Abstract
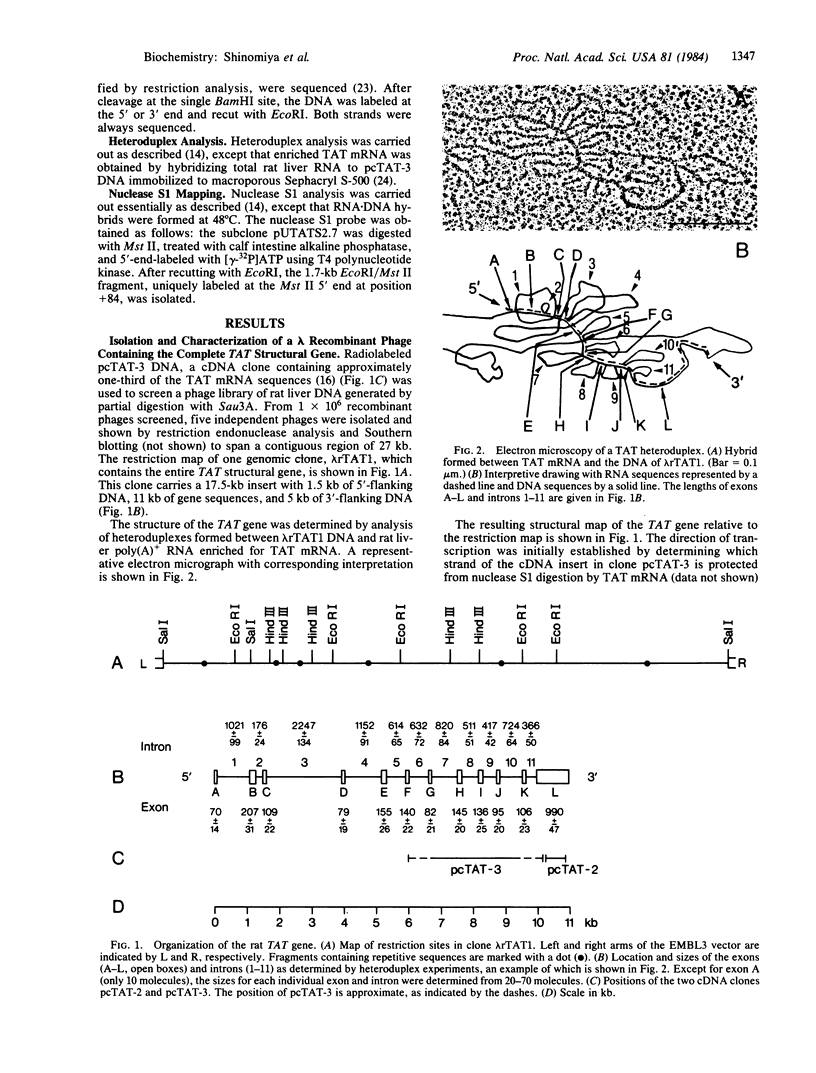
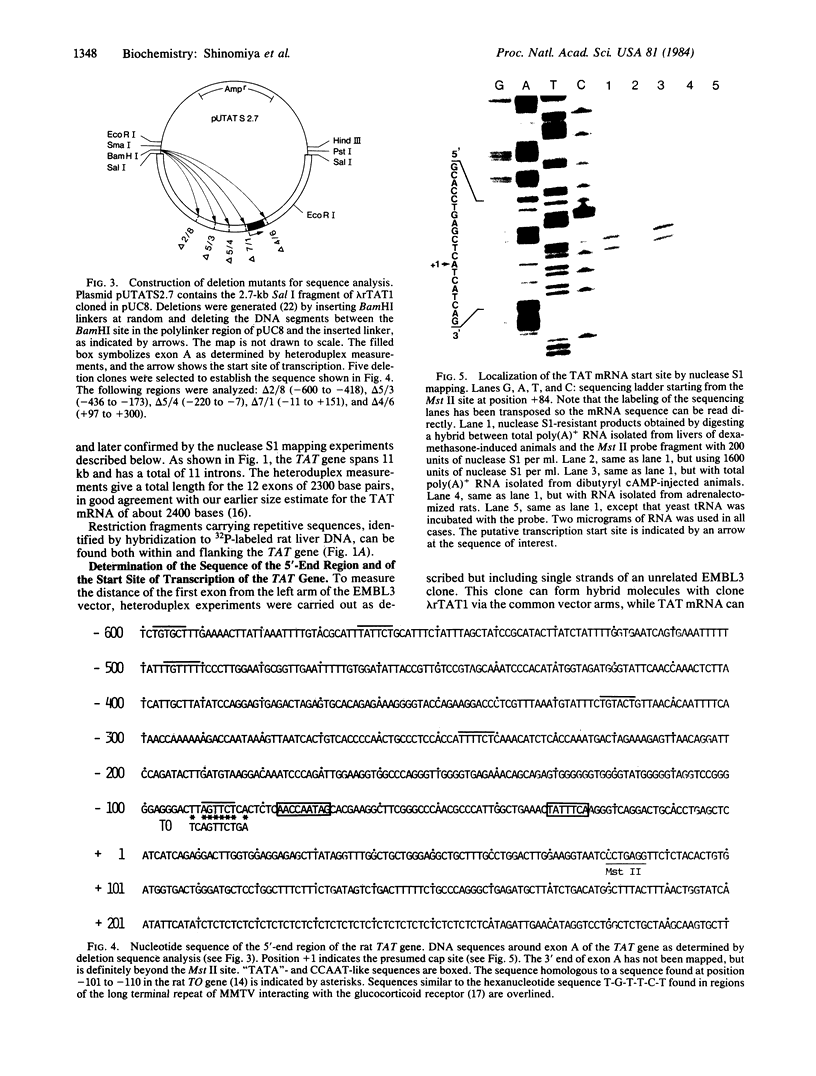
Tyrosine aminotransferase (TAT; L-tyrosine:2-oxoglutarate aminotransferase, EC 2.6.1.5) from rat liver is subject to glucocorticoid, cAMP, and developmental control. To study the underlying regulatory mechanisms, the TAT structural gene was isolated from a lambda bacteriophage rat DNA library. Heteroduplex analysis revealed that the 2.4-kilobase-long TAT mRNA is encoded by a gene that extends over 11 kilobases and is interrupted by 11 introns. To characterize the presumptive control region, the DNA sequence around the 5' end of the gene was determined and the start site of transcription was identified by nuclease S1 protection experiments. A short sequence homology in an equivalent position relative to the cap site was detected between TAT and tryptophan oxygenase, another glucocorticoid-controlled gene from rat liver. This sequence is related to the sequence 5' T-G-T-T-C-T 3' found in regions of the long terminal repeat of mouse mammary tumor virus, which has been shown to interact with the glucocorticoid receptor [Scheidereit, C., Geisse, J., Westphal, H. M. & Beato, M. (1983) Nature (London) 304, 749-752].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Bünemann H., Westhoff P., Herrmann R. G. Immobilization of denatured DNA to macroporous supports: I. Efficiency of different coupling procedures. Nucleic Acids Res. 1982 Nov 25;10(22):7163–7180. doi: 10.1093/nar/10.22.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet M., Chang A. C., Cohen S. N. Characterization of the structural gene and putative 5'-regulatory sequences for human proopiomelanocortin. Nature. 1982 May 27;297(5864):335–339. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Baxter J. D. Structural analysis of the prolactin gene suggests a separate origin for its 5' end. Nature. 1982 Jun 17;297(5867):603–606. doi: 10.1038/297603a0. [DOI] [PubMed] [Google Scholar]
- Cori C. F., Gluecksohn-Waelsch S., Klinger H. P., Pick L., Schlagman S. L., Teicher L. S., Wang-Chang H. F. Complementation of gene deletions by cell hybridization. Proc Natl Acad Sci U S A. 1981 Jan;78(1):479–483. doi: 10.1073/pnas.78.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesch U., Hashimoto S., Renkawitz R., Schütz G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J Biol Chem. 1983 Apr 25;258(8):4750–4753. [PubMed] [Google Scholar]
- Franz J. M., Knox W. E. The effect of development and hydrocortisone on tryptophan oxygenase, formamidase, and tyrosine aminotransferase in the livers of young rats. Biochemistry. 1967 Nov;6(11):3464–3471. doi: 10.1021/bi00863a017. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Grez M., Land H., Giesecke K., Schütz G., Jung A., Sippel A. E. Multiple mRNAs are generated from the chicken lysozyme gene. Cell. 1981 Sep;25(3):743–752. doi: 10.1016/0092-8674(81)90182-3. [DOI] [PubMed] [Google Scholar]
