Abstract
Background:
The objective of this study was to assess the frequency of, and risk factors for, periprosthetic fractures during and after shoulder arthroplasty.
Methods:
All adults treated with a primary total shoulder arthroplasty or humeral head replacement at the Mayo Clinic Medical Center from 1976 to 2008 were identified. Periprosthetic fractures were validated by medical record review. Univariate and multivariable-adjusted logistic regression analyses were used to assess the association of demographic factors (age, sex, and body mass index [BMI]), underlying diagnosis, implant fixation (cemented or uncemented), American Society of Anesthesiologists (ASA) class, and comorbidity as assessed with the Deyo-Charlson index.
Results:
The cohort consisted of 2207 patients treated with a total of 2588 primary total shoulder arthroplasties and 1349 patients treated with 1431 humeral head replacements. Seventy-two medical-record-confirmed periprosthetic fractures occurred in association with the total shoulder arthroplasties. These consisted of forty-seven intraoperative fractures (forty humeral fractures, five glenoid fractures, and two fractures for which the site was unclear) and twenty-five postoperative fractures (twenty humeral fractures, three glenoid fractures, and two fractures for which the site was unclear). There were thirty-three fractures associated with the humeral head replacements. Fifteen were intraoperative (eight humeral fractures and seven glenoid fractures), and eighteen were postoperative (sixteen humeral fractures and two glenoid fractures). In the multivariable regression analysis of the total shoulder arthroplasties, female sex (odds ratio [OR], 4.19; 95% confidence interval [CI], 1.82 to 9.62; p < 0.001; a 2.4% rate for women versus 0.6% for men) and the underlying diagnosis (p = 0.04; posttraumatic arthritis: OR, 2.55; 95% CI, 0.92 to 7.12) were associated with a significantly higher risk of intraoperative humeral fracture in general, and female sex was associated with the risk of intraoperative humeral shaft fracture (OR, infinity; p < 0.001). In combined analyses of all patients (treated with either total shoulder arthroplasty or humeral head replacement), a higher Deyo-Charlson index was significantly associated with an increased risk of postoperative periprosthetic humeral shaft fracture (OR, 1.27; 95% CI, 1.11 to 1.45); p < 0.001), after adjusting for the type of surgery (total shoulder arthroplasty or humeral head replacement).
Conclusions:
The overall risk of periprosthetic fractures after total shoulder arthroplasty or humeral head replacement was low. Women had a significantly higher risk of intraoperative humeral shaft fracture. The underlying diagnosis (especially posttraumatic arthritis) was significantly associated with the risk of intraoperative humeral fracture, and comorbidity was significantly associated with the risk of postoperative humeral shaft fracture.
Level of Evidence:
Prognostic Level II. See Instructions for Authors for a complete description of levels of evidence.
Periprosthetic fracture during or following shoulder arthroplasty is an important but rare complication. The frequency of periprosthetic fractures after total shoulder arthroplasty ranges from 1.6% to 2.8%1-4. In a systematic review of forty studies of humeral head replacement or total shoulder arthroplasty that included 3584 patients, 1.2% (range, 0% to 8%) had a perioperative periprosthetic fracture5. In studies of more than 3000 patients who had undergone primary or revision humeral head replacement or total shoulder arthroplasty at the Mayo Clinic, 0.6% had a postoperative humeral fracture6 and 1.5% had a perioperative humeral fracture7.
While there are high-quality data on the frequency, types, and outcomes of periprosthetic fractures after shoulder arthroplasty, our knowledge of its risk factors is very limited. In our earlier univariate analyses of the same database as was used in the present study, but with a shorter follow-up period, female sex, press-fit humeral components, and revision surgery were significant correlates of higher perioperative fracture rates7, while the underlying diagnosis was not associated. Two other reviews identified poor bone quality, female sex, advanced age, and a history of rheumatoid arthritis as risk factors for periprosthetic fractures8,9. Periprosthetic fractures have been found to be more common with total shoulder arthroplasty than with humeral head replacement8. These observations were based on univariate associations from large series of patients from a few high-volume centers. To our knowledge, none of the previous studies included enough events to make it possible to control for confounders and perform multivariable analyses.
We sought to examine the frequency and predictors of periprosthetic fractures during and following humeral head replacement or total shoulder arthroplasty using thirty-three-year data from one large-volume medical center. We examined the hypotheses whether sex, underlying diagnosis, body mass index (BMI), comorbidity, age, American Society of Anesthesiologists (ASA) class, and implant fixation were associated with a greater risk of periprosthetic fractures in patients treated with humeral head replacement or total shoulder arthroplasty.
Materials and Methods
Data Sources and Case Identification
We performed a retrospective cohort study using the data from the Mayo Clinic Medical Center Total Joint Registry. This registry was established in 1969 for systematic follow-up of patients after joint arthroplasty and has collected data on every patient who underwent arthroplasty at the Mayo Clinic Medical Center, Rochester, Minnesota, since that time10. The registry has prospectively captured data on every shoulder arthroplasty since the surgery was performed at the Mayo Clinic Medical Center beginning in 1976. A clinical follow-up with a physician recording a history and performing an examination and radiographs made is planned for all patients at one, two, and five years after an arthroplasty and every five years thereafter. When patients were unable to return for a follow-up visit, we asked them to respond to a standard questionnaire11 and to provide radiographs to us. When patients did not respond to mailed requests, they were contacted and interviewed on the telephone with use of a standardized questionnaire by trained joint registry staff. Complications including fracture, infection, and additional surgery were particularly captured with these encounters and interviews.
