Abstract
Aberrant repetitive behaviors are commonly observed in a variety of neurodevelopmental, neurological, and neuropsychiatric disorders. Little is known about the specific neurobiological mechanisms that underlie such behaviors, however, and effective treatments are lacking. Valid animal models can aid substantially in identifying pathophysiological factors mediating aberrant repetitive behavior and aid in treatment development. The C58 inbred mouse strain is a particularly promising model, and we have further characterized its repetitive behavior phenotype. Compared to C57BL/6 mice, C58 mice exhibit high rates of spontaneous hindlimb jumping and backward somersaulting reaching adult frequencies by 5 weeks post-weaning and adult temporal organization by 2 weeks post-weaning. The development of repetitive behavior in C58 mice was markedly attenuated by rearing these mice in larger, more complex environments. In addition to characterizing repetitive motor behavior, we also assessed related forms of inflexible behavior that reflect restricted and perseverative responding. Contrary to our hypothesis, C58 mice did not exhibit increased marble burying nor did they display reduced exploratory behavior in the holeboard task. The C58 strain appears to be a very useful model for the repetitive motor behavior characteristic of a number of clinical disorders. As an inbred mouse strain, studies using the C58 model can take full advantage of the tool kit of modern genetics and molecular neuroscience. This technical advantage makes the model a compelling choice for use in studies designed to elucidate the etiology and pathophysiology of aberrant repetitive behavior. Such findings should, in turn, translate into effective new treatments.
Keywords: repetitive behavior, stereotypy, environmental enrichment
1. INTRODUCTION
Repetitive behