Abstract
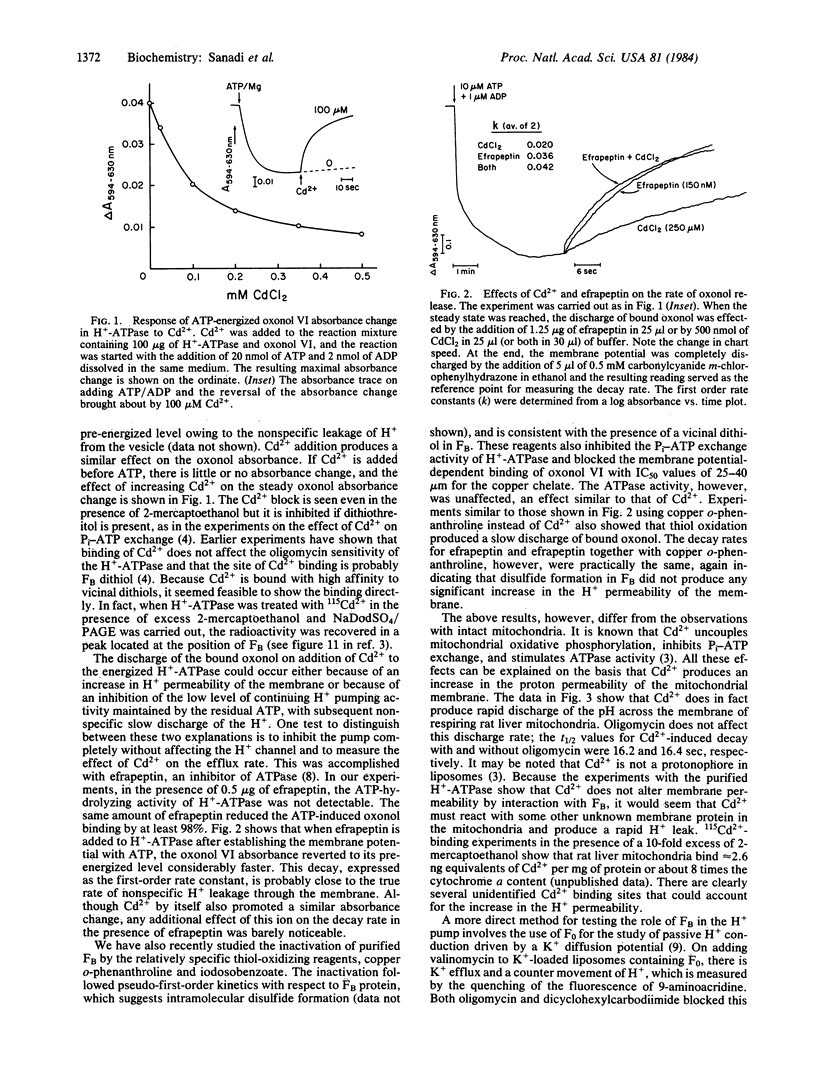
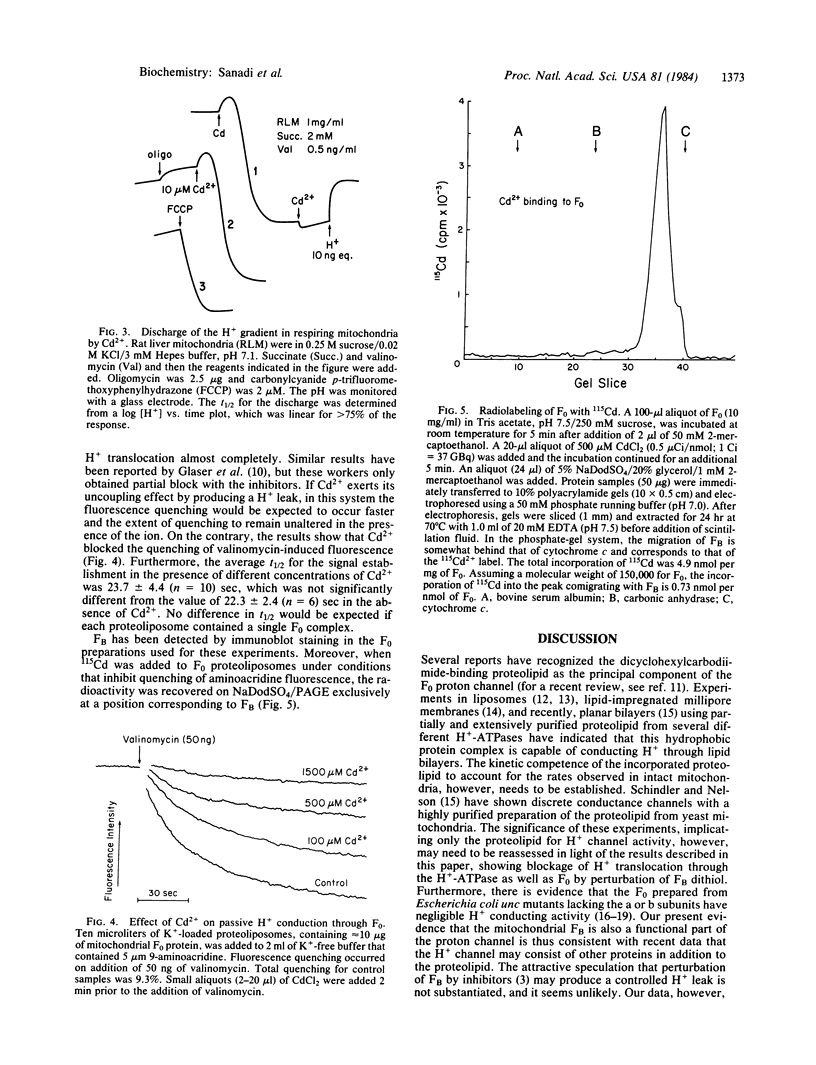
Membrane energization by ATP has been measured in vesicles containing purified bovine heart mitochondrial H+-ATPase (ATP synthase) with the voltage-sensitive dye oxonol VI. The dithiol chelator, Cd2+, and the thiol oxidant, copper o-phenanthroline, produced discharge of the membrane potential when added at the steady state and inhibited its establishment when added prior to energization by ATP. These effects, which were reversed by dithiothreitol, were not accompanied by an increase in the nonspecific H+ permeability of the membrane. Passive H+ conduction in proteoliposomes containing F0 (hydrophobic segment of ATP synthase) was assayed by the quenching of 9-aminoacridine fluorescence after establishing a K+ diffusion potential. This conductance was blocked by Cd2+, an inhibitor of coupling factor B (FB). Labeling of F0 with 115Cd2+ at the concentrations that inhibited the F0 conductance followed by gel electrophoresis yielded a single radioactive band with a molecular weight corresponding to FB, the presence of which in the F0 preparation was confirmed by immunoblot staining. The data offer strong evidence that FB is an essential component of the H+ channel of F0, because H+ conduction through the channel is inhibited by chemical modification of FB.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Criddle R. S., Packer L., Shieh P. Oligomycin-dependent ionophoric protein subunit of mitochondrial adenosinetriphosphatase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4306–4310. doi: 10.1073/pnas.74.10.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Célis H. 1-Butanol extracted proteolipid. Proton conducting properties. Biochem Biophys Res Commun. 1980 Jan 15;92(1):26–31. doi: 10.1016/0006-291x(80)91514-4. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Mosher M. E., Negrin R. S., Peters L. K. H+-ATPase of Escherichia coli uncB402 mutation leads to loss of chi subunit of subunit of F0 sector. J Biol Chem. 1983 Jan 10;258(1):604–609. [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Friedl P., Bienhaus G., Hoppe J., Schairer H. U. The dicyclohexylcarbodiimide-binding protein c of ATP synthase from Escherichia coli is not sufficient to express an efficient H+ conduction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6643–6646. doi: 10.1073/pnas.78.11.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Hoppe J., Gunsalus R. P., Michelsen O., von Meyenburg K., Schairer H. U. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 1983;2(1):99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser E., Norling B., Ernster L. Reconstitution of mitochondrial oligomycin and dicyclohexylcarbodiimide-sensitive ATPase. Eur J Biochem. 1980 Sep;110(1):225–235. doi: 10.1111/j.1432-1033.1980.tb04859.x. [DOI] [PubMed] [Google Scholar]
- Hughes J., Joshi S., Torok K., Sanadi D. R. Isolation of a highly active H+-ATPase from beef heart mitochondria. J Bioenerg Biomembr. 1982 Dec;14(5-6):287–295. doi: 10.1007/BF00743058. [DOI] [PubMed] [Google Scholar]
- Joshi S., Hughes J. B. Inhibition of coupling factor B activity by cadmium ion, arsenite-2,3-dimercaptopropanol, and phenylarsine oxide, and preferential reactivation by dithiols. J Biol Chem. 1981 Nov 10;256(21):11112–11116. [PubMed] [Google Scholar]
- Joshi S., Hughes J. B., Shaikh F., Sanadi D. R. On the role of coupling factor B in the mitochondrial Pi-ATP exchange reaction. J Biol Chem. 1979 Oct 25;254(20):10145–10152. [PubMed] [Google Scholar]
- Loo T. W., Bragg P. D. The DCCD-binding polypeptide alone is insufficient for proton translocation through F0 in membranes of Escherichia coli. Biochem Biophys Res Commun. 1981 Nov 16;103(1):52–59. doi: 10.1016/0006-291x(81)91659-4. [DOI] [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E. Mechanisms of energy transformations. Annu Rev Biochem. 1977;46:1006–1014. doi: 10.1146/annurev.bi.46.070177.005042. [DOI] [PubMed] [Google Scholar]
- Schindler H., Nelson N. Proteolipid of adenosinetriphosphatase from yeast mitochondria forms proton-selective channels in planar lipid bilayers. Biochemistry. 1982 Nov 9;21(23):5787–5794. doi: 10.1021/bi00266a010. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Resolution of the membrane moiety of the H+-ATPase complex into two kinds of subunits. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4219–4223. doi: 10.1073/pnas.75.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susa J. B., Lardy H. A. Antibiotics as tools for metabolic studies. XVIII. Inhibition of sodium- and potassium-dependent adenosine triphosphatase. Mol Pharmacol. 1975 Mar;11(2):166–173. [PubMed] [Google Scholar]