Our study cohort consisted of every patient who had had a primary shoulder arthroplasty performed when they were eighteen years of age or older at the Mayo Clinic Medical Center, Rochester, Minnesota, in a thirty-three-year period from 1976 to 2008. The periprosthetic shoulder fractures were identified from the total joint registry, which captures these events prospectively for all shoulder arthroplasties. For each fracture that was identified, the lead author (J.A.S.), a physician-epidemiologist, reviewed the medical records for confirmation of the occurrence of a periprosthetic fracture on the basis of a priori criteria as follows. Documentation of a periprosthetic fracture in the surgeon's operative note, surgeon's clinical follow-up note, or surgeon's letter to the patient was considered the “gold standard.” (In almost all cases, this information was available in the operative procedure note and/or surgeon's notes.) Radiographic documentation of a periprosthetic fracture occurring either intraoperatively or postoperatively, in the absence of any other documentation, was also considered evidence of a periprosthetic fracture; however, there were no cases of radiographic evidence of a periprosthetic fracture in the absence of surgeon's documentation. The final designation of each fracture type was reviewed by the senior orthopaedic surgeon. Intraoperative and postoperative fractures were evaluated separately, since the mechanism and outcomes often differ between the two types of fractures. Humeral fractures were categorized as proximal humeral or humeral shaft fractures. Our a priori decision was to analyze humeral fractures as a group and also focus on humeral shaft fractures. We did not anticipate that the number of glenoid fractures or fenestrations would be high enough to allow meaningful analyses of risk factors, but we anticipated that some of the events captured as periprosthetic fractures in the registry would be glenoid-related.
Predictors of Interest
Predictors of interest for the risk of periprosthetic fracture included both unmodifiable factors, including sex, age, underlying diagnosis, and ASA class, and modifiable factors, including BMI, comorbidities, and implant fixation. These data were obtained from the total joint registry and other institutional electronic databases, linked to the total joint registry by the use of unique patient identifiers. Age was categorized as sixty years of age or younger, sixty-one to seventy years of age, and more than seventy years of age on the basis of the a priori clinical impression of the two senior orthopaedic surgeons that these age groups might represent different risk levels and that it was unlikely that an increase in the risk of fracture would be linear to allow the age at the time of treatment to be a continuous variable. The underlying diagnosis was categorized as osteoarthritis, trauma-related, rheumatoid arthritis or another inflammatory arthritis, and other diagnoses. The trauma category included acute-trauma-related fractures or dislocations and posttraumatic arthritis as a sequela of an old injury; none of these trauma cases were classified as osteoarthritis. The ASA classification, a validated measure of perioperative mortality and immediate postoperative morbidity12,13, was categorized as class 1 or 2 compared with class 3 or 412. BMI (kg/m2) was treated as a continuous variable. Comorbidity was measured with use of a validated comorbidity measure, the Deyo-Charlson index14. This is the most commonly used comorbidity measure, and it consists of a weighted scale of seventeen comorbidities (including cardiac, pulmonary, renal, and hepatic disease; diabetes; cancer; HIV; and others), expressed as a summative score15,16. A higher score indicates a greater number of disease conditions, and a score of zero indicates an absence of these disease conditions. Implant fixation was categorized as cemented or uncemented.
Statistical Analyses
Summary statistics for the frequency of intraoperative and postoperative periprosthetic fractures are reported as the mean and standard deviation and as proportions. Variables of interest assessed for their associations with both intraoperative and postoperative fractures included age, sex, BMI, the Deyo-Charlson index as the comorbidity measure, ASA class, implant fixation, and the underlying diagnosis.
For intraoperative humeral fractures during total shoulder arthroplasty, we performed univariate and multivariable logistic regression analyses. Only variables that were associated with intraoperative humeral fracture at p < 0.10 in the univariate analysis and for which data were available for most cases were considered for entry into the multivariable model. Inclusion of only significant factors was done to avoid overfitting the models, given that the number of events was forty. Since the ASA class was missing in 22% (563) of the 2588 cases, it was not included in the multivariable model, as its inclusion would likely have led to potential bias due to exclusion of 563 cases. For the subgroup of intraoperative humeral shaft fracture, we performed only univariate logistic regression analysis because of the small number of fractures.
The univariate analyses of postoperative humeral shaft fractures, a subset of all postoperative humeral fractures, were assessed with use of Cox regression. We assessed each univariate model by including one variable of interest for predicting postoperative humeral shaft fracture and adjusting for the type of surgery (total shoulder arthroplasty versus humeral head replacement). A multivariable model included as potential predictors those variables with a univariate significance of p < 0.10. The resulting multivariable model included a single predictor and therefore is not described further.
For the multivariable models, we anticipated a potential issue of collinearity between variables and set an a priori rule to exclude variables with correlation coefficients of 0.50 or higher. The correlation coefficients were as follows: Deyo-Charlson index and ASA class, 0.27; ASA class and age, 0.24; and Deyo-Charlson index and age, 0.03. Therefore, these variables were considered for inclusion in the multivariable models on the basis of significance in the univariate models. The hazard ratio and 95% confidence interval are reported. The alpha level was set at 0.05 for significance.
Kaplan-Meier survival analysis was used to obtain postoperative fracture-free survival estimates (with 95% confidence intervals) at five, ten, and twenty years after shoulder arthroplasty.
Source of Funding
The funding agencies (National Institutes of Health and Veterans Administration) did not play any role in protocol development, analysis, manuscript preparation, or the decision to submit the article for publication.
