapy for each regimen is shown in Table 2.
Figure 1. Study Participant Recruitment, Cohort Distribution and Retention.
Rx = therapy, DI = delayed intensification, IM = interim maintenance
Contin Dex = continuous dexamethasone, AWD = alternate-week dexamethasone
* CNS disease, Ph+ status, patient refusal
Table 1.
Characteristics of the Patients at Diagnosis Rapid Early Response Regimens
| Characteristic* | Continuous Dexamethasone # (%), N = 652 |
Alternate-Week Dexamethasone # (%), N = 649 |
P, t |
|---|---|---|---|
|
| |||
| Age, y | 0·55 | ||
| 1 to 9 | 249 (38·2) | 229 (35·3) | — |
| 10 to 15 | 324 (49·7) | 336 (51·8) | — |
| 16+ | 79 (12·1) | 84 (12·9) | |
|
| |||
| White cells, × 109/L | 0·37 | ||
| Less than 50 | 324 (49·7) | 341 (52·5) | — |
| 50 to 199 | 254 (39·0) | 248 (38·2) | — |
| 200+ | 74 (11·3) | 60 (9·3) | |
|
| |||
| Sex | 0·58 | ||
| Male | 376 (57·7) | 384 (59·2) | — |
| Female | 276 (42·3) | 265 (40·8) | |
|
| |||
| Race | 0·32 | ||
| White | 454 (70·5) | 441 (69·2) | — |
| Black | 29 (4·5) | 41 (6·4) | — |
| Other | 161 (25·0) | 155 (24·4) | |
|
| |||
| Liver | 0·86 | ||
| Normal | 204 (46·4) | 207 (48·0) | — |
| Moderately enlarged | 204 (46·4) | 192 (44·5) | — |
| Markedly enlarged | 32 (7·2) | 32 (7·5) | |
|
| |||
| Spleen | 0·85 | ||
| Normal | 266 (41·1) | 271 (42·0) | — |
| Moderately enlarged | 311 (48·1) | 301 (46·6) | — |
| Markedly enlarged | 70 (10·8) | 74 (11·4) | |
|
| |||
| Lymph nodes | 0·16 | ||
| Normal | 310 (47·9) | 299 (46·3) | — |
| Moderately enlarged | 275 (42·4) | 300 (46·4) | — |
| Significantly enlarged | 63 (9·7) | 47 (7·3) | |
|
| |||
| Mediastinal mass | 0·74 | ||
| Absent | 547 (84·5) | 541 (83·9) | — |
| Present | 100 (15·5) | 104 (16·1) | |
|
| |||
| Hemoglobin, g/L | 0·72 | ||
| 10 to 79 | 286 (45·4) | 291 (46·6) | — |
| 80 to 110 | 198 (31·4) | 201 (32·1) | — |
| More than 110 | 146 (23·2) | 133 (21·3) | |
|
| |||
| Platelets, × 109/L | 0·03 | ||
| 1 to 49 | 325 (50·4) | 357 (55·4) | — |
| 50 to 149 | 212 (32·9) | 211 (32·8) | — |
| 150+ | 108 (16·7) | 76 (11·8) | |
|
| |||
| Immunophenotyping | 0·39 | ||
| B-cell lineage | 439 (77·8) | 442 (79·9) | — |
| T-cell lineage | 125 (22·2) | 111 (20·1) | |
|
| |||
| Karyotypic features† | |||
| No. | 0·06 | ||
| Diploid (46) | 111 (31·9) | 110 (32·2) | — |
| Pseudodiploid (46) | 112 (32·2) | 118 (34·5) | — |
| Hypodiploid (less than 46) | 25 (7·2) | 42 (12·3) | — |
| Hyperdiploid (47 to 50) | 48 (13·8) | 32 (9·4) | — |
| Hyperdiploid (more than 50) | 52 (14·9) | 40 (11·7) | |
|
| |||
| Translocations | 0·59 | ||
| t(4;11) present | 7 (2·0) | 9 (2·6) | — |
| t(4;11) absent | 341 (98·0) | 333 (97·4) | — |
| t(1;19) present | 14 (4·0) | 21 (6·1) | 0·21 |
| t(1;19) absent | 334 (96·0) | 321 (93·9) | — |
The global χ 2 test for homogeneity was used
— indicates not applicable
Because of rounding, not all percentages total 100; percentages were based on the number of patients for whom there were data on the various characteristics
The centrally reviewed and accepted cytogenetic data were available for a subgroup of patients
Table 2.