- KENNEY F. T. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. IV. Evidence for an increase in the rate of enzyme synthesis. J Biol Chem. 1962 Nov;237:3495–3498. [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek L. T., Eschenfeldt W. H., Munns T. W., Rhoads R. E. Heterogeneity of the 5' terminus of hen ovalbumin messenger ribonucleic acid. Nucleic Acids Res. 1981 Apr 10;9(7):1657–1673. doi: 10.1093/nar/9.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
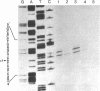
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch G. H., Rosenfeld M. G. Eukaryotic transcriptional regulation and chromatin-associated protein phosphorylation by cyclic AMP. Science. 1982 Dec 24;218(4579):1315–1317. doi: 10.1126/science.6293056. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Diesterhaft M., Granner D. Dibutyryl cyclic AMP increases the amount of functional messenger RNA coding for tyrosine aminotransferase in rat liver. J Biol Chem. 1978 Mar 10;253(5):1332–1335. [PubMed] [Google Scholar]
- Roewekamp W. G., Hofer E., Sekeris C. E. Translation of mRNA from rat-liver polysomes into tyrosine aminotransferase and tryptophan oxygenase in a protein-synthesizing system from wheat germ. Effects of cortisol on the translatable levels of mRNA for these two enzymes. Eur J Biochem. 1976 Nov 1;70(1):259–268. doi: 10.1111/j.1432-1033.1976.tb10977.x. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Scherer G., Schmid W., Strange C. M., Röwekamp W., Schütz G. Isolation of cDNA clones coding for rat tyrosine aminotransferase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7205–7208. doi: 10.1073/pnas.79.23.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W., Scherer G., Danesch U., Zentgraf H., Matthias P., Strange C. M., Röwekamp W., Schütz G. Isolation and characterization of the rat tryptophan oxygenase gene. EMBO J. 1982;1(10):1287–1293. doi: 10.1002/j.1460-2075.1982.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz G., Beato M., Feigelson P. Messenger RNA for hepatic tryptophan oxygenase: its partial purification, its translation in a heterologous cell-free system, and its control by glucocorticoid hormones. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1218–1221. doi: 10.1073/pnas.70.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz G., Killewich L., Chen G., Feigelson P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1017–1020. doi: 10.1073/pnas.72.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks W. D., Kenney F. T., Lee K. L. Induction of hepatic enzyme synthesis in vivo by adenosine 3', 5'-monophosphate. J Biol Chem. 1969 Nov 10;244(21):6008–6013. [PubMed] [Google Scholar]