Results
Study Population Characteristics
The total shoulder arthroplasty cohort included 2207 patients with a total of 2588 primary shoulder arthroplasties performed between 1976 and 2008. The mean duration of follow-up was seven years (range, one day to thirty-one years) after the total shoulder arthroplasties. The mean age was sixty-five years (median, sixty-seven years); 53% were women, 63% had an underlying diagnosis of osteoarthritis, and 61% had an ASA class of 1 or 2 (see Appendix). Of the 2588 total shoulder arthroplasties, 96% (2485) were done with cemented implant(s); in one arthroplasty only the humeral component was cemented, and in five only the glenoid component was cemented.
The humeral head replacement cohort included 1349 patients with a total of 1431 shoulder arthroplasties, followed for a mean of eight years (range, one day to thirty-two years). The mean age was sixty-three years (range, eighteen to ninety-seven years; median, sixty-six years); 63% were women, 24% had an underlying diagnosis of osteoarthritis, and 49% had an ASA class of 1 or 2 (see Appendix).
The follow-up duration for the majority (98%) of the cohort (total shoulder arthroplasties and humeral head replacements combined) without any periprosthetic fracture of the cohort was adequate (two years or longer): it was more than two years for 78% (3150), one to two years for 6% (231), and less than one year for 16% (638).
Frequency of Periprosthetic Fractures
A total of 134 periprosthetic fractures were identified in association with total shoulder arthroplasty, and medical charts were missing for five patients. Of the remaining 129, only seventy-two were confirmed as fractures on medical record review (a 54% confirmation rate) since fifty-seven were glenoid fenestrations and not fractures (Table I). Of the forty-seven medical-record-confirmed intraoperative fractures, forty were humeral fractures (thirteen humeral shaft, twenty-one greater tuberosity, three humeral metaphysis, and three humeral head and neck fractures), five were glenoid fractures, and two fractures were at unknown sites in the shoulder. Of the twenty-five confirmed postoperative fractures, twenty were humeral fractures (all involving the humeral shaft), three were glenoid fractures, and two were at unknown sites. Glenoid fenestrations were not analyzed.
TABLE I.
Periprosthetic Shoulder Fractures Identified in the Total Joint Registry from 1976 to 2008
| Total Shoulder Arthroplasty (N = 134 Fractures in 133 Patients)* |
Humeral Head Replacement (N = 44 Fractures in 43 Patients)† |
|||||
| Intraop. | Postop. | Total | Intraop. | Postop. | Total | |
| Total fractures identified from joint registry (no.) | 99 | 35 | 134 | 21 | 23 | 44 |
| No fracture‡ | 0 | 0 | 0 | 4 | 3 | 7 |
| Confirmed fracture‡ | 47 | 25 | 72 | 15 | 18 | 33 |
| Glenoid fenestration‡ | 52 | 5 | 57 | 1 | 2 | 3 |
| Missing chart‡ | 0 | 5 | 5 | 1 | 0 | 1 |
| Details of confirmed fractures on medical record review (no.) | 47 | 25 | 72 | 15 | 18 | 33 |
| Any humeral fracture | 40 | 20 | 60 | 8 | 16 | 24 |
| Glenoid fracture | 5 | 3 | 8 | 7 | 2 | 9 |
| Site unclear | 2 | 2 | 4 | 0 | 0 | 0 |
| Type of humeral fracture | ||||||
| Humeral shaft fracture | 13 | 20 | 33 | 5 | 15 | 20 |
| Proximal humeral fracture | 24 | 0 | 24 | 3 | 1 | 4 |
| Greater tuberosity | 21 | 0 | 21 | 2 | 1 | 3 |
| Humeral head and neck | 3 | 0 | 3 | 1 | 0 | 1 |
| Humeral metaphysis fracture | 3 | 0 | 3 | 0 | 0 | 0 |
One patient with a total shoulder arthroplasty had a glenoid fenestration and a proximal humeral fracture intraoperatively.
One patient with a humeral head replacement had a glenoid fracture and a proximal humeral fracture intraoperatively.
All fractures coded in the total joint registry were validated by examining the medical records of each patient. The four categories show the number of fractures that could or could not be confirmed. For example, only seventy-two of the 134 fractures in the patients with total shoulder arthroplasty and thirty-three of the forty-four fractures in the patients with humeral head replacement were confirmed by medical record review.
Of the forty-four periprosthetic fractures identified in patients with humeral head replacement, only thirty-three could be confirmed by medical record review (a 75% confirmation rate) since medical records were not available for the dates of interest for one, seven patients had no fracture, and three had glenoid fenestration only (Table I). Of the fifteen medical-record-confirmed intraoperative fractures, eight were humeral fractures (five involved the humeral shaft; two, the greater tuberosity; and one, the humeral head and neck) and seven were glenoid fractures. Of the eighteen medical-record-confirmed postoperative fractures, sixteen were humeral fractures (fifteen were of the humeral shaft and one involved the greater tuberosity) and two were glenoid fractures.