Total corticosteroid therapy (mg/m2)
| Rapid Response Regimens | Slow Response Regimensd | ||||
|---|---|---|---|---|---|
| Therapy Phase (Corticosteroid) | Aa | Bb | Cc | Dd | |
| Induction (prednisone) | 1815 | 1815 | 1815 | 1815 | 1815 |
| Delayed Intensification (dexamethasone) | 210 | 280 | 210 | 280 | 280 |
| Maintenance (prednisone) | |||||
| Males | 7050 | 6300 | 7000 | 6200 | 6200 |
| Females | 4450 | 3700 | 4400 | 3600 | 3600 |
|
| |||||
| Total Corticosteroid Exposure* | |||||
| Males | 10230 | 9935 | 10180 | 9835 | 9835 |
| Females | 7630 | 7335 | 7580 | 7235 | 7235 |
Expressed in prednisone equivalents (1 mg dexamethasone = 6·5 mg prednisone)16
Standard post-induction therapy; single interim maintenance and delayed intensification phases
Standard post-induction therapy; double interim maintenance and delayed intensification phases
Intensified post-induction therapy; single interim maintenance and delayed intensification phases
Intensified post-induction therapy; double interim maintenance and delayed intensification phases
Patients randomized to regimens A (standard) and C (intensified) post-induction therapy with single interim maintenance and delayed intensification phases received continuous dexamethasone during delayed intensification (10 mg/m2/day on days 0-20); those randomized to regimens B (standard) and D (intensified) post-induction therapy with two interim maintenance and delayed intensification phases received alternate-week dexamethasone during each delayed intensification phase (10 mg/m2/day on days 0-6 and 14-20). Maintenance therapy included prednisone pulses (40 mg/m2/day x5 every 4 weeks) with females receiving one year less of maintenance therapy than males. No modifications were made in steroid dose if osteonecrosis developed prior to maintenance. Steroids were held during maintenance until the pain resolved (off analgesics) and MRI returned to baseline or close to baseline. This protocol was approved by the National Cancer Institute and Institutional Review Boards of the participating institutions. Informed consent was obtained from the patients, their parents, or both.
Osteonecrosis Detection, Diagnosis, and Reporting
Patients were prospectively monitored for clinical signs and symptoms of osteonecrosis (e.g. pain, limited range of motion, joint collapse, arthritis). Institutions were required to report the presence or absence of such findings, in known and/or newly identified sites, at the end of each treatment phase, and annually after completion of protocol therapy. For patients with osteonecrosis, a detailed toxicity form was submitted upon initial symptom onset and serially thereafter. Required data for each affected joint included: estimated date of symptom onset, date of imaging diagnosis, imaging results, and surgical interventions. Reporting ended upon progressive disease, death, loss to follow up, or voluntary removal from study. All skeletal sites reported as “positive” for osteonecrosis were confirmed by diagnostic imaging; modalities utilized were according to local practice and interpreted by institutional radiologists. Radiographic staging of ON lesions was not reported.
Statistical Analysis
Osteonecrosis joint and surgical data are current through December 2010. As there have been no additional patients identified with ON since November 2005, statistical analyses were performed on the data set used for reporting the study’s primary results.18 Primary analyses presented are estimation and comparison of cumulative incidence rates of ON both overall and in various subgroups of patients (by age, gender, and early response status). ON rates are given for the slow early responders and rapid early responders both overall and by gender and age group; additional analyses by randomized regimens were restricted to rapid response patients who were randomized to continous versus alternate-week dexamethasone. All slow early responders were assigned to alternate-week dexamethasone in the same intensive therapy backbone with a separate randomized question as detailed above, and no difference in ON incidence was found between these regimens. The randomization procedure is described below. Cumulative incidence rates were estimated using the method of Gray.19 Event-free survival rates were estimated using the Kaplan-Meier method and standard errors of the estimate were obtained by the Peto method.20, 21 Event-free survival time was computed as time from enrollment on study to occurrence of first event (induction failure, induction death, relapse at any site, death in remission, or a second malignant neoplasm). Patients who had not had an event were censored at the time of last contact. The log rank test was used to compare survival curves between groups.22 The Chi-square test was used to compare proportions between groups.
A randomization schedule was used to assign the patients to the treatment regimens. The randomization sequence was generated using permuted blocks within strata defined at the time of randomization. Strata for this study consisted of Rapid Early Responders (RERs) (block size of 8) and Slow Early Responders (SERs) (block size of 4). The randomized regimens for the RERs (4 arms) were different from those for the SERs (2 arms). This sequence was generated prior to trial activation, based on an algorithm programmed by the Information Technology personnel in the Children’s Cancer Group (CCG) who were responsible for generating these for all randomized clinical trials coordinated by CCG. Access to the schedule was restricted to the CCG Registrar located in the central operations center for CCG and hence concealed from all others. Clinical research associates from the participating centers contacted the Registrar at the time of enrollment and randomization; eligibility criteria were confirmed by the Registrar and the patient assigned to the next random treatment assignment per the randomization schedule. There was no masking of treatment assignment; since the therapies on the different regimens were so different, it was impossible to conceal or mask the assignment from participants, treating physicians, those assessing outcomes, and those conducting the data analyses.
All data analyses were performed using the SAS System (SAS Institute Inc. 2007. SAS OnlineDoc® 9.2. Cary, NC: SAS Institute Inc.). This study is registered with ClinicalTrials.gov, number NCT00002812.
Role of the Funding Source
Funding for this study derived from grants awarded to the Children’s Cancer Group and the COG from the Cancer Treatment Evaluation Program (CTEP) of the National Cancer Institute. The CTEP approved the final protocol design, but played no role in these analyses.
RESULTS
Symptomatic osteonecrosis was diagnosed in 143 of 2056 enrolled, eligible patients who had a median follow up of 93·1 months. Symptoms developed during therapy in almost all affected patients. Onset was within one year of diagnosis in 59 (41%), during the second year in 67 (47%), during years three and four in 13 (9%) and four (3%) respectively, and none thereafter. Symptom onset occurred during pre-maintenance therapy in 31 patients (22%), during maintenance in 104 (73%), and following completion of therapy in eight (5%). Median age at osteonecrosis symptom onset was 14·6 years, occurring earlier in females versus males (14·1 versus 15·4 years) and in those receiving continuous versus alternate-week dexamethasone (14·0 versus 16·2 years). There was no difference between rapid and slow responders, and no correlation between osteonecrosis incidence and baseline patient, laboratory, or ALL characteristics (data not shown).
