Abstract
α-fetoprotein (AFP) producing adenocarcinoma of endometrium is a rare tumour. It is mostly high grade and has poor prognosis. Lung metastases are common. In this article, the authors present a case of a 57-year-old woman with AFP producing adenocarcinoma of endometrium and history of bilateral metachronous breast cancer, with lung, subcutaneous and brain metastases.
Background
α-fetoprotein (AFP) is a fetal serum protein synthesised by fetal liver, yolk sac and gastrointestinal tract. After birth, AFP disappears from the circulation.1 Finding of AFP after birth is abnormal and mostly due to hepatocellular carcinoma, yolk sac tumour, but recent studies have indicated that AFP producing adenocarcinoma may originate from somatic cells of various origin. These included prostate, lung, stomach, colon, ovary, kidney, pancreas, billiary tract, duodenum, kidney, bladder, ovary, vagina and uterus.1–3 In this article, we present a woman with endometroid adenocarcinoma of uterus producing high level of AFP.
Case presentation
A 57-year-old woman was referred to our gynaecology oncology clinic due to 2 months of abnormal vaginal bleeding. The patient had a history of bilateral metachoronous breast cancer 9 and 7 years ago. Since both of these tumours were hormone positive, the patient was taking tamoxifen for over 5 years in a private clinic. Unfortunately however, she did not underwent regular pelvic examination and Pap smear test. Pelvic examination and vaginal sonography revealed increasing of endometrial thickness about 18 mm. Abdominopelvic CT scanning showed enlarged uterus with a 20 mm mass in the endometrium. The gynaecologist oncologist decided to perform surgery. Reported chest x-ray (CXR) was normal and laboratory tests were as follows: CA125=11.96 U/ml, β human chorionic gonadotropin=4.57 mlU/ml, lactate dehydrogenase=351U/ml (all of them being in normal range) but AFP=465.3 ng/ml.
Differential diagnosis
Primary yolk sac tumour of uterus and ovarian yolk sac tumour with endometrial metastasis are in differential diagnosis however, pathologic feature in this case ruled out them.
Treatment
Total abdominal hysterectomy and bilateral salpigo-oophorectomy were performed. Pathology showed a 5 cm mass originated from endometrium. Microscopic examination (figure 1) showed endometrioid adenocarcinoma grade III with more than 50% involvement of uterus wall and extension to cervical stroma. Pelvic lymph nodes and omental sampling did not show any evidence of malignancy. According to these findings the disease consisted of Figo stage II. The patient was referred to our centre for adjuvant radiation therapy. The patient was fairly well and repeated AFP before starting radiation therapy and 1 month after surgery was 188.32 ng/ml. Considering half life of AFP (about 5–7 days), we expected this level to became less than 30 ng/ml after this period and it could mean a residual disease however, physical examination and imaging did not show any abnormal findings at the time. We started radiation therapy using two parallel opposed anterior posterior/posterior anterior pelvic fields with 9MV photon. In the fifth week of treatment course the patient had cough and dyspnoea, and repeated CXR and thoracic CT scanning (figure 2) showed multiple pulmonary metastases, and repeated physical examination showed two attached subcutaneous masses in her buttock. Therefore, the treatment was discontinued and she was referred for chemotherapy. At this time AFP was 6464 ng/ml and CA125 was 77.6 U/ml.
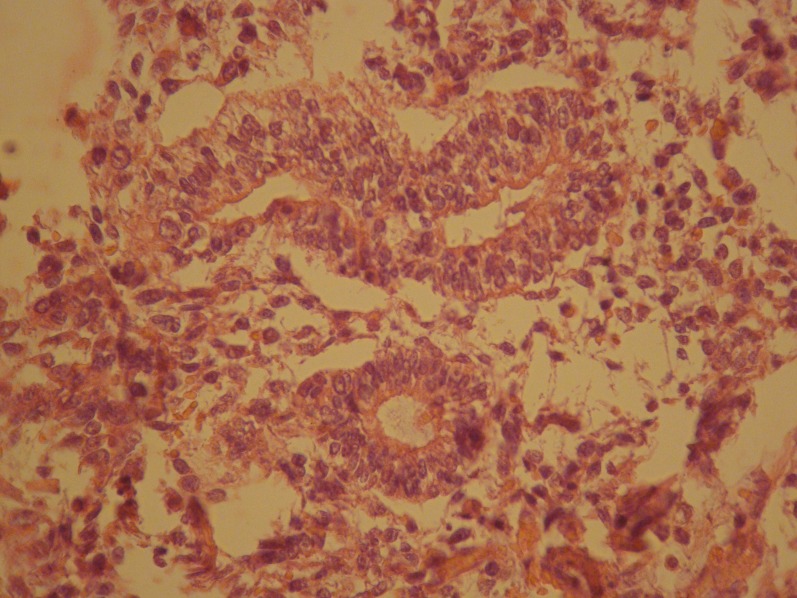
Figure 1.