Of all twenty-four proximal humeral fractures associated with total shoulder arthroplasty, seven were minimally displaced greater tuberosity fractures, which were repaired with sutures with no additional complications. One patient had a nondisplaced fracture in the back wall of the proximal portion of the humerus, which was repaired with a Steinmann pin without any complications. Two patients had a fracture that required reduction and use of sutures; one of them had a fracture of the posterior aspect of the greater tuberosity and the other had a fracture of both the lesser tuberosity and the greater tuberosity. One patient had a fracture of the anterior-inferior quadrant of the proximal part of the humerus that required bone graft and sutures. One patient had avulsion of the external rotators with a flake of bone intraoperatively, during trial implant placement, and this required repair of the external rotators to the bone with four interrupted sutures. The remaining twelve patients had a nondisplaced fracture of the greater tuberosity, without any additional intervention. None of the twenty-four patients had any additional complications or required additional surgery.
Similarly, no additional intervention or surgery was required for any of the four proximal humeral fractures in the patients with a humeral head replacement. Three patients had an intraoperative fracture: one of them had an undisplaced greater tuberosity fracture, one had a posterior-inferior metaphyseal fracture, and one had a metaphyseal fracture without capsulotendinous tissue. All were managed conservatively. One patient had a postoperative fracture of the proximal part of the humerus beneath and extending into the humeral head replacement twenty-three years after the humeral head replacement. Callus formation was noted at the time of presentation, and the fracture was managed without surgery.
Risk and Predictors of Intraoperative Periprosthetic Fractures During Total Shoulder Arthroplasty
There were forty intraoperative humeral fractures in the patients treated with total shoulder arthroplasty. Thirteen of them involved the humeral shaft; twenty-one, the greater tuberosity; three, the humeral head and neck; and three, the humeral metaphysis. In the univariate analyses, female sex and an ASA class of 3 or 4 were each significantly associated (p values of <0.05) with higher odds of intraoperative humeral fracture during total shoulder arthroplasty (Table II); cemented implant fixation (p = 0.07) and the underlying diagnosis (p = 0.08) had a nonsignificant association. In a multivariable analysis that included these variables (with a p value of <0.10), female sex remained significantly associated with a higher risk of intraoperative humeral fracture, the underlying diagnosis was now significantly associated with a higher risk (with the highest risk in patients with posttraumatic arthritis and no fractures in patients with acute trauma), and cement status was no longer associated (Table II).
TABLE II.
Factors Associated with Intraoperative Humeral Fractures (N = 40) During Total Shoulder Arthroplasty
| Univariate Logistic Regression Analysis |
Multivariable Logistic Regression Analysis |
|||||
| Intraop. Humeral Fracture* | No Intraop. Humeral Fracture* | Odds Ratio (95% Confidence Interval) | P Value† | Odds Ratio (95% Confidence Interval) | P Value† | |
| Age at surgery‡ (yr) | 68 ± 10 | 66 ± 23 | 1.27 (0.93, 1.72) | 0.13 | ||
| BMI§# (kg/m2) | 30 ± 7 | 30 ± 6 | 1.01 (0.96, 1.07) | 0.61 | ||
| Deyo-Charlson index§ | 0.6 ± 0.8 | 0.8 ± 1.4 | 0.92 (0.70, 1.20) | 0.52 | ||
| Sex | ||||||
| Male (n = 1236) | 7 (0.6%) | 1229 (99.4%) | 1.0 (ref) | 1.0 (ref) | ||
| Female (n = 1352) | 33 (2.4%) | 1319 (97.6%) | 4.39 (1.94, 9.97) | <0.001 | 4.19 (1.82, 9.62) | <0.001 |
| ASA class** | ||||||
| 1 or 2 (n = 1236) | 30 (2.4%) | 1206 (97.6%) | 1.0 (ref) | NA** | ||
| 3 or 4 (n = 780) | 9 (1.2%) | 771 (98.8%) | 0.47 (0.22, 0.99) | 0.048 | ||
| Implant fixation | ||||||
| No cement (n = 103) | 0 (0%) | 103 (100%) | 1.0 (ref) | 1.0 (ref) | 0.10 | |
| Cement (n = 2485) | 40 (1.6%) | 2445 (98.4%) | ∞ | 0.07 | ∞ | |
| Underlying diagnosis | 0.08 | 0.04 | ||||
| Rheumatoid/inflammatory arthritis (n = 452) | 7 (1.6%) | 445 (98.4%) | 1.0 (ref) | 1.0 (ref) | ||
| Acute trauma (n = 40) | 0 (0%) | 40 (100%) | 0 | 0 | ||
| Posttraumatic arthritis (n = 334) | 11 (3.3%) | 323 (96.7%) | 2.17 (0.83, 5.65) | 2.55 (0.92, 7.12) | ||
| Osteoarthritis (n = 1640) | 19 (1.2%) | 1621 (98.8%) | 0.75 (0.31, 1.78) | 0.79 (0.31, 2.03) | ||
| Other†† (n = 122) | 3 (2.5%) | 119 (97.5%) | 1.60 (0.41, 6.29) | 2.07 (0.50, 8.60) | ||
The values are given as the number (percent) of patients, except those for continuous variables (age, BMI, and Deyo-Charlson index), which are given as the mean and standard deviation.
Significant p values are in bold.
The odds ratios are for an increase in age by ten years.
The odds ratios are for each unit change.
The BMI was available from 9/1/87 to the time of the study.
The ASA class was available from 11/1/88 to the time of the study. Since it was missing in 22% (563) of the 2588 cases, it was not included in the multivariable model to avoid further bias. NA = not applicable.
Includes osteonecrosis, ankylosing spondylitis, psoriatic arthritis, gout, Charcot arthropathy, dislocation, old injury, and a history of septic arthritis.
A subgroup of these intraoperative humeral fractures were humeral shaft fractures (n = 13), which were analyzed separately with univariate logistic regression analyses as well. All intraoperative humeral shaft fractures occurred in women (Table III). A higher BMI showed a nonsignificant association with intraoperative humeral shaft fracture (p = 0.10). The underlying diagnosis was not significantly associated.
TABLE III.
Factors Associated with Intraoperative Humeral Shaft Fractures (N = 13) During Total Shoulder Arthroplasty as Demonstrated by Univariate Analysis
| Intraop. Humeral Fracture* | No Intraop. Humeral Fracture* | Odds Ratio (95% Confidence Interval) | P Value | |
| Age at surgery† (yr) | 69 ± 8 | 66 ± 12 | 1.03 (0.98, 1.09) | 0.29 |
| BMI‡§ (kg/m2) | 32 ± 9 | 30 ± 6 | 1.07 (0.99, 1.15) | 0.10 |
| Deyo-Charlson index‡ | 0.5 ± 0.8 | 0.8 ± 1.4 | 0.78 (0.42, 1.45) | 0.43 |
| Sex | ||||
| Male (n = 1236) | 0 | 1236 (100%) | 1.0 (ref) | <0.001 |
| Female (n = 1352) | 13 (1.0%) | 1339 (99.0%) | ∞ | |
| ASA class# | ||||
| 1 or 2 (n = 1236) | 9 (0.7%) | 1227 (99.3%) | 1.0 (ref) | |
| 3 or 4 (n = 780) | 4 (0.5%) | 776 (99.5%) | 0.70 (0.22, 2.29) | 0.56 |
| Implant fixation | ||||
| No cement (n = 103) | 0 | 103 (100%) | 1.0 (ref) | 0.30 |
| Cement (n = 2485) | 13 (0.5%) | 2472 (99.5%) | ∞ | |
| Underlying diagnosis | 0.96 | |||
| Rheumatoid/inflammatory arthritis (n = 452) | 2 (0.4%) | 450 (99.6%) | 1.0 (ref) | |
| Acute trauma (n = 40) | 0 (0%) | 40 (100%) | 0 | |
| Posttraumatic arthritis (n = 334) | 1 (0.3%) | 333 (99.7%) | 0.68 (0.06, 7.48) | |
| Osteoarthritis (n = 1640) | 9 (0.5%) | 1631 (99.5%) | 1.24 (0.27, 5.77) | |
| Other** (n = 122) | 1 (0.8%) | 121 (99.2%) | 1.86 (0.17, 20.68) |
The values are given as the number (percent) of patients, except those for continuous variables (age, BMI, and Deyo-Charlson index), which are given as the mean and standard deviation.
The odds ratio is for an increase in age by ten years.
The odds ratio is for each unit change.
The BMI was available from 9/1/87 to the time of the study.
The ASA class was available from 11/1/88 to the time of the study.
Includes osteonecrosis, ankylosing spondylitis, psoriatic arthritis, gout, Charcot arthropathy, dislocation, old injury, and a history of septic arthritis.
Survivorship Analyses of Postoperative Humeral Periprosthetic Fractures
There were twenty postoperative humeral fractures after total shoulder arthroplasty and sixteen postoperative humeral fractures after humeral head replacement. The patients with a total shoulder arthroplasty had a humeral-fracture-free survival rate (and 95% confidence interval) of 99.5% (99.2, 99.8) at five years, 98.9% (98.4, 99.5) at ten years, and 97.1% (95.2, 99.1) at twenty years (Fig. 1).
Fig. 1.
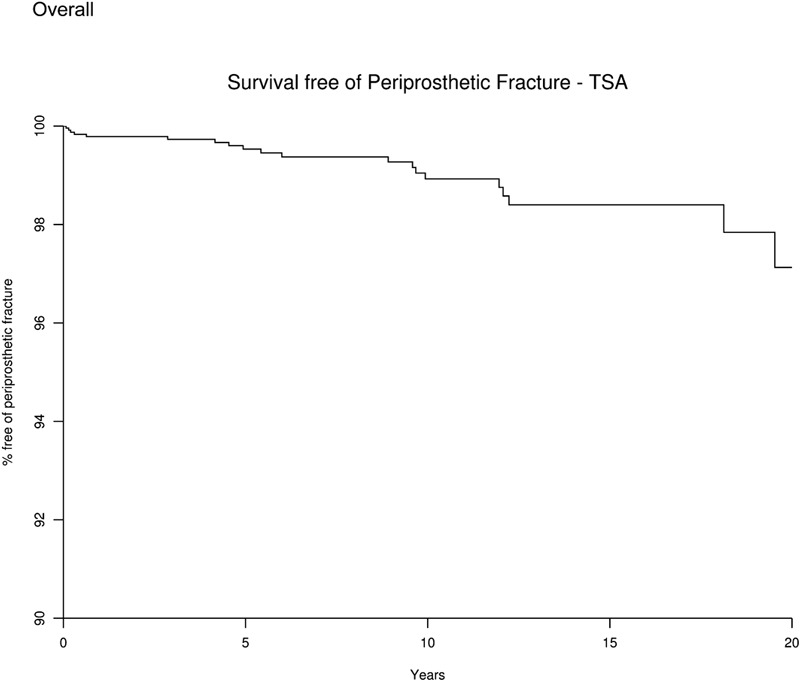
Survival curve showing the percentage of patients free of periprosthetic fracture after total shoulder arthroplasty (TSA). The x axis represents the number of years since the total shoulder arthroplasty, and the y axis shows the percentage of patients without periprosthetic fracture.
For the patients with humeral head replacement, the humeral-fracture-free survival rage (and 95% confidence interval) was 99.2% (98.6, 99.7) at five years, 98.6% (97.9, 99.4) at ten years, and 97.8% (96.4, 99.3) at twenty years (Fig. 2). Fracture-free survival at five, ten, and twenty years according to clinical characteristics in the total shoulder arthroplasty and humeral head replacement cohorts is shown in the Appendix.
Fig. 2.
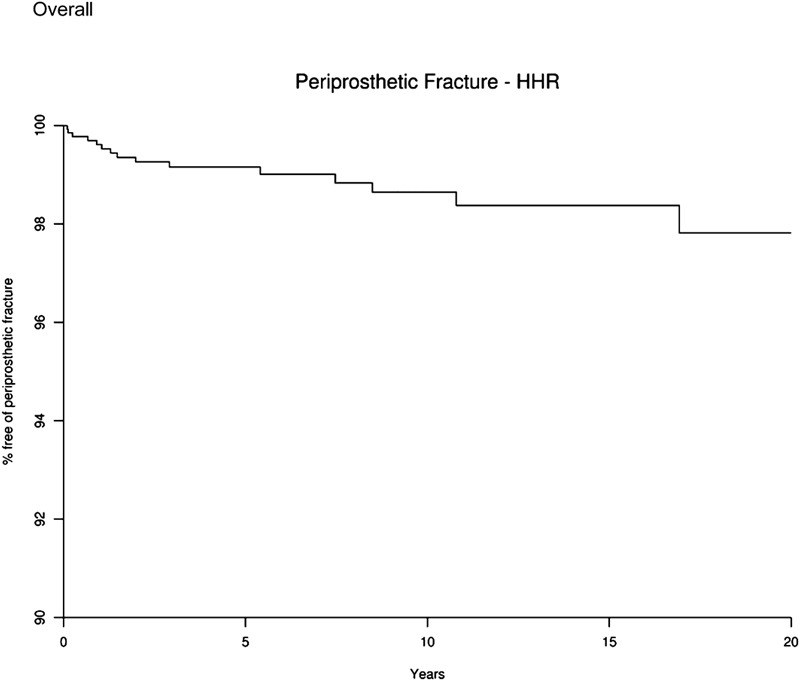
Survival curve showing the percentage of patients free of periprosthetic fracture after humeral head replacement (HHR). The x axis represents the number of years since the humeral head replacement, and the y axis shows the percentage of patients without periprosthetic fracture.
Predictors of Postoperative Humeral Shaft Periprosthetic Fractures
Multivariable analyses were performed for thirty-five postoperative humeral shaft fractures by combining them across the total shoulder arthroplasty and humeral head replacement groups. (All twenty postoperative humeral fractures after total shoulder arthroplasty were humeral shaft fractures, and fifteen of the sixteen postoperative humeral fractures after humeral head replacement were humeral shaft fractures). A higher Deyo-Charlson index was significantly associated with an increased risk of postoperative periprosthetic humeral shaft fracture (hazard ratio, 1.27 per unit increase in score; 95% confidence interval, 1.11 to 1.45; p < 0.001) (Table IV). Older age showed a nonsignificant association with postoperative humeral shaft fracture (p = 0.13). Diagnosis was not significantly associated with the risk of postoperative periprosthetic humeral shaft fracture.
TABLE IV.
Factors Associated with Postoperative Humeral Shaft Fractures (N = 35) After Total Shoulder Arthroplasty or Humeral Head Replacement*
| Univariate Cox Regression† |
|||
| No. of Postop. Humeral Shaft Fractures | Hazard Ratio (95% Confidence Interval) | P Value | |
| Age at surgery, per 10-yr increase | 35 | 1.28 (0.93, 1.75) | 0.13 |
| BMI‡ | 23 | 0.96 (0.86, 1.06) | 0.38 |
| Deyo-Charlson index, per 1-unit change | 35 | 1.27 (1.11, 1.45) | <0.001 |
| Sex | |||
| Female (n = 2250) | 23 | 1.43 (0.71, 2.86) | 0.32 |
| Male (n = 1769) | 12 | 1.0 (ref) | |
| ASA class§ | |||
| 1 or 2 (n = 1725) | 11 | 1.0 (ref) | |
| 3 or 4 (n = 1279) | 9 | 1.25 (0.51, 3.04) | 0.63 |
| Implant fixation | |||
| No cement (n = 677) | 4 | 1.0 (ref) | |
| Cement (n = 3342) | 31 | 0.64 (0.30, 1.35) | 0.24 |
| Underlying diagnosis | 0.48 | ||
| Rheumatoid/inflammatory arthritis (n = 685) | 6 | 1.0 (ref) | |
| Acute trauma (n = 305) | 2 | 0.81 (0.16, 4.03) | 0.80 |
| Posttraumatic arthritis (n = 562) | 7 | 1.66 (0.56, 4.93) | 0.36 |
| Osteoarthritis (n = 1988) | 14 | 1.22 (0.46, 3.21) | 0.69 |
| Other# (n = 479) | 6 | 2.07 (0.68, 6.30) | 0.20 |
| Type of surgery** | |||
| Humeral head replacement (n = 1431) | 15 | 1.0 (ref) | |
| Total shoulder arthroplasty (n = 2588) | 20 | 0.76 (0.38, 1.49) | 0.42 |
Each variable was adjusted for the type of surgery (total shoulder arthroplasty versus humeral head replacement).
All models included the type of surgery (total shoulder arthroplasty versus humeral head replacement) as a predictor in addition to the variable listed in the table.
The BMI was available from 9/1/87 to the time of the study.
The ASA class was available from 11/1/88 to the time of the study; therefore the total number of fractures is less than thirty-five.
Includes osteonecrosis, ankylosing spondylitis, psoriatic arthritis, gout, Charcot arthropathy, dislocation, old injury, and a history of septic arthritis.
This model included only the type of surgery as a predictor.
Discussion
We studied more than 2500 primary total shoulder arthroplasties and 1400 humeral head replacements performed over a thirty-three-year period at a single institution with a mean of seven years of follow-up. The rate of intraoperative humeral fractures was 1.2% (forty-eight of 4019) and the rate of postoperative humeral fractures was 0.9% (thirty-six of 4019). There were very few glenoid fractures (0.4% [seventeen of 4019] overall; 0.3% [twelve] intraoperative and 0.1% [five] postoperative). Female sex and the underlying diagnosis were significantly associated with a higher odds of intraoperative humeral fractures during total shoulder arthroplasty. Female sex was significantly associated with a higher odds of intraoperative humeral fracture during total shoulder arthroplasty, with 2.4% of women versus 0.6% of men having an intraoperative humeral fracture. We also found that greater comorbidity (a higher Deyo-Charlson index) was associated with a significantly increased risk of postoperative periprosthetic humeral shaft fractures after total shoulder arthroplasty and humeral head replacement. For example, a hazard ratio of 1.27 of postoperative humeral fracture for a 1-unit increase in the Deyo-Charlson index would be interpreted as a patient with dementia having a 27% higher hazard of postoperative humeral fracture compared with a patient without dementia.
The strengths of this study are its large sample size and the long duration of prospective follow-up of all patients included in an institutional registry. A particular strength of this study is the confirmation of fracture occurrence, the time of its occurrence (intraoperatively or postoperatively), and the confirmation of each fracture by a review of medical records. It was reassuring that we could confirm 96% of all periprosthetic fractures recorded in the total joint registry.
This study has several limitations. The data are from a single institution and, therefore, generalizability to other cohorts may be limited; however, this cohort appears to be representative of patients undergoing shoulder arthroplasty. Replication of these findings in other cohorts will strengthen their validity. Even with a large sample over a thirty-three-year period, in several instances the number of postoperative fractures was not adequate to perform multivariable-adjusted analyses. This problem is most likely related to the rarity of this complication. Another limitation is that several patients were lost to follow-up, and the results may be generalizable only to patients who continue to be followed for years after their shoulder replacement. We may have underestimated the number of postoperative fractures, since some patients were lost to follow-up. Another reason for underestimation may be related to an intermediate follow-up duration of seven years in this study. A longer follow-up will likely demonstrate a higher frequency of fractures. The two factors most likely contributing to a median follow-up duration that was lower than expected are a higher annual arthroplasty volume in the latter years and loss to follow-up despite systematic follow-up. Very few total shoulder arthroplasties were performed with uncemented implants (4% versus 40% of the humeral head replacements), which likely led to unstable estimates; however, cement status was not associated with the risk of intraoperative fractures. We did not extract the exact time of fracture for the intraoperative fractures; if we had, it may have provided us with information to potentially prevent them in the future. Nevertheless, the clinical relevance of many of the intraoperative fractures was minimal. Loss to follow-up was likely minimized by active prospective data collection with clinical follow-up supplemented by data collection by the registry staff contacting every patient (by mail and/or telephone) with a shoulder arthroplasty who failed to return for a follow-up visit.
The intraoperative fracture rate in our study was similar to the prevalences in two previously published series, with shorter time periods of observation, from our institution. In those studies, 1.2% of all primary shoulder arthroplasties performed from 1980 to 20027 and 1.4% of those done with a cemented all-polyethylene glenoid component from 1990 to 20004 were associated with an intraoperative fracture. The postoperative humeral fracture rate (0.9%) in our study is comparable with rates reported in other studies (range, 1.6% to 2.4%)1,2,6,17 and in a systematic review of forty studies (1.2%) with a total of 3584 patients5. In a previous study from our institution, the use of a press-fit humeral component was associated with a borderline significantly increased risk of intraoperative humeral fracture (p = 0.046) in a cohort treated with total shoulder arthroplasty, humeral head replacement, or revision shoulder arthroplasty from 1980 to 20027. In our study of a cohort consisting of only primary total shoulder arthroplasties and humeral head replacements, done from 1976 to 2008, cement status was not a significant factor in either the univariate (p = 0.07) or the multivariable analyses (p = 0.10); this was consistent with differences between the two cohorts. An important observation in our study was that most of the intraoperative fractures did not require special treatment and therefore did not substantially impact postoperative management and outcomes.
Female sex and the underlying diagnosis (especially posttraumatic arthritis) were significantly associated with four-times and two-times higher odds of intraoperative humeral fracture during total shoulder arthroplasty, respectively. This finding is consistent with our previous study's finding of a significantly increased risk of intraoperative humeral fractures in women treated from 1980 to 2002 with total shoulder arthroplasty, humeral head replacement, or revision shoulder arthroplasty7. There are several potential explanations for this increased risk in women, including the fact that they have a higher prevalence of osteoporosis and rheumatoid arthritis, both of which are risk factors for periprosthetic fractures18,19. Similarly, we also found that female sex was associated with a higher risk of intraoperative humeral shaft fractures, a subset of all humeral fractures. Additional studies should be performed to investigate whether any modifiable risk factor in women can be identified for targeted interventions to reduce the fracture risk. Our finding of an association of the underlying diagnosis with a higher risk of intraoperative humeral fracture during total shoulder arthroplasty adds to the literature and seems to be related to a higher risk in those with posttraumatic arthritis. We found that increasing comorbidity was associated with an increased risk of postoperative humeral shaft fracture after total shoulder arthroplasty and humeral head replacement. This finding is not surprising since higher comorbidity indicates more frailty and use of multiple pharmaceutical agents, both of which may be associated with a higher risk of falls and fractures20-23. A small sample size prevented us from examining individual comorbidities (diabetes, heart disease, etc.) as risk factors.
In summary, we found a low rate of intraoperative and postoperative periprosthetic humeral fractures. Most intraoperative fractures were clinically irrelevant and did not require substantial postoperative management. Female sex and underlying diagnosis were significantly associated with a higher risk of intraoperative fractures, and comorbidity was significantly associated with a higher risk of postoperative fractures. Intraoperative fracture prevention may be more realistic than postoperative fracture prevention, although modifiable factors for both should be examined for targeted interventions in the future.
Appendix
Tables showing the clinical and demographic characteristics of the study population and the univariate risk factors for postoperative periprosthetic fractures after humeral head replacement and total shoulder arthroplasty as well as figures showing survival curves by sex and age group are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Tables showing the clinical and demographic characteristics of the study population and the univariate risk factors for postoperative periprosthetic fractures after humeral head replacement and total shoulder arthroplasty as well as figures showing survival curves by sex and age group
Footnotes
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Worland RL, Kim DY, Arredondo J. Periprosthetic humeral fractures: management and classification. J Shoulder Elbow Surg. 1999;8:590-4 [DOI] [PubMed] [Google Scholar]
- 2.Cameron B, Iannotti JP. Periprosthetic fractures of the humerus and scapula: management and prevention. Orthop Clin North Am. 1999;30:305-18 [DOI] [PubMed] [Google Scholar]
- 3.Tammachote N, Sperling JW, Vathana T, Cofield RH, Harmsen WS, Schleck CD. Long-term results of cemented metal-backed glenoid components for osteoarthritis of the shoulder. J Bone Joint Surg Am. 2009;91:160-6 [DOI] [PubMed] [Google Scholar]
- 4.Chin PY, Sperling JW, Cofield RH, Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15:19-22 [DOI] [PubMed] [Google Scholar]
- 5.van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35:426-34 [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86:680-9 [DOI] [PubMed] [Google Scholar]
- 7.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am. 2009;91:594-603 [DOI] [PubMed] [Google Scholar]
- 8.Steinmann SP, Cheung EV. Treatment of periprosthetic humerus fractures associated with shoulder arthroplasty. J Am Acad Orthop Surg. 2008;16:199-207 [DOI] [PubMed] [Google Scholar]
- 9.McDonough EB, Crosby LA. Periprosthetic fractures of the humerus. Am J Orthop (Belle Mead NJ). 2005;34:586-91 [PubMed] [Google Scholar]
- 10.Berry DJ, Kessler M, Morrey BF. Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res. 1997;344:61-8 [DOI] [PubMed] [Google Scholar]
- 11.Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88:508-13 [DOI] [PubMed] [Google Scholar]
- 12.Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178:261-6 [DOI] [PubMed] [Google Scholar]
- 13.Weaver F, Hynes D, Hopkinson W, Wixson R, Khuri S, Daley J, Henderson WG. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18:693-708 [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-9 [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-83 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Sax FL, MacKenzie CR, Braham RL, Fields SD, Douglas RG., Jr Morbidity during hospitalization: can we predict it? J Chronic Dis. 1987;40:705-12 [DOI] [PubMed] [Google Scholar]
- 17.Thomas SR, Wilson AJ, Chambler A, Harding I, Thomas M. Outcome of Copeland surface replacement shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14:485-91 [DOI] [PubMed] [Google Scholar]
- 18.Boyd AD, Jr, Thornhill TS, Barnes CL. Fractures adjacent to humeral prostheses. J Bone Joint Surg Am. 1992;74:1498-504 [PubMed] [Google Scholar]
- 19.Campbell JT, Moore RS, Iannotti JP, Norris TR, Williams GR. Periprosthetic humeral fractures: mechanisms of fracture and treatment options. J Shoulder Elbow Surg. 1998;7:406-13 [DOI] [PubMed] [Google Scholar]
- 20.Kojima T, Akishita M, Nakamura T, Nomura K, Ogawa S, Iijima K, Eto M, Ouchi Y. Association of polypharmacy with fall risk among geriatric outpatients. Geriatr Gerontol Int. 2011;11:438-44 [DOI] [PubMed] [Google Scholar]
- 21.Lai SW, Liao KF, Liao CC, Muo CH, Liu CS, Sung FC. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore). 2010;89:295-9 [DOI] [PubMed] [Google Scholar]
- 22.Baranzini F, Diurni M, Ceccon F, Poloni N, Cazzamalli S, Costantini C, Colli C, Greco L, Callegari C. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Serv Res. 2009;9:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziere G, Dieleman JP, Hofman A, Pols HA, van der Cammen TJ, Stricker BH. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61:218-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
Tables showing the clinical and demographic characteristics of the study population and the univariate risk factors for postoperative periprosthetic fractures after humeral head replacement and total shoulder arthroplasty as well as figures showing survival curves by sex and age group