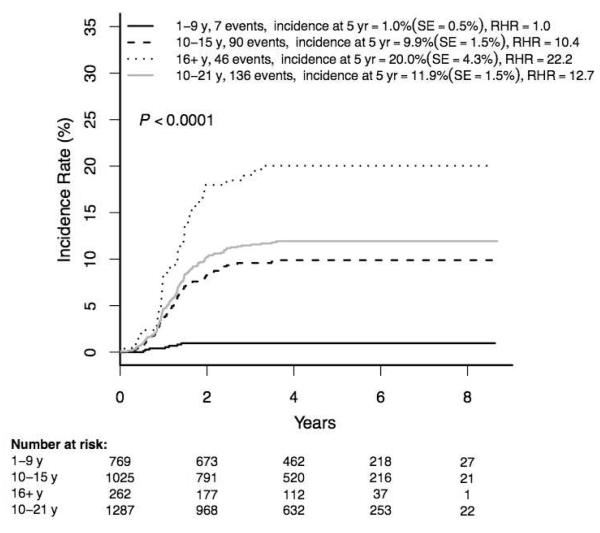
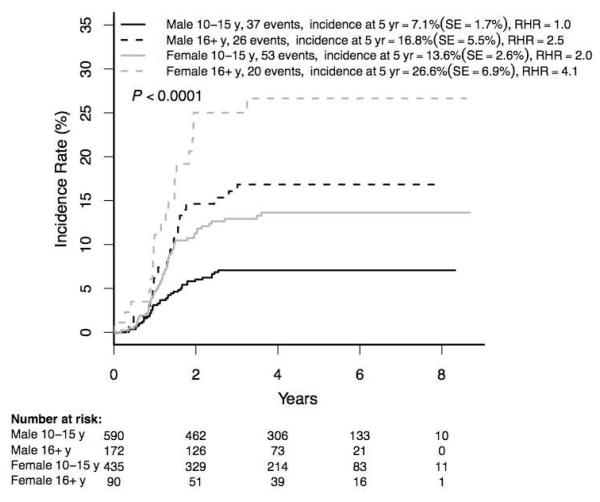
The 5-year overall cumulative incidence of osteonecrosis was 7·7±0·9% (143/2056). Incidence strongly correlated with age at ALL diagnosis (1·0±0·5% (7/769) in children 1-9 years versus 11·9±1·5% (136/1287) (relative hazard ratio (RHR) 12·7, p<0·0001) in the 10-21 year group (Figure 2)). Osteonecrosis rates were higher in those 16-21 versus 10-15 years (20·0±4·3% (46/262) versus 9·9±1·5% (90/1025); p<0·0001). Despite the fact that females received one year less of maintenance therapy with monthly 5-day prednisone pulses than males, the osteonecrosis incidence was higher in females than males overall (10·3±1·6 (77/828) versus 6·0±1·1% (66/1228), p=0·0006), among those 10-21 years (15·7±2·5 (73/525) versus 9·3±1·7% (63/762), p=0·0010), and in age subgroups 10-15 and 16-21 years (Figure 3). Given the very low incidence of osteonecrosis among patients <10 years old at initial ALL diagnosis, the remaining analyses were limited to patients ≥10 years old.
Figure 2. Cumulative osteonecrosis incidence by age at diagnosis of ALL (N=2056).
ALL = acute lymphoblastic leukemia
RHR = relative hazard ratio
Figure 3. Cumulative osteonecrosis incidence by age and gender (N=1287).
RHR = relative hazard ratio
F = female, M = male
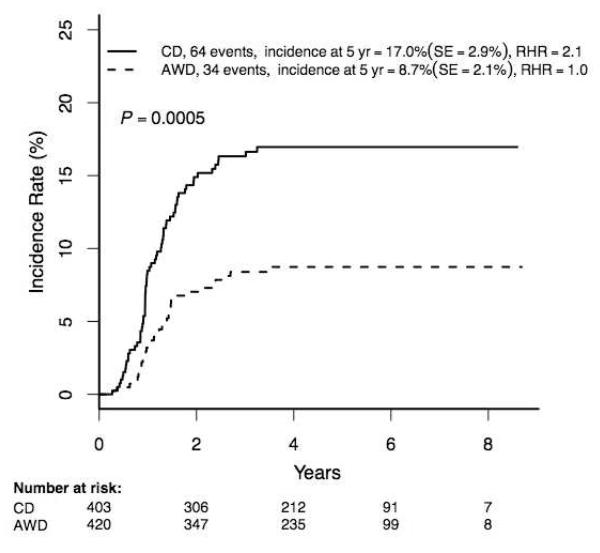
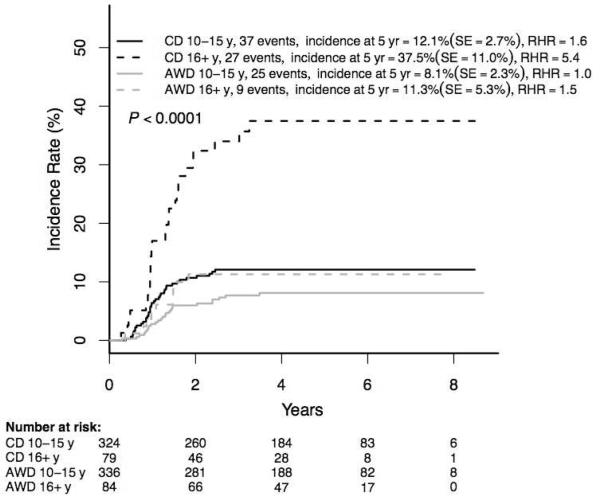
The incidence of osteonecrosis among rapid responder patients 10-21 years was 12·8±1·8% (98/823), with rates significantly higher in females and among those 16-21 versus 10-15 years (Supplemental Table 3). Among the randomized rapid responder patients ≥10 years, those randomized to regimens with single delayed intensification phases and continuous dose dexamethasone had significantly higher osteonecrosis rates than those randomized to receive two delayed intensification phases with alternate-week dexamethasone dosing (17·0±2·9 (64/403) versus 8·7±1·2% (34/420), p=0·0005) (Figure 4A), despite the fact that patients given alternate-week dosing received more dexamethasone overall (280 versus 210 mg/m2). The osteonecrosis incidence was higher among those receiving continuous dexamethasone in both 10-15 and 16+ year age groups (Figure 4B; p<0·0001) with a highly significant difference in the latter cohort (37·5±11·1% (27/79) versus 11·3±5·3 (9/84), p=0·0003). There was no difference in osteonecrosis incidence between patients randomized to standard (regimens A+B) or intensified (regimens C+D) post-induction therapy (p=0·13; data not shown).