Section shows that the tumour forms well-defined glands and shows moderate cytologic atypia, stroma is loose.
Figure 2.
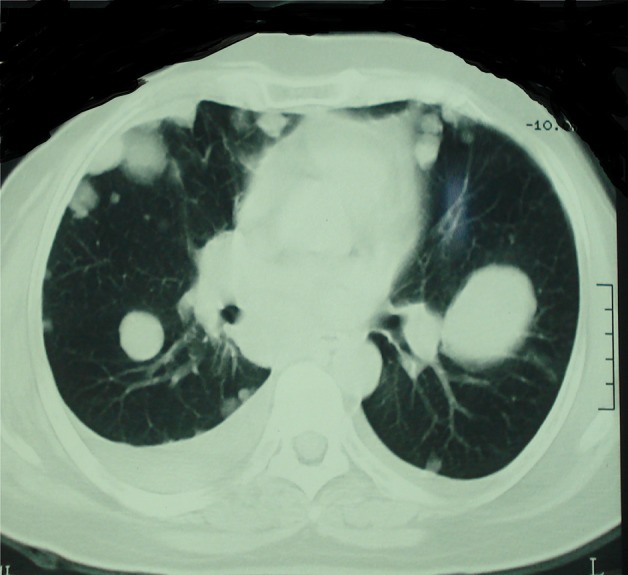
Thoracic CT scan shows multiple lung metastases.
Outcome and follow-up
After the first course of taxane based chemotherapy AFP level reached to more than 9000 ng/ml but with continuing the same regimen her pulmonary symptoms subsided but radiologic response was less than 50% and surprisingly after four courses AFP became 2 ng/ml (normal). However, after that and before starting the next chemotherapy course the patient had headache, blurred vision and right hand weakness and two episodes of seizure. Brain MRI (figure 3) showed multiple brain metastases and brain radiotherapy (3000cGy) was performed. Four weeks later, her AFP level was 600 ng/ml. The patient deceased 6 weeks later due to progressive central nervous system complications.
Figure 3.
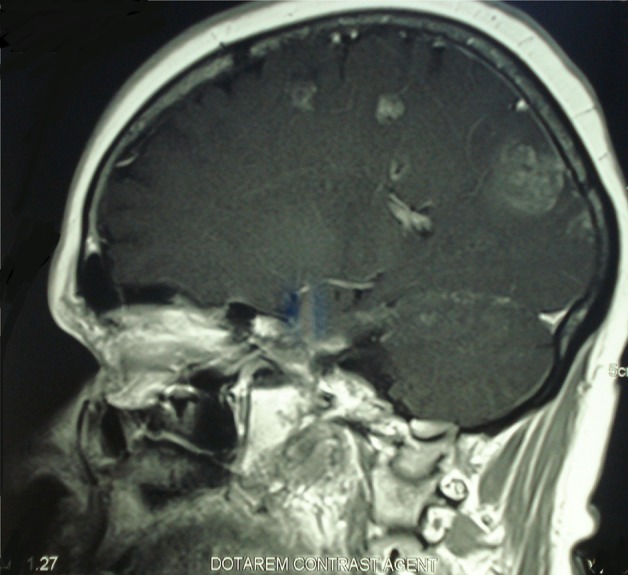
Brain MRI shows multiple brain metastases.
Discussion
AFP is a significant serum protein with a half life of 5.8 days being synthesised during fetal life, and the expression of the AFP gene is dramatically reduced after birth.4 AFP is synthesised only to a very limited extent in normal adult tissues. Besides hepatocellular carcinoma and yolk sac tumour adenocarcinoma from different origin can produce AFP.1–4 Hepatoid carcinomas are a rare group of extrahepatic tumours and have been found in the stomach, pancreas, kidney, bladder and ovary. These tumours show liver differentiation and produce fetoprotein,5 however the cases of endometrial adenocarcinoma producing AFP are very rare. Reports of AFP producing adenocarcinoma in other parts of female genital tracts such as ovary, fallopian tube and cervix were reviewed by El-Bahrawy.6 Hepatoid carcinoma should essentially be diagnosed on the basis of histological features of the tumour. It is characterised by the medullary or papillotubular arrangement of tumour cells with eosiophilic and granular cytoplasm, thus resembling hepatocellular carcinoma.4 But in some cases such as ours and the case reported by Kodama et al4 there was not any evidence of hepatoid differentiation despite AFP production. AFP producing adenocarcinoma can be carcinomatous part of a malignant mixed mullerian tumour.3 5 7 In most studies, these tumours were seen in women in their 60s but an 83-year-old woman was reported by Ishibashi et al.8 Most of them were high grade1–3 5 although a case reported by Kodama et al4 was not high grade. Lung metastases are common, and the disease has poor prognosis,1–3 5 and tumours recurred or lead to death within 12 months of diagnosis in most of cases,8 however few patients with long-time survival have been reported.4 8 In all patients total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed. Various chemotherapy regimens used were as follows: cycophosphamide, adriamycin, cisplatin,1 2 8 carboplatin, paclitaxel,3 6 carboplatin, paclitaxel, therarubicin,4 carboplatin, adriamycin5 and oral etoposide.9 Radiation therapy was performed in some cases.9 To our knowledge, it is the first case of AFP producing adenocarcinoma of endometrium with brain and subcutaneous metastases and history of bilateral breast cancer in which the patient was taking tamoxifen for more than 5 years. In our patient, despite the level of AFP being very high it became normal temporarily after chemotherapy, however the disease progressed and brain metastases occurred and AFP was raised once again.
Learning points.
AFP producing adenocarcinoma of endometrium is a rare poor prognostic disease.
It can metastasis to lung and brain and subcutaneous.
Pathology may not show hepatoid feature.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Yamamoto R, Ishikura H, Azuma M, et al. Alpha-fetoprotein production by a hepatoid adenocarcinoma of the uterus. J Clin Pathol 1996;49:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toyoda H, Hirai T, Ishii E. Alpha-fetoprotein producing uterine corpus carcinoma: A hepatoid adenocarcinoma of the endometrium. Pathol Int 2000;50:847–52. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi R, Furukawa N, Yamada Y, et al. Carcinosarcoma of the uterine corpus with alpha-fetoprotein-producing hepatoid adenocarcinoma: a report of two cases. Case Rep Oncol 2011;4:358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodama J, Seki N, Yanai H, et al. α-fetoprotein-producing endometrial adenocarcinoma without an obvious hepatoid component. Oncology letters 2010;1:243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips KA, Scurry JP, Toner G. Alpha-fetoprotein production by a malignant mixed müllerian tumour of the uterus. J Clin Pathol 1996;49:349–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Bahrawy M. Alpha-fetoprotein-producing non-germ cell tumours of the female genital tract. Eur J Cancer 2010;46:1317–22. [DOI] [PubMed] [Google Scholar]
- 7.Takano M, Shibasaki T, Sato K, et al. Malignant mixed Mullerian tumor of the uterine corpus with alpha-fetoprotein-producing hepatoid adenocarcinoma component. Gynecol Oncol 2003;91:444–8. [DOI] [PubMed] [Google Scholar]
- 8.Ishibashi K, Kishimoto T, Yonemori Y, et al. Primary hepatoid adenocarcinoma of the uterine corpus: A case report with immunohistochemical study for expression of liver-enriched nuclear factors. Pathol Res Pract 2011;207:332–6. [DOI] [PubMed] [Google Scholar]
- 9.Adams SF, Yamada SD, Montag A, et al. An alpha-fetoprotein-producing hepatoid adenocarcinoma of the endometrium. Gynecol Oncol 2001;83:418–21. [DOI] [PubMed] [Google Scholar]