Abstract
Background:
Prediction of negative postoperative outcomes after long-bone fracture treatment may help to optimize patient care. We recently completed the Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures (SPRINT), a large, multicenter trial of reamed and unreamed intramedullary nailing of tibial shaft fractures in 1226 patients. Using the SPRINT data, we conducted an investigation of baseline and surgical factors to determine any associations with an increased risk of adverse events within one year of intramedullary nailing.
Methods:
Using multivariable logistic regression analysis, we investigated fifteen baseline and surgical factors for any associations with an increased risk of negative outcomes.
Results:
There was an increased risk of negative events in patients with a high-energy mechanism of injury (odds ratio [OR] = 1.57; 95% confidence interval [CI], 1.05 to 2.35), a stainless steel compared with a titanium nail (OR = 1.52; 95% CI, 1.10 to 2.13), a fracture gap (OR = 2.40; 95% CI, 1.47 to 3.94), and full weight-bearing status after surgery (OR = 1.63; 95% CI, 1.00 to 2.64). There was no increased risk with the use of nonsteroidal anti-inflammatory agents, late or early time to surgery, or smoking status. Open fractures had a higher risk of events among patients treated with reamed nailing (OR = 3.26; 95% CI, 2.01 to 5.28) but not in patients treated with unreamed nailing (OR = 1.50; 95% CI, 0.92 to 2.47). Patients with open fractures who had wound management either without any additional procedures or with delayed primary closure had a decreased risk of events compared with patients who required subsequent, more complex reconstruction (OR = 0.18 [95% CI, 0.09 to 0.35] and 0.29 [95% CI, 0.14 to 0.62], respectively).
Conclusions:
We identified several baseline fracture and surgical characteristics that may increase the risk of adverse events in patients with tibial shaft fractures. Surgeons should consider the predictors identified in our analysis to inform patients treated for tibial shaft fractures.
Level of Evidence:
Prognostic Level II. See Instructions for Authors for a complete description of levels of evidence.
Several widely accepted systems classify long-bone injuries according to the nature and severity of damage to the bone1-3 and surrounding soft tissue2,3. Intramedullary nailing is the most common repair method for tibial shaft fractures2-5. The choice between the use of reamed or unreamed intramedullary nailing, however, has been controversial4-18. Following tibial shaft fracture repair with use of nails, annual reoperation rates have been reported to be between 12% and 44%5. This substantial problem is due to nonunion, malunion, knee pain, osteomyelitis, infection, and/or broken implants2,3.
The question of which characteristics are most predictive of risk of a negative outcome following tibial shaft fracture repair remains unresolved19-26. Investigators have assessed a number of potential prognostic factors, such as age, sex, fracture morphology, injury mechanism, severity of soft-tissue damage, surgical delay, diabetes, vasculopathy, alcohol use, smoking, corticosteroids, antibiotics, anticoagulants, anticonvulsants, and anti-inflammatory medications27-47. However, prior investigations were limited methodologically by small sample sizes, few participating health-care centers, lack of adjustment for confounders, and/or nonstandardized perioperative patient-care regimens.
Accurate prediction of patients who are at an increased risk for poor outcomes following tibial nailing may facilitate optimal patient care. We recently completed the Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures (SPRINT), a large, multicenter trial of reamed and unreamed intramedullary nailing in patients with tibial shaft fractures48. This trial suggested a benefit for reamed intramedullary nailing in patients with closed tibial shaft fractures, largely because of fewer dynamizations, and a potential advantage for unreamed intramedullary nailing in open tibial fractures48,49. Using the SPRINT data, we conducted an investigation of baseline and surgical factors previously documented to determine which were associated with increased risk of negative events within one year of tibial intramedullary nailing.
Materials and Methods
Study Design
The standardized protocol for the SPRINT study was approved by the human subjects committees (REB #99-077—Research Ethics Boards/Institutional Review Boards) at each participating site. The study was registered at www.ClinicalTrials.gov (identifier: NCT00038129). The methodological details and the results of the primary SPRINT analysis of reamed compared with unreamed nailing has been published previously48,49.
Briefly, the SPRINT study involved twenty-nine clinical centers in Canada, the United States, and the Netherlands. We standardized the surgical protocols for reamed and unreamed nailing, and all patients underwent the same perioperative protocol. One thousand two hundred and twenty-six patients met the eligibility criteria and completed one year of follow-up (Fig. 1).
Fig. 1.
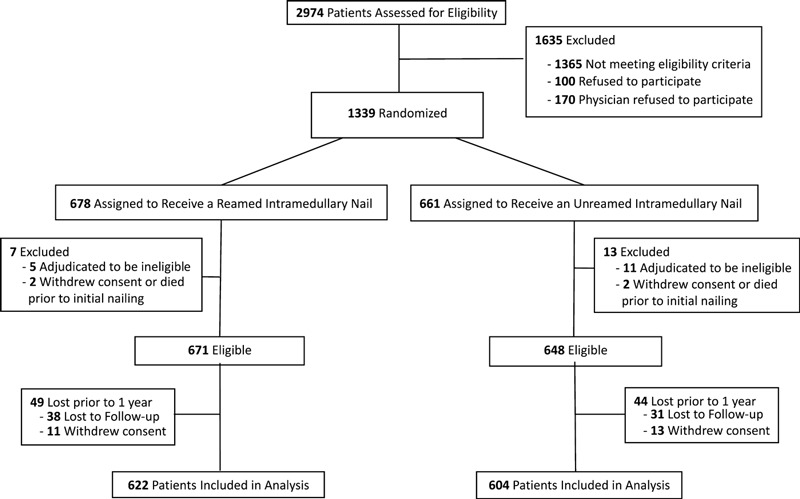
Flow diagram showing patient enrollment and follow-up throughout the SPRINT trial.
Inclusion criteria included skeletal maturity, an open or closed tibial shaft fracture (Tscherne Type 0 to 3 and Gustilo-Anderson Type I to IIIB)1-3,50-52, amenability of the fracture to surgical repair with an intramedullary nail, and informed consent. Exclusion criteria included tibial shaft fractures not amenable to reamed or unreamed nailing, pathologic fractures, patients likely to be lost before completing adequate follow-up, patients who were not skeletally mature, and patients who had not provided consent. Patients were followed for one year after injury.
In the SPRINT trial, the primary outcome was a composite including bone-grafting, implant exchange or removal, debridement of bone and soft tissue because of deep infection, fracture dynamization (due to locking screw removal), removal of locking screws because of screw breakage or loosening, autodynamization (breaking of a locking screw that resulted in the fracture collapsing), fasciotomy, failure of the construct (broken nail), and hematoma drainage. This composite is the primary outcome for the current analysis as well. The events included in our composite vary in severity, but are included in the composite as they are all deemed detrimental to the patient.
Briefly, the SPRINT investigation found that there was a significant interaction between the randomized intervention and open and closed fractures (p = 0.01). In patients with closed fractures, we found a significant decrease in risk for those who had reamed nailing compared with those who had unreamed nailing. This effect was not seen in patients with open fractures.
Selection of Prognostic Factors
On the basis of previous reports in the literature47-49 and variables collected in the SPRINT study, two investigators (M.B. and E.H.S.) independently identified two different types of factors: (1) baseline factors measured before the intramedullary nailing (age, sex, race, mechanism of injury, smoking status, insulin-dependent diabetes, unilateral or bilateral injury, open or closed fracture, anticoagulation medication use, nonsteroidal anti-inflammatory drugs [NSAIDs] use, isolated fracture, AO/OTA [Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association] classification, and location of fracture) and (2) surgical factors (reamed or unreamed nailing, surgeon or resident performing the surgery, nail material, number of locking screws used, patellar tendon split or tendon retraction, superior or inferior surgical approach for nail insertion, postoperative fracture gap, time from injury to surgery, fasciotomy at the time of initial surgery, postoperative weight-bearing status, and type of wound coverage in open fractures). With input from the Steering Committee, we agreed that factors needed to have thirty occurrences to be included in our model to ensure stability of the model. As a result, the following factors were not included in the analysis: bilateral fractures (twenty-two patients) and insulin-dependent diabetes (eleven patients).
For our adjusted model, we used multivariable logistic regression. Having fewer than ten events for each factor (predictor variable) can result in overfitted, unstable models53. We had 219 events in the SPRINT trial; therefore, we reduced the candidate list of variables so that there were only twenty-one factors in our analyses, as we describe below.
Steering Committee members independently rated their perceived importance of each factor on a scale of one to ten, with ten being top priority to include in the analysis and one being the lowest priority. We then chose the fifteen highest ranked factors to include in our analysis, which were, in descending order: (1) smoking status, (2) open fracture, (3) fracture gap, (4) mechanism of injury, (5) reamed intramedullary nailing, (6) age, (7) location of fracture, (8) isolated fracture, (9) type of wound coverage, (10) NSAID use, (11) AO/OTA fracture classification, (12) number of locking screws, (13) postoperative weight-bearing status, (14) time from injury to surgery, and (15) nail material. Because some of the variables had more than two categories (e.g., AO/OTA fracture classification), these fifteen factors accounted for twenty-one factors in the model.
Definition of Orthopaedic Factors
We classified the mechanism of injury as either high energy or low energy. High-energy injuries included motor vehicle accidents, pedestrian-motor vehicle accidents, motorcycle accidents, snowmobile accidents, crush injuries, and direct blunt trauma. Low-energy injuries included falls, twists, and direct penetrating trauma. We classified smoking status as current smokers versus previous smokers or nonsmokers. The fracture location data were recorded in five categories: proximal, proximal-middle, middle, distal-middle, and distal. For the current analysis, we classified fracture location as proximal and proximal-middle versus middle versus distal and distal-middle.
Nail material was recorded and analyzed as either stainless steel or titanium. We categorized the number of locking screws as two or more on both the proximal and distal sides compared with less than two on at least one side. The size of the fracture gap was assessed by the Central Adjudication Committee. The Committee reviewed the radiographs of each patient and determined whether there was no fracture gap, a fracture gap of <1 cm, or a fracture gap of ≥1 cm. Fracture gap refers to the magnitude of circumferential bone loss as judged by the adjudicator on review of the postoperative radiograph, or noncircumferential bone loss as judged by the adjudicator in patients with cortical continuity of up to 25% as judged by the surgeon at the time of the operation and recorded on the study case report forms. The time to surgery after injury was recorded as a continuous variable. For this analysis, we classified the time to surgery after injury into three categories: early (less than six hours from injury to surgery), middle (six hours to twenty-four hours), and late (a greater than twenty-four-hour surgical delay).
We classified postoperative weight-bearing as full weight-bearing postoperatively compared with partial or non-weight-bearing. Type of wound coverage was defined as primary closure, delayed primary closure, and additional soft-tissue reconstruction for open fractures. Primary closure was performed at the time of the intramedullary nailing. Patients in the delayed primary closure group had an open wound with a repeat irrigation and debridement and no other documented wound procedures, although they may have had negative-pressure wound therapy. Patients in the additional soft-tissue reconstruction group had documentation of a delayed wound closure procedure, including split-thickness skin grafts, fasciocutaneous flaps, rotational muscle flaps, or free flaps.
Statistical Analysis
Our primary analysis was a multivariable logistic regression using the SPRINT primary outcome as the dependent variable. All tests were two-tailed and p values of <0.05 were considered significant. Because the primary analysis of the SPRINT investigation found that reamed nailing reduced events in patients with closed but not open fractures, we included in the current analysis open or closed fractures, treatment status, and the interaction between the two in our logistic regression model. This was the only interaction investigated. The main multivariable model included fourteen characteristics. The fifteenth characteristic, type of wound closure, could not be included because of confounding with open versus closed fracture. Therefore, a second logistic regression was performed to investigate the type of wound closure. This model included only patients with open fractures and included the type of wound closure variable and all other variables except open compared with closed fracture. The main multivariable analysis was repeated with use of the following revised outcomes: (1) our primary outcome but without including autodynamization in the composite and (2) our primary outcome but without including dynamization and autodynamization in the composite. All analyses were performed with use of SAS software (version 9.1; SAS Institute, Cary, North Carolina).
Source of Funding
Research grants were received from the following: Canadian Institutes of Health Research (MCT-38140); National Institutes of Health (NIAMS-072 and R01-AR48529); Orthopaedic Research and Education Foundation of the American Academy of Orthopaedic Surgeons; Orthopaedic Trauma Association; Hamilton Health Sciences Research Grant; Zimmer; and, in part, by a Canada Research Chair in Musculoskeletal Trauma at McMaster University. The funding sources had no role in influencing the trial or the manuscript.
Results
A total of 1226 patients met the eligibility criteria and completed one year of follow-up (Fig. 1). Patients were relatively young (mean age [and standard deviation], 39.5 ± 16.0 years) and predominantly white (80.4%) (see Appendix). Over half (52.8%) were in an accident involving a motor vehicle or motorcycle, and the majority (98.2%) had an isolated tibial fracture. A table in the Appendix shows the distribution of the potential predictors. Most patients (67.4%) had closed injuries. Six hundred and twenty-two patients were treated with reamed intramedullary nailing, and 604 patients were treated with unreamed intramedullary nailing. The majority of the tibial fractures (88.8%) did not have a fracture gap, and >90% of the patients were restricted to partial or non-weight-bearing in the postoperative period.
There was an increased risk of an event for high-energy injuries (adjusted odds ratio [OR] = 1.57; 95% confidence interval [CI], 1.05 to 2.35), stainless steel nails (OR = 1.52; 95% CI, 1.10 to 2.13), a fracture gap of <1 cm compared with no fracture gap (OR = 2.40; 95% CI, 1.47 to 3.94), and full postoperative weight-bearing (OR = 1.63; 95% CI, 1.00 to 2.64) (Table I). The increase in risk associated with weight-bearing and nail material was attributable to the autodynamization component of the SPRINT composite outcome. The autodynamization rate was 2.3% with titanium nails and 10.1% with stainless steel nails, and it was 12.8% for full weight-bearing and 3.9% for partial or non-weight-bearing. Nail diameter, bone loss, or nail manufacturer had no effect on the result of the comparison of stainless steel and titanium nails. When autodynamization and both dynamization and autodynamization were removed as components of the composite outcome, stainless steel and full weight-bearing were no longer significant predictors.
TABLE I.
Results for Risk of Negative Events
| Factor* | Adjusted Odds Ratio (95% Confidence Interval) | P Value† |
| Patient factors | ||
| Age (10-yr increment) | 1.07 (0.97-1.18) | 0.19 |
| Mechanism of injury | ||
| High energy vs. low energy | 1.57 (1.05-2.35) | 0.03 |
| Current smoker vs. nonsmoker | 1.13 (0.82-1.57) | 0.45 |
| Nonsteroidal anti-inflammatory medication | 0.97 (0.53-1.78) | 0.93 |
| Isolated fracture vs. additional injuries | 0.79 (0.56-1.11) | 0.18 |
| AO/OTA fracture classification | ||
| B vs. A | 1.14 (0.80-1.63) | 0.47 |
| C vs. A | 0.76 (0.45-1.26) | 0.28 |
| Location of fracture | ||
| Proximal and proximal-middle vs. middle | 0.91 (0.52-1.57) | 0.72 |
| Distal and distal-middle vs. middle | 0.81 (0.56-1.16) | 0.25 |
| Open vs. closed | ||
| Reamed | 3.26 (2.01-5.28) | <0.001 |
| Unreamed | 1.50 (0.92-2.47) | 0.11 |
| Surgical factors | ||
| Reamed vs. unreamed nailing | ||
| Open | 1.31 (0.82-2.09) | 0.27 |
| Closed | 0.60 (0.40-0.92) | 0.02 |
| Nail material | ||
| Stainless steel vs. titanium | 1.52 (1.10-2.13) | 0.01 |
| Number of locking screws | ||
| ≥2 on both sides vs. <2 on at least 1 side | 0.98 (0.71-1.35) | 0.90 |
| Fracture gap (adjudicated) | ||
| Gap of ≥1 cm vs. no gap | 0.71 (0.28-1.83)† | 0.48 |
| Gap of <1 cm vs. no gap | 2.40 (1.47-3.94) | <0.001 |
| Time from injury to surgery | ||
| Late vs. early | 1.00 (0.58-1.72) | 0.99 |
| Middle vs. early | 1.12 (0.73-1.73) | 0.59 |
| Postoperative weight-bearing status | ||
| Full vs. partial or non-weight-bearing | 1.63 (1.00-2.64) | 0.048 |
| Type of coverage | ||
| Primary vs. additional soft-tissue reconstruction | 0.18 (0.09-0.35) | <0.001 |
| Delayed primary vs. additional soft-tissue reconstruction | 0.29 (0.14-0.62) | 0.001 |
All predictor variables were included in one model except for type of wound closure, which was included in a second model involving only patients with open fractures. AO/OTA = Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association.
The level of significance was set at a p value of <0.05.
Open fractures increased risk only for patients who had reamed nailing (OR = 3.26; 95% CI, 2.01 to 5.28). Patients with open fractures who had primary closure (OR = 0.18; 95% CI, 0.09 to 0.35) or delayed primary closure (OR = 0.29; 95% CI, 0.14 to 0.62) had decreased risk of an event compared with patients requiring additional soft-tissue reconstruction. Reamed compared with unreamed nailing had decreased risk for closed fractures (OR = 0.60; 95% CI, 0.40 to 0.92). However, this relationship was no longer significant when autodynamization and both dynamization and autodynamization were removed from the composite outcome. All other variables included in the model were not significant predictors.
Discussion
Reoperation rates following tibial shaft fracture repair with use of intramedullary nails have been reported to range between 12% and 44%5. To address this problem, we used multivariable analysis to identify prognostic factors for negative events (components of the composite outcome) from the first large-scale, multicenter, multinational, blinded, standardized, and randomized controlled trial of tibial shaft fracture repair with use of nails. An increased risk for a negative event was found for high-energy trauma, stainless steel compared with titanium nails, fracture gaps, postoperative full weight-bearing, and open fractures for reamed nailing only. Open fractures with wound management that included no additional procedures or delayed primary closure had lower risk compared with patients needing further more complex reconstruction. Other variables proved nonpredictive.
Patients with open fractures had a higher risk of events if they had reamed nailing but not if they had unreamed nailing. These results are consistent with those reported in our previous investigation48. There was an increased risk of poor outcomes with stainless steel nails compared with titanium nails, which may be surprising considering that stainless steel is a stronger material than titanium. Given these surprising results, we further reviewed the SPRINT data to investigate possible explanations. Nail diameter, bone loss, or nail manufacturer had no effect on the result of the comparison of stainless steel and titanium nails. This result was primarily driven by autodynamization, and when autodynamization was removed from the composite end point, stainless steel was no longer a significant predictor. Autodynamizations occurred more frequently with stainless steel nails than with titanium nails.
There was an increased risk of an event for fracture gaps of <1 cm but not for those with gaps of ≥1 cm or no fracture gap. Our findings for fracture gaps of ≥1 cm were seen because any reoperations to promote fracture-healing in patients with fracture gaps of ≥1 cm were not considered study events as per the SPRINT protocol49. The SPRINT Steering Committee believed that patients with large fracture gaps would have had an increased risk of reoperation and these events would not be related to the type of intervention. In addition, there were few patients included in the SPRINT trial with fracture gaps of ≥1 cm.
Postoperative full weight-bearing was also a significant predictor of events compared with partial weight-bearing and non-weight-bearing after surgery. This finding contradicts the belief of most surgeons that full weight-bearing following intramedullary nailing is appropriate. This result was driven by autodynamization. While full weight-bearing may stimulate fracture union, our results indicate that it may have a role in autodynamization of screws. On the basis of our findings, weight-bearing should not necessarily be limited following intramedullary nailing of the tibia; however, if all patients are able to bear full weight following surgery, the rate of autodynamizations will likely increase. Autodynamization may be associated with patient-important outcomes such as retained broken screws, temporary pain, and potential problems with revision surgery. If autodynamization is a concern, consideration should be given to some limitation of postoperative weight-bearing. Unfortunately, the clinical relevance of a potentially improved union rate compared with an increase in risk of screw breakage is unknown. When autodynamization was removed from the SPRINT investigation composite, weight-bearing status was not a significant predictor.
Prior studies have also shown an increased risk for reoperation because of soft-tissue injury (i.e., open fracture) and injury mechanism (i.e., high-energy injuries, the majority of which cause transverse fractures), which are further supported by the current study. Similar to our investigation, two prognostic studies found that patients with open compared with closed fractures were at higher risk for reoperation34,54. Of several studies that have evaluated fracture type as a risk factor40-42, Sarmiento et al. found that segmental fractures increased the risk of nonunion and reoperation41. Tytherleigh-Strong et al. found an association of risk with fracture type (segmental and comminuted proximal tibial shaft fractures)46. However, segmental fractures and fractures with comminution were not predictive of reoperation in the current analysis. Bhandari et al.47 found an increased risk with open fracture, fracture gap after fixation, and transverse fracture, which are very similar to our findings.
Smoking was not associated with reoperation in the present analysis, whereas other reports have suggested an association38,43. Given the strong biological rationale that smoking is detrimental to fracture-healing, we had hypothesized that smoking would be predictive of reoperation, as 33% of the patients in our sample were current smokers. As previous research suggests, fracture-healing time may be delayed in smokers; however, the detrimental effect of smoking may not be strong enough to affect the reoperation rate. In addition, surgeons may treat patients who smoke differently from patients who do not smoke, which would also help to explain why smoking was not predictive of reoperation in the current analysis.
While prior clinical and experimental studies have assessed numerous factors for reoperation risk, these efforts have been limited by small patient sample sizes, few participating clinics, lack of concealment, lack of adjustment for multiple variables, and/or nonstandardized patient-care regimens27-47,54. Therefore, comparison of their results with this investigation showed no clear consensus. Consequently, for proper interstudy comparison and clinical applicability, future investigations should be broader in scope and standardized, as in the present study. Such research should be encouraged since this remains an important, yet elusive, issue in orthopaedic trauma19-26.
The strengths of this investigation include, first, the largest sample size of patients with tibial shaft fracture (1226) and fractures (1248). Second, a substantial number of trauma clinics (twenty-nine) from three nations participated, thereby increasing the generalizability of the findings. Third, perioperative care was standardized with use of a uniform protocol for clinical management. Fourth, all events were centrally adjudicated. Fifth, patient follow-up was sufficiently long (one year) to ensure adequate management and recovery. Last, multivariable analysis minimized the effects of confounding.
The limitations of the current analysis are typical of initial attempts to develop a predictive model55,56. First, regression models capitalize on the play of chance; thus, a model’s application to new data often fails to confirm initial results. Second, results may be anomalous to the patients studied, exacerbating problems of replicability. Third, not all potential predictor variables, including alcohol consumption, corticosteroid use, antibiotic use, and obesity, were collected as part of the SPRINT investigation. Therefore, the results of the current analysis are limited to the variables that were collected as part of the SPRINT trial. In addition, the study population was relatively young, and the results may not be generalizable to older patients with osteoporosis or osteopenia.
To our knowledge, this is the first large-scale, multicenter, multinational, standardized, and randomized controlled trial with use of multivariate analysis to have investigated this important issue. There are significant, potentially modifiable factors that have an impact on patient-important outcomes. Autodynamization may be reduced, in some instances, by not choosing stainless steel nails and avoiding full weight-bearing after surgery. Severity of injury plays the most important role in outcome following intramedullary nailing of the tibia. High-energy injuries, the need for soft-tissue reconstruction, and a fracture gap were predictive of a higher risk of a poor outcome. In conclusion, the predictors identified in our analysis may be used to help to inform patients with tibial shaft fractures on their risk of a possible negative outcome.
Appendix
A table showing the patient characteristics and the incidence of potential predictors is available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A table showing the patient characteristics and the incidence of potential predictors
Acknowledgments
Note: Data were analyzed by Diane Heels-Ansdell, MSc (Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Ontario, Canada), under the supervision of Stephen Walter (Senior Biostatistician). Details regarding the authors and investigators are provided below.
Author Contributions: Writing Committee: Emil H. Schemitsch, MD, FRCS(C) (Chair), Mohit Bhandari, MD, PhD, FRCS(C), Gordon Guyatt, MD, David W. Sanders, MD, MSc, FRCS(C), Marc Swiontkowski, MD, Paul Tornetta III, MD, Stephen D. Walter, PhD, Rad Zdero, PhD, J.C. Goslings, MD, PhD, David Teague, MD, Kyle Jeray, MD, and Michael D. McKee, MD, FRCS(C).
SPRINT Investigators: The following persons participated in the SPRINT Study:
Study trial co-principal investigators: Mohit Bhandari, Gordon Guyatt.
Steering Committee: Gordon Guyatt (Chair), Mohit Bhandari, David W. Sanders, Emil H. Schemitsch, Marc Swiontkowski, Paul Tornetta III, Stephen D. Walter.
Central Adjudication Committee: Gordon Guyatt (Chair), Mohit Bhandari, David W. Sanders, Emil H. Schemitsch, Marc Swiontkowski, Paul Tornetta III, Stephen D. Walter.
SPRINT Methods Center staff: McMaster University, Hamilton, Ontario: Sheila Sprague, Diane Heels-Ansdell, Lisa Buckingham, Pamela Leece, Helena Viveiros, Tashay Mignott, Natalie Ansell, Natalie Sidorkewicz. University of Minnesota, Minneapolis, Minnesota: Julie Agel.
Data Safety and Monitoring Board (DSMB): Claire Bombardier (Chair), Jesse A. Berlin, Michael Bosse, Bruce Browner, Brenda Gillespie, Alan Jones, Peter O’Brien.
Site Audit Committee: Julie Agel, Sheila Sprague, Rudolf Poolman, Mohit Bhandari.
Investigators: London Health Sciences Center/University of Western Ontario, London, Ontario: David W. Sanders, Mark D. Macleod, Timothy Carey, Kellie Leitch, Stuart Bailey, Kevin Gurr, Ken Konito, Charlene Bartha, Isolina Low, Leila V. MacBean, Mala Ramu, Susan Reiber, Ruth Strapp, Christina Tieszer.
Sunnybrook Health Sciences Center/University of Toronto, Toronto, Ontario: Hans J. Kreder, David J. G. Stephen, Terry S. Axelrod, Albert J.M. Yee, Robin R. Richards, Joel Finkelstein, Wade Gofton, John Murnaghan, Joseph Schatztker, Michael Ford; Beverly Bulmer, Lisa Conlan.
Hospital du Sacre Coeur de Montreal, Montreal, Quebec: G. Yves Laflamme, Gregory Berry, Pierre Beaumont, Pierre Ranger, Georges-Henri Laflamme, Sylvain Gagnon, Michel Malo, Julio Fernandes, Marie-France Poirier.
St. Michael’s Hospital/University of Toronto, Toronto, Ontario: Emil H. Schemitsch, Michael D. McKee, James P. Waddell, Earl R. Bogoch, Timothy R. Daniels, Robert R. McBroom, Milena R. Vicente, Wendy Storey, Lisa M. Wild.
Royal Columbian Hospital/University of British Columbia, New Westminster/Vancouver, British Columbia: Robert McCormack, Bertrand Perey, Thomas J. Goetz, Graham Pate, Murray J. Penner, Kostas Panagiotopoulos, Shafique Pirani, Ian G. Dommisse, Richard L. Loomer, Trevor Stone, Karyn Moon, Mauri Zomar.
Wake Forest Medical Center/Wake Forest University Health Sciences, Winston-Salem, North Carolina: Lawrence X. Webb, Robert D. Teasdall, John Peter Birkedal, David Franklin Martin, David S. Ruch, Douglas J. Kilgus, David C. Pollock, Mitchel Brion Harris, Ethan Ron Wiesler, William G. Ward, Jeffrey Scott Shilt, Andrew L. Koman, Gary G. Poehling, Brenda Kulp.
Boston Medical Center/Boston University School of Medicine, Boston, Massachusetts: Paul Tornetta III, William R. Creevy, Andrew B. Stein, Christopher T. Bono, Thomas A. Einhorn, T. Desmond Brown, Donna Pacicca, John B. Sledge III, Timothy E. Foster, Ilva Voloshin, Jill Bolton, Hope Carlisle, Lisa Shaughnessy.
Wake Medical Center, Raleigh, North Carolina: William T. Obremskey, C. Michael LeCroy, Eric G. Meinberg, Terry M. Messer, William L. Craig III, Douglas R. Dirschl, Robert Caudle, Tim Harris, Kurt Elhert, William Hage, Robert Jones, Luis Piedrahita, Paul O. Schricker, Robin Driver, Jean Godwin.
Vanderbilt University Medical Center, Nashville, Tennessee: William T. Obremskey, Philip James Kregor, Gregory Tennent, Lisa M. Truchan, Marcus Sciadini, Franklin D. Shuler, Robin E. Driver, Mary Alice Nading, Jacky Neiderstadt, Alexander R. Vap.
MetroHealth Medical Center, Cleveland, Ohio: Heather A. Vallier, Brendan M. Patterson, John H. Wilber, Roger G. Wilber, John K. Sontich, Timothy Alan Moore, Drew Brady, Daniel R. Cooperman, John A. Davis, Beth Ann Cureton.
Hamilton Health Sciences, Hamilton, Ontario: Scott Mandel, R. Douglas Orr, John T.S. Sadler, Tousief Hussain, Krishan Rajaratnam, Bradley Petrisor, Mohit Bhandari, Brian Drew, Drew A. Bednar, Desmond C.H. Kwok, Shirley Pettit, Jill Hancock, Natalie Sidorkewicz.
Regions Hospital, St. Paul, Minnesota: Peter A. Cole, Joel J. Smith, Gregory A. Brown, Thomas A. Lange, John G. Stark, Bruce A. Levy, Marc F. Swiontkowski, Mary J. Garaghty, Joshua G. Salzman, Carol A. Schutte, Linda Tastad, Sandy Vang.
University of Louisville School of Medicine, Louisville, Kentucky: David Seligson, Craig S. Roberts, Arthur L. Malkani, Laura Sanders, Carmen Dyer, Jessica Heinsen, Langan Smith, Sudhakar Madanagopal, Linda Frantz-Bush.
Memorial Hermann Hospital, Houston, Texas: Kevin J. Coupe, Jeffrey J. Tucker, Allen R. Criswell, Rosemary Buckle, Alan Jeffrey Rechter, Dhiren Shaskikant Sheth, Brad Urquart, Thea Trotscher.
Erie County Medical Center/University of Buffalo, Buffalo, New York: Mark J. Anders, Joseph M. Kowalski, Marc S. Fineberg, Lawrence B. Bone, Matthew J. Phillips, Bernard Rohrbacher, Philip Stegemann, William M. Mihalko, Cathy Buyea.
University of Florida–Jacksonville, Jacksonville, Florida: Stephen J. Augustine, William Thomas Jackson, Gregory Solis, Sunday U. Ero, Daniel N. Segina, Hudson B. Berrey, Samuel G. Agnew, Michael Fitzpatrick, Lakina C. Campbell, Lynn Derting, June McAdams.
Academic Medical Center, Amsterdam, The Netherlands: J. Carel Goslings, Kees Jan Ponsen, Jan Luitse, Peter Kloen, Pieter Joosse, Jasper Winkelhagen, Raphaël Duivenvoorden.
University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma: David C. Teague, Joseph Davey, J. Andy Sullivan, William J.J. Ertl, Timothy A. Puckett, Charles B. Pasque, John F. Tompkins II, Curtis R. Gruel, Paul Kammerlocher, Thomas P. Lehman, William R. Puffinbarger, Kathy L. Carl.
University of Alberta/University of Alberta Hospital/Royal Alexandra Hospital, Edmonton, Alberta: Donald W. Weber, Nadr M. Jomha, Gordon R. Goplen, Edward Masson, Lauren A. Beaupre, Karen E. Greaves, Lori N. Schaump.
Greenville Hospital System, Greenville, South Carolina: Kyle J. Jeray, David R. Goetz, David E. Westberry, J. Scott Broderick, Bryan S. Moon, Stephanie L. Tanner.
Foothills General Hospital, Calgary, Alberta: James N. Powell, Richard E. Buckley, Leslie Elves.
Saint John Regional Hospital, Saint John, New Brunswick: Stephen Connolly, Edward P. Abraham, Trudy Steele.
Oregon Health & Sciences University, Portland, Oregon: Thomas Ellis, Alex Herzberg, George A. Brown, Dennis E. Crawford, Robert Hart, James Hayden, Robert M. Orfaly, Theodore Vigland, Maharani Vivekaraj, Gina L. Bundy.
University of California, San Francisco, San Francisco General Hospital, San Francisco, California: Theodore Miclau III, Amir Matityahu, R. Richard Coughlin, Utku Kandemir, R. Trigg McClellan, Cindy Hsin-Hua Lin.
Detroit Receiving Hospital, Detroit, Michigan: David Karges, Kathryn Cramer, J. Tracy Watson, Berton Moed, Barbara Scott.
Deaconess Hospital Regional Trauma Center and Orthopaedic Associates, Evansville, Indiana: Dennis J. Beck, Carolyn Orth.
Thunder Bay Regional Health Science Center, Thunder Bay, Ontario: David Puskas, Russell Clark, Jennifer Jones.
Jamaica Hospital, Jamaica, New York: Kenneth A. Egol, Nader Paksima, Monet France.
Ottawa Hospital–Civic Campus, Ottawa, Ontario: Eugene K. Wai, Garth Johnson, Ross Wilkinson, Adam T. Gruszczynski, Liisa Vexler.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Fracture and dislocation compendium Orthopaedic Trauma Association Committee for Coding and Classification. J Orthop Trauma. 1996;10 Suppl 1:v-ix, 1-154 [PubMed] [Google Scholar]
- 2.Anglen J. Tibial shaft fractures. : Sanders R, editor Core knowledge in orthopaedics: trauma. Philadelphia, PA: Mosby-Elsevier; 2007. p 326-43 [Google Scholar]
- 3.Appleton P, Court-Brown CM. Diaphyseal fractures of the tibia and fibula. : Elstrom JA, Virkus WW, Pankovich AM, Handbook of fractures. New York: McGraw-Hill; 2006. p 340-52 [Google Scholar]
- 4.Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001 Jan;83(1):62-8 [DOI] [PubMed] [Google Scholar]
- 5.Bhandari M, Guyatt GH, Tong D, Adili A, Shaughnessy SG. Reamed versus nonreamed intramedullary nailing of lower extremity long bone fractures: a systematic overview and meta-analysis. J Orthop Trauma. 2000 Jan;14(1):2-9 [DOI] [PubMed] [Google Scholar]
- 6.Forster MC, Bruce AS, Aster AS. Should the tibia be reamed when nailing? Injury. 2005 Mar;36(3):439-44 [DOI] [PubMed] [Google Scholar]
- 7.Rhinelander FW. Tibial blood supply in relation to fracture healing. Clin Orthop Relat Res. 1974 Nov-Dec;(105):34-81 [PubMed] [Google Scholar]
- 8.Rhinelander FW. The vascular response of bone to internal fixation. : Browner BD, Edwards CC, The science and practice of intramedullary nailing. Philadelphia: Lea & Febiger; 1987. p 25-59 [Google Scholar]
- 9.Olerud S, Strömberg L. Intramedullary reaming and nailing: its early effects on cortical bone vascularization. Orthopedics. 1986 Sep;9(9):1204-8 [DOI] [PubMed] [Google Scholar]
- 10.Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-6 [DOI] [PubMed] [Google Scholar]
- 11.Hupel TM, Aksenov SA, Schemitsch EH. Cortical bone blood flow in loose and tight fitting locked unreamed intramedullary nailing: a canine segmental tibia fracture model. J Orthop Trauma. 1998 Feb;12(2):127-35 [DOI] [PubMed] [Google Scholar]
- 12.Schemitsch EH, Turchin DC, Kowalski MJ, Swiontkowski MF. Quantitative assessment of bone injury and repair after reamed and unreamed locked intramedullary nailing. J Trauma. 1998 Aug;45(2):250-5 [DOI] [PubMed] [Google Scholar]
- 13.Schemitsch EH, Kowalski MJ, Swiontkowski MF. Soft-tissue blood flow following reamed versus unreamed locked intramedullary nailing: a fractured sheep tibia model. Ann Plast Surg. 1996 Jan;36(1):70-5 [DOI] [PubMed] [Google Scholar]
- 14.Utvåg SE, Grundnes O, Reikerås O. Effects of degrees of reaming on healing of segmental fractures in rats. J Orthop Trauma. 1998 Mar-Apr;12(3):192-9 [DOI] [PubMed] [Google Scholar]
- 15.Grundnes O, Utvåg SE, Reikerås O. Restoration of bone flow following fracture and reaming in rat femora. Acta Orthop Scand. 1994 Apr;65(2):185-90 [DOI] [PubMed] [Google Scholar]
- 16.Fairbank AC, Thomas D, Cunningham B, Curtis M, Jinnah RH. Stability of reamed and unreamed intramedullary tibial nails: a biomechanical study. Injury. 1995 Sep;26(7):483-5 [DOI] [PubMed] [Google Scholar]
- 17.Whittle AP, Wester W, Russell TA. Fatigue failure in small diameter tibial nails. Clin Orthop Relat Res. 1995 Jun;(315):119-28 [PubMed] [Google Scholar]
- 18.Bhandari M, Schemitsch EH. Bone formation following intramedullary femoral reaming is decreased by indomethacin and antibodies to insulin-like growth factors. J Orthop Trauma. 2002 Nov-Dec;16(10):717-22 [DOI] [PubMed] [Google Scholar]
- 19.Bach AW, Hansen ST., Jr Plates versus external fixation in severe open tibial shaft fractures. A randomized trial. Clin Orthop Relat Res. 1989 Apr;(241):89-94 [PubMed] [Google Scholar]
- 20.Bostrom MP, Camacho NP. Potential role of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998 Oct;(355 Suppl):S274-82 [DOI] [PubMed] [Google Scholar]
- 21.Court-Brown CM, Will E, Christie J, McQueen MM. Reamed or unreamed nailing for closed tibial fractures. A prospective study in Tscherne C1 fractures. J Bone Joint Surg Br. 1996 Jul;78(4):580-3 [PubMed] [Google Scholar]
- 22.Finkemeier CG, Schmidt AH, Kyle RF, Templeman DC, Varecka TF. A prospective, randomized study of intramedullary nails inserted with and without reaming for the treatment of open and closed fractures of the tibial shaft. J Orthop Trauma. 2000 Mar-Apr;14(3):187-93 [DOI] [PubMed] [Google Scholar]
- 23.Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998 Jan;12(1):1-7 [DOI] [PubMed] [Google Scholar]
- 24.Holbrook JL, Swiontkowski MF, Sanders R. Treatment of open fractures of the tibial shaft: Ender nailing versus external fixation. A randomized, prospective comparison. J Bone Joint Surg Am. 1989 Sep;71(8):1231-8 [PubMed] [Google Scholar]
- 25.Keating JF, O’Brien PJ, Blachut PA, Meek RN, Broekhuyse HM. Locking intramedullary nailing with and without reaming for open fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997 Mar;79(3):334-41 [DOI] [PubMed] [Google Scholar]
- 26.Tornetta P, 3rd, Bergman M, Watnik N, Berkowitz G, Steuer J. Treatment of grade-IIIb open tibial fractures. A prospective randomised comparison of external fixation and non-reamed locked nailing. J Bone Joint Surg Br. 1994 Jan;76(1):13-9 [PubMed] [Google Scholar]
- 27.Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9(5):392-400 [DOI] [PubMed] [Google Scholar]
- 28.Cozen L. Does diabetes delay fracture healing? Clin Orthop Relat Res. 1972 Jan-Feb;82:134-40 [PubMed] [Google Scholar]
- 29.Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemp Orthop. 1995 Jun;30(6):489-93 [PubMed] [Google Scholar]
- 30.Dodds RA, Catterall A, Bitensky L, Chayen J. Effects on fracture healing of an antagonist of the vitamin K cycle. Calcif Tissue Int. 1984 Mar;36(2):233-8 [DOI] [PubMed] [Google Scholar]
- 31.Engesaeter LB, Sudmann B, Sudmann E. Fracture healing in rats inhibited by locally administered indomethacin. Acta Orthop Scand. 1992 Jun;63(3):330-3 [DOI] [PubMed] [Google Scholar]
- 32.Frymoyer JW. Fracture healing in rats treated with diphenylhydantoin (Dilantin). J Trauma. 1976 May;16(5):368-70 [DOI] [PubMed] [Google Scholar]
- 33.Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res. 2000 Jan;18(1):126-32 [DOI] [PubMed] [Google Scholar]
- 34.Gaston P, Will E, Elton RA, McQueen MM, Court-Brown CM. Fractures of the tibia. Can their outcome be predicted? J Bone Joint Surg Br. 1999 Jan;81(1):71-6 [DOI] [PubMed] [Google Scholar]
- 35.Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000 Jul;82(5):655-8 [DOI] [PubMed] [Google Scholar]
- 36.Høgevold HE, Grøgaard B, Reikerås O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing. A mechanical study of osteotomies in rats. Acta Orthop Scand. 1992 Dec;63(6):607-11 [DOI] [PubMed] [Google Scholar]
- 37.Huddleston PM, Steckelberg JM, Hanssen AD, Rouse MS, Bolander ME, Patel R. Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg Am. 2000 Feb;82(2):161-73 [DOI] [PubMed] [Google Scholar]
- 38.Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-62 [PubMed] [Google Scholar]
- 39.Nyquist F, Berglund M, Nilsson BE, Obrant KJ. Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcohol. 1997 Jan-Feb;32(1):91-5 [DOI] [PubMed] [Google Scholar]
- 40.Oni OO, Dunning J, Mobbs RJ, Gregg PJ. Clinical factors and the size of the external callus in tibial shaft fractures. Clin Orthop Relat Res. 1991 Dec;(273):278-83 [PubMed] [Google Scholar]
- 41.Sarmiento A, Sharpe FE, Ebramzadeh E, Normand P, Shankwiler J. Factors influencing the outcome of closed tibial fractures treated with functional bracing. Clin Orthop Relat Res. 1995 Jun;(315):8-24 [PubMed] [Google Scholar]
- 42.Sarmiento A. On the behavior of closed tibial fractures: clinical/radiological correlations. J Orthop Trauma. 2000 Mar-Apr;14(3):199-205 [DOI] [PubMed] [Google Scholar]
- 43.Schmitz MA, Finnegan M, Natarajan R, Champine J. Effect of smoking on tibial shaft fracture healing. Clin Orthop Relat Res. 1999 Aug;(365):184-200 [DOI] [PubMed] [Google Scholar]
- 44.Stinchfield FE, Sankaran B, Samilson R. The effect of anticoagulant therapy on bone repair. J Bone Joint Surg Am. 1956 Apr;38-A(2):270-82 [PubMed] [Google Scholar]
- 45.Templeman DC, Gulli B, Tsukayama DT, Gustilo RB. Update on the management of open fractures of the tibial shaft. Clin Orthop Relat Res. 1998 May;(350):18-25 [PubMed] [Google Scholar]
- 46.Tytherleigh-Strong GM, Keating JF, Court-Brown CM. Extra-articular fractures of the proximal tibial diaphysis: their epidemiology, management and outcome. J R Coll Surg Edinb. 1997 Oct;42(5):334-8 [PubMed] [Google Scholar]
- 47.Bhandari M, Tornetta P, 3rd, Sprague S, Najibi S, Petrisor B, Griffith L, Guyatt GH. Predictors of reoperation following operative management of fractures of the tibial shaft. J Orthop Trauma. 2003 May;17(5):353-61 [DOI] [PubMed] [Google Scholar]
- 48.Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, Schemitsch EH, Swiontkowski M, Sanders D, Walter SD. J Bone Joint Surg Am. 2008 Dec;90(12):2567-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Study to prospectively evaluate reamed intramedullary nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, Schemitsch E, Swiontkowski M, Sanders D, Walter SD. BMC Musculoskelet Disord. 2008 Jun 23;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990 Feb;72(2):299-304 [PubMed] [Google Scholar]
- 51.Oestern HJ, Tscherne H. Pathophysiology and classification of soft tissue injuries associated with fractures. : Tscherne H, Gotzen L, Fractures with soft tissue injuries. Telger TC, translator. New York: Springer; 1984. p 1-9 [Google Scholar]
- 52.Tscherne H, Oestern HJ. [A new classification of soft-tissue damage in open and closed fractures (author’s transl)]. Unfallheilkunde. 1982 Mar;85(3):111-5 German [PubMed] [Google Scholar]
- 53.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996 Dec;49(12):1373-9 [DOI] [PubMed] [Google Scholar]
- 54.Court-Brown CM, Keating JF, Christie J, McQueen MM. Exchange intramedullary nailing. Its use in aseptic tibial nonunion. J Bone Joint Surg Br. 1995 May;77(3):407-11 [PubMed] [Google Scholar]
- 55.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000 Jul 5;284(1):79-84 [DOI] [PubMed] [Google Scholar]
- 56.Guyatt GH, Walter S, Shannon H, et al. Basic statistics for clinicians: IV. Regression and correlation. Can Med Assoc J. 1995;152:497-504 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
A table showing the patient characteristics and the incidence of potential predictors