Figure 4A. Cumulative osteonecrosis incidence by dexamethasone group in RER patients Age ≥10 years (N=823).
RER = rapid early response
CD = continuous dexamethasone
AWD = alternate-week dexamethasone
RHR = relative hazard ratio
Cumulative osteonecrosis incidence by dexamethasone group in RER patients age ≥10 years, gender subgroups:
Females CD: 20·3±4·6%, AWD: 11·6±3·6% (RHR 1·9, p=0·022)
Males CD: 14·6±3·6%, AWD: 6·6±2·5% (RHR 2·3, p=0·0064)
Figure 4B. Cumulative osteonecrosis incidence by dexamethasone group in RER patients Age subsets 10-15 years, 16-21 years (N=823).
RER = rapid early response
CD = continuous dexamethasone
AWD = alternate-week dexamethasone
RHR = relative hazard ratio
Cumulative osteonecrosis incidence by dexamethasone group in RER patients age ≥16 years, gender subgroups:
Females CD: 43·9±14·1%, AWD: 17·2±8·1% (RHR 2·9, p=0·050)
Males CD: 34·6±11·6%, AWD: 7·7±5·9% (RHR 4·9, p=0·0014)
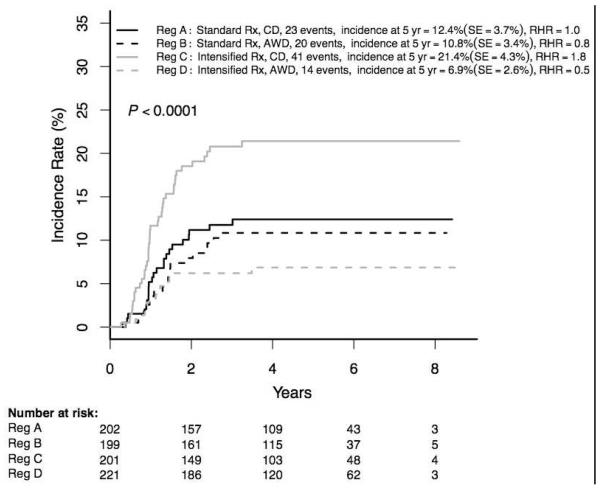
The highest incidence of osteonecrosis among patients ≥10 years occurred in those receiving intensified therapy with single interim maintenance and delayed intensification phases (regimen C: 21·4±4·3% (41/201); Figure 4C). Despite small numbers in the 16-21 year cohorts, differences in incidences between the four regimens were significant overall, and within the female and male subsets.
Figure 4C. Cumulative osteonecrosis incidence by RER regimen Age ≥10 years (N=823).
RER = rapid early response
CD = continuous dexamethasone
AWD = alternate-week dexamethasone
Rx = therapy
IM = interim maintenance
DI = delayed intensification
RHR = relative hazard ratio
Cumulative osteonecrosis incidence by RER regimen in patients age ≥16 years:
All A: 38·1±14·4%, B: 16·5±9·1%, C: 37·3±15·6%, D: 6·8±5·7% (p=0·0028)
Females A: 57·6±18·6%, B: 33·9±17·2%, C: 31·8±19·2%, D: 5·6±7·0% (p=0·035)
Males A: 28·3±19·1%, B: 8·2±8·8%, C: 38·7±22·0%, D: 7·7±9·1%, 2/27 (p=0·011)
Slow responder patients all received intensified post-induction therapy and alternate-week dexamethasone during each of two delayed intensification phases. Among all slow responder patients ≥10 years the osteonecrosis incidence was 11·8±3·3% (30/271), and was significantly higher in females than males (17·2±5·9% (18/111) versus 7·9±3·6% (12/160); RHR 2·2, p=0·026). Incidences appeared similar for patients in age subgroups 10-15 and 16-21 years.
Among all patients ≥10 years, those who developed osteonecrosis had a better 5-year event-free survival rate than those who did not (86·3% versus 68·8%; p<0·0001, HR 0·32). These differences were present among both females (90·3% versus 69·9%; p=0·0001, HR 0·23) and males (81·2 versus 68·0%; p=0·0086, HR 0·43).
Overall, 441 osteonecrosis sites were reported among the 143 patients (Table 3), with 377 sites confirmed by radiographic imaging, primarily MRI. An additional 64 sites were diagnosed by clinical signs and symptoms, all in patients who had at least one other site confirmed by imaging. Multiple sites were involved in 80% of affected patients, and 6/28 patients with only a single site confirmed by imaging had a total of 13 additional sites diagnosed clinically. Imaging-documented osteonecrosis of hip(s) and/or knee(s) comprised 74% of joints and were present in 90% of patients; 5% of patients had ankle(s) and/or heel(s) involvement without hip/knee osteonecrosis. The average number of confirmed osteonecrosis joints was 2·6/patient (median 2, maximum 12). Two, three, or four confirmed joints were identified in 63, 13, and 28 patients, respectively; 11 patients had five or more affected joints. There was no significant difference by treatment regimen or manner of dexamethasone dosing.
Table 3.
Joints affected by osteonecrosis in 143 patients*
| Radiologically Diagnosed | Clinically Diagnosed | Total | |
|---|---|---|---|
| Joint | # Joints (%) | # Joints (%) | # Joints (%) |
| Hip | 132 (35·0) | 14 (21·9) | 146 (33·1) |
| Knee | 147 (39·0) | 19 (29·7) | 166 (37·6) |
| Ankle | 32 (8·5) | 12 (18·8) | 44 (10·0) |
| Shoulder | 38 (10·1) | 9 (14·1) | 47 (10·7) |
| Elbow | 9 (2·4) | 5 (7·8) | 14 (3·2) |
| Wrist | 8 (2·1) | 5 (7·8) | 13 (2·9) |
| Other† | 11 (2·9) | 0 (0) | 11 (2·5) |
| Total | 377 (100) | 64 (100) | 441 (100) |
Includes all data received by Children’s Oncology Group through December 2010
Heel (5), pelvis (1), femoral diaphysis (4), rib (1)
Surgical procedures (total 139) reported in 62 patients included: 55 total joint replacements (hip 49, knee 6), 23 arthroscopic repairs (hip 2, knee 16, shoulder 5), 43 core decompressions ± bone grafting (hip 22, knee 17, shoulder 4), 1 joint pinning (knee), 2 arthrotomies (1 hip, 1 elbow), and 15 unspecified (hip 4, knee 7, ankle 2, shoulder 1, elbow 1). In addition, 5 joints were treated with steroid injections.
As of December 2010, 139 surgical procedures were reported for 62 patients; most were performed after completion of ALL therapy (Table 3). Surgical procedures were performed more frequently in those ≥16 years at ALL diagnosis (28/46, 61% of patients) compared with those ≤15 years (34/97, 35% of patients). There was no correlation between surgery and either treatment regimen or manner of dexamethasone dosing.
DISCUSSION
An unanticipated consequence of the successful treatment of childhood ALL has been the emergence of osteonecrosis as a significant toxicity with unacceptable morbidity.4, 6, 9, 10 Our prospective findings in this trial demonstrate that the use of alternate-week rather than continuous dexamethasone during delayed intensification results in a two-fold reduction in the relative risk of symptomatic ON among rapid responder patients ≥10 years old, particularly those 16+ years old, and a four-fold reduction among those randomized to intensified therapy, even though those treated with alternate week dexamethasone received a higher total dexamethasone exposure (Figures 4A-4C). Additionally, osteonecrosis incidence was lower among slow responder patients ≥10 years old assigned to double delayed intensification with alternate-week dexamethasone on this study, as compared to a similar historical cohort treated on the prior CCG-1882 trial who received two delayed intensification phases with continuous dose dexamethasone (11·8% versus 23·2%).10 These results establish that dexamethasone dosing manner supersedes cumulative exposure as a key factor in the development of treatment-related osteonecrosis and can be modified to limit osteonecrosis risk while achieving superior ALL outcomes.18 The alternate-week dexamethasone schedule was devised based on the pathophysiology of glucocorticoid-induced osteonecrosis, which is associated with marrow lipid infiltration and osteocyte lipid hypertrophy. Such changes are dramatic and occur early during glucocorticoid exposure, causing increased intramedullary pressure and consequent blood flow stasis, which is exacerbated by pubertal bone growth and epiphyseal closure.4, 23, 24 Liver-to-marrow lipid emboli contribute to the development of thrombotic ischemia.25 Glucocorticoids are also directly toxic to osteocytes, inducing apoptosis and exacerbating bone necrosis.26 Alternate-week dosing may moderate these effects, allow for dissipation of intramedullary pressure, and avert osteocyte death. The positive effect of a “steroid holiday” was demonstrated in a mouse model.13 Bone toxicity may also occur with methotrexate and asparaginase due to hypercoagulability, vascular endothelial damage, and perturbations of bone formation, possibly influenced by host genetic polymorphisms.5, 12, 14, 27-29
While the pathogenesis of treatment-related osteonecrosis is presumed multifactorial, it is significant that dexamethasone dose modification was sufficient to reduce osteonecrosis incidence despite exposure to intensified therapy and two interim maintenance and delayed intensification phases (Figure 4C). The magnitude of osteonecrosis risk reduction associated with alternate-week dexamethasone was greater among patients receiving intensified therapy, which included additional vincristine, pegaspargase, and escalating-dose methotrexate, than among those receiving standard therapy; this benefit was especially notable in both groups for patients ≥16 years. Although patients assigned to continuous dexamethasone during single delayed intensification entered maintenance somewhat earlier than those assigned to alternate-week dexamethasone during double delayed intensification, we believe that an uninterupted three-week dexamethasone exposure constitutes a greater ON risk than four additional monthly prednisone pulses. In addition to the physiologic, pharmacologic, and pharmacogenomic factors discussed earlier, it is notable that males had significantly less ON than females despite receiving an additional year of maintenance, including approximately 13 prednisone pulses. There are many potential reasons for the difference in ON rates between the genders, but our findings do not provide compelling evidence that the prednisone pulses are a major factor.
Interestingly, among patients ≥10 years given continuous dexamethasone the osteonecrosis incidence was significantly higher with intensified versus standard therapy (21·4 versus 12·4%, p=0·018). In contrast, for patients given alternate-week dexamethasone the osteonecrosis incidence trended lower on the intensified than the standard regimen (6·9 versus 10·8%, p=0·18). Bone toxicity is likely initiated during induction by prednisone and exacerbated during delayed intensification by dexamethasone. It is plausible that the longer scheduled glucocorticoid-free interval between induction and delayed intensification on the intensified regimens (17 versus 13 weeks) was able to provide partial protection against the degree of additional toxicity associated with alternate-week but not that of continuous dexamethasone. These and other potential interactions between glucocorticoid schedule and components of intensified therapy are being further evaluated in the COG successor trial AALL0232 (http://clinicaltrials.gov/ct/show/NCT00075725).
Dexamethasone exposure is influenced by host drug clearance, and poor clearance is strongly associated with ON risk.5 Reduced clearance is also associated with older age and concurrent asparaginase treatment, which would predict increased dexamethasone levels and toxicity among older patients and among those receiving intensive asparaginase therapy. Our findings substantiate this prediction, and suggest that alternate-week dexamethasone may ameliorate these effects. In addition, because intensified therapy includes more potentially hepatotoxic agents during interim maintenance, it is possible that residual hepatotoxicity leading to altered dexamethasone metabolism and increased host exposure during delayed intensification contributed to the observed differences in osteonecrosis between regimens, particularly with continous dexamethasone dosing.
Osteonecrosis incidence strongly correlated with age at ALL diagnosis and female gender, despite females receiving one year less of maintenace therapy than males. The median age of symptom onset was younger in females than males and among those receiving continuous dexamethasone, suggesting a contribution of growth and hormonal factors in the development of osteonecrosis. Pubertal status was not assessed in this trial.
The burden of osteonecrosis was substantial. While only seven patients younger than ten years developed osteonecrosis, all but one had multiple joints diagnosed; 15 of the 18 joints were weight bearing, with three total hip arthroplasties having been performed in two patients. Of note, four of seven received alternate-week dexamethasone, and all became symptomatic relatively early, either during delayed intensification (two) or within the first six months of maintenance therapy (five). This warrants further study since young children who develop osteonecrosis during ALL therapy may possess unique pharmacogenetic or other predisposing factors. Almost all (95%) of the 136 patients ≥10 years with osteonecrosis had symptomatic involvement of weight-bearing joints. While there were no clinical or therapy-related features predictive of surgical need, more patients ≥16 years at ALL diagnosis underwent surgical procedures than those younger (61 versus 35%). However, this may reflect physician preference to delay surgery pending skeletal maturity and therapy completion rather than osteonecrosis severity. While the protocol provided corticosteroid dose modification guidelines for patients who developed osteonecrosis, surgical and other management approaches were at institutional discretion. Although initial osteonecrosis symptom onset was within two years of ALL diagnosis in 88% of patients, in many cases additional symptomatic and asymptomatic sites were identified over time; in fact, across the trial, 10% of joints were diagnosed after the last patient had completed protocol therapy. The trial was not designed to assess long-term functional outcome, nor was presymptomatic MRI screening performed. As observed by others, the ultimate outcome of asymptomatic osteonecrosis is variable.1, 4
An intriguing finding was the apparent event-free survival advantage among older patients of both genders diagnosed with osteonecrosis compared to those without osteonecrosis. This may be attributable to the superior outcomes achieved with intensified therapy and/or the fact that all slow responder patients, who had an inferior event-free survival compared to rapid responder patients, were non-randomly assigned to receive double interim maintenance and delayed intensification phases including alternate-week dexamethasone. However, we believe that these explanations are unlikely to account fully for this difference given the similar osteonecrosis incidence rates observed between standard and intensified therapy cohorts, and the absence of therapeutic advantage for double vs single delayed intensification as previously reported.18 Further subset comparisons are limited by patient numbers. Instead, this finding may reflect inherent differences of host glucocorticoid metabolism and/or response that might impact both anti-leukemia efficacy and toxicity.10, 30 These observations are noteworthy given that maintenance corticosteroid discontinuation or modification was allowed for patients diagnosed with osteonecrosis, and suggest that this practice does not compromise ALL outcome.
One limitation of our study was to allow institutional choice of imaging modality used to confirm each osteonecrosis site. While this was a prudent approach from the perspective of patient management and health economics, it is acknowledged that the true osteonecrosis incidence may have been underestimated due to the variability in diagnostic imaging sensitivity. A second limitation was that data were not captured on acquired or underlying clotting abnormalities that may have contributed to osteonecrosis risk, the potential significance of which was yet to be elucidated when the study was designed in the mid-1990s. Finally, the study was neither designed nor did it have adequate power to directly compare osteonecrosis incidences between individual rapid response regimens within age and gender subsets; statistical analyses are thus limited to overall comparisons as reported (Figure 4C).
In conclusion, this study provides new insight into patient- and treatment-related risk factors for osteonecrosis, highlights the burden of this toxicity among affected patients, and identifies simple dose modifications of dexamethasone administration that can significantly decrease the incidence of osteonecrosis in the context of highly effective chemotherapy.18 Alternate-week dexamethasone dosing during delayed intensification has been incorporated into successor COG ALL clinical trials with prospective toxicity monitoring. Further studies to better characterize the natural history and predisposing factors for osteonecrosis in ALL are ongoing by the COG.
Supplementary Material
PANEL.
Research in Context
Systematic Review
We searched Medline for full papers related to “osteonecrosis,” “avascular necrosis,” “bone changes and leukemia,” and the terms “chemotherapy,” “asparaginase,” “methotrexate,” “corticosteroids” (including prednisone and dexamethasone) individually with “osteonecrosis” in humans and animals. We did not limit our search by date and have searched at multiple timepoints including during the development of the trial in 1995, and following completion of the trial in 2003, 2009, and 2011. Additionally, on January 25-26, 2010, we held a workshop* co-sponsored by the Office of Rare Diseases, National Cancer Institute and the Children’s Oncology Group entitled, “Osteonecrosis in Pediatric and Adolescent Acute Lymphoblastic Leukemia (ALL): Insights into the Etiology of this Emerging Toxicity,” to further investigate the incidence, etiology and treatment of this entity. We did not identify any previously reported randomized prospective studies utilizing an intervention to reduce the development of osteonecrosis in children or adolescents with ALL.
Interpretation
Before the use of post-induction intensification for treatment of acute lymphoblastic leukemia, osteonecrosis was infrequent. The improvement in outcome for children and adolescents 10 years of age and older has been accompanied by an increased incidence of osteonecrosis in patients. Previously, dexamethasone was thought to be the sole cause of osteonecrosis, however it is now recognized that numerous factors play a role. Our current results, built upon prior COG experience in sequential ALL clinical trials,10 establish that the dexamethasone dosing manner supersedes cumulative exposure as a key factor in the development of therapy-related osteonecrosis and identifies a simple dose modification of dexamethasone administration that can significantly decrease the incidence of osteonecrosis while achieving superior ALL outcomes. This is also the first study to suggest a superior event-free survival for patients who developed osteonecrosis as compared to those who did not. These findings have changed clinical practice in North America and the United Kingdom. In the current COG high-risk ALL study, AALL1131, we hope to gain further insight into the natural history of clinically silent ON by determining ON incidence and severity via MR imaging at defined timepoints, and to assess the role of drugs in addition to corticosteroids (i.e., asparaginase and methotrexate) in the risk for development of ON.
* http://rarediseases.info.nih.gov/ScientificConferences.aspx?PageID=5&ID=984
ACKNOWLEDGMENTS
In memory of James B Nachman (1948-2011), an exemplary healer, learner, teacher, humanitarian, and friend. We thank William L Carroll and Michael E Trigg for providing insightful, stalwart leadership in COG ALL research strategy. Research is supported by the Chair’s Grant CA98543, Statistics and Data Center Grant U10 CA98413, and Grant CA13539 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online (http://applications.childrensoncologygroup.org/admin/grantinfo.htm).
Funding Children’s Oncology Group Chair’s Grant CA98543-08 and Statistics and Data Center Grant CA98413-08; U.S. National Cancer Institute at the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS NLS and PGS were study chair and vice-chair, respectively. LAM, JBN, HNS, PGS, PSG, NLS designed the study. LAM, JBN, PGS, NLS monitored the study. LAM, MD, HNS, NLS collected the data. LAM, MD, JBN, HNS, SPH, NLS analyzed the data. LAM, MD, JBN, HNS, SPH, PGS, PSG, NLS interpreted the data. LAM and NLS conducted the literature search. LAM, MD, NLS prepared the figures and tables. LAM, MD, JBN, SPH, PSG, NLS wrote the report. All authors had full access to the final version of the report and agreed to the submission with the exception of JBN, who reviewed, contributed to, and approved a near-final draft prior to his untimely death.
CONFLICTS OF INTEREST LAM was employed by Pfizer 2009-2012 and owns Pfizer stock. PSG acts as a consultant to Hospira. All other authors report that they have no conflicts of interest.
Contributor Information
Leonard A Mattano, Jr, Michigan State University College of Human Medicine MSU Kalamazoo Center for Medical Studies.
Meenakshi Devidas, COG & Department of Biostatistics University of Florida College of Medicine, Public Health & Health Professions Address: 104 North Main Street, Suite 600, Gainesville FL 32601 USA mdevidas@cog.ufl.edu.
James B Nachman, Address: 5839 S. Maryland Avenue, MC 4060, Chicago IL 60637 USA.
Harland N Sather, Address: 440 E. Huntington Drive, 4th Floor, Arcadia, CA 91006 USA harland_sather@sbcglobal.net.
Stephen P Hunger, University of Colorado School of Medicine Ergen Family Chair in Pediatric Cancer Director, Center for Cancer and Blood Disorders Children’s Hospital Colorado Chief, Section of Pediatric Hematology/Oncology/Bone Marrow Transplantation Address: 13123 East 16th Street, B115, Aurora CO 80045 USA stephen.hunger@childrenscolorado.org.
Peter G. Steinherz, Weill Medical College of Cornell University Member & Attending Pediatrician Memorial Sloan-Kettering Cancer Center Address: 1275 York Avenue, Box 411, New York NY 10065 USA steinhep@mskcc.org.
Paul S Gaynon, University of Southern California Children’s Hospital Los Angeles Address: 4650 Sunset Boulevard, Los Angeles CA 90027 USA pgaynon@chla.usc.edu.
Nita L Seibel, Cancer Therapy Evaluation Program National Cancer Institute Address: 6130 Executive Boulevard, Room 7025, Bethesda MD 20852 USA seibelnl@mail.nih.gov.
REFERENCES
- 1.te Winkel ML, Pieters R, Hop WC, et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(31):4143–50. doi: 10.1200/JCO.2011.37.3217. Epub 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 2.Gaynon PS, Trigg ME, Heerema NA, et al. Children’s Cancer Group trials in childhood acute lymphoblastic leukemia: 1983-1995. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2000;14(12):2223–33. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 3.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24(2):265–84. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 4.Kaste SC, Karimova EJ, Neel MD. Osteonecrosis in children after therapy for malignancy. AJR American journal of roentgenology. 2011;196(5):1011–8. doi: 10.2214/AJR.10.6073. Epub 2011/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117(8):2340–7. doi: 10.1182/blood-2010-10-311969. quiz 556. Epub 2010/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchese VG, Connolly BH, Able C, et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys Ther. 2008;88(3):341–50. doi: 10.2522/ptj.20070108. [DOI] [PubMed] [Google Scholar]
- 7.Elmantaser M, Stewart G, Young D, Duncan R, Gibson B, Ahmed SF. Skeletal morbidity in children receiving chemotherapy for acute lymphoblastic leukaemia. Archives of disease in childhood. 2010;95(10):805–9. doi: 10.1136/adc.2009.172528. Epub 2010/06/26. [DOI] [PubMed] [Google Scholar]
- 8.Arico M, Boccalatte MF, Silvestri D, et al. Osteonecrosis: An emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica. 2003;88(7):747–53. [PubMed] [Google Scholar]
- 9.Burger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)--experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44(3):220–5. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 10.Mattano LA, Jr., Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(18):3262–72. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 11.Patel B, Richards SM, Rowe JM, Goldstone AH, Fielding AK. High incidence of avascular necrosis in adolescents with acute lymphoblastic leukaemia: a UKALL XII analysis. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22(2):308–12. doi: 10.1038/sj.leu.2405032. [DOI] [PubMed] [Google Scholar]
- 12.Fan C, Cool JC, Scherer MA, et al. Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone. 2009;44(1):61–70. doi: 10.1016/j.bone.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Boyd K, Kaste SC, Kamdem Kamdem L, Rahija RJ, Relling MV. A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday. J Orthop Res. 2009;27(2):169–75. doi: 10.1002/jor.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(12):1932–9. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 15.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101(10):3809–17. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 16.Ito C, Evans WE, McNinch L, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(8):2370–6. doi: 10.1200/JCO.1996.14.8.2370. [DOI] [PubMed] [Google Scholar]
- 17.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338(23):1663–71. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 18.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111(5):2548–55. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–54. [Google Scholar]
- 20.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 21.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. British journal of cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. Epub 1977/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breslow NE. Analysis of survival data under the proportional hazards model. Int Stat Rev. 1975;43:45–58. [Google Scholar]
- 23.Miyanishi K, Yamamoto T, Irisa T, et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30(1):185–90. doi: 10.1016/s8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 24.Motomura G, Yamamoto T, Miyanishi K, Yamashita A, Sueishi K, Iwamoto Y. Bone marrow fat-cell enlargement in early steroid-induced osteonecrosis--a histomorphometric study of autopsy cases. Pathol Res Pract. 2005;200(11-12):807–11. doi: 10.1016/j.prp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Laroche M. Intraosseous circulation from physiology to disease. Joint Bone Spine. 2002;69(3):262–9. doi: 10.1016/s1297-319x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85(8):2907–12. doi: 10.1210/jcem.85.8.6714. [DOI] [PubMed] [Google Scholar]
- 27.te Winkel ML, Appel IM, Pieters R, van den Heuvel-Eibrink MM. Impaired dexamethasone-related increase of anticoagulants is associated with the development of osteonecrosis in childhood acute lymphoblastic leukemia. Haematologica. 2008;93(10):1570–4. doi: 10.3324/haematol.12956. [DOI] [PubMed] [Google Scholar]
- 28.Spencer JD, Brookes M. Avascular necrosis and the blood supply of the femoral head. Clin Orthop Relat Res. 1988;(235):127–40. [PubMed] [Google Scholar]
- 29.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(19):3930–6. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 30.McNeer JL, Nachman JB. The optimal use of steroids in paediatric acute lymphoblastic leukaemia: no easy answers. Br J Haematol. 2010;149(5):638–52. doi: 10.1111/j.1365-2141.2010.08192.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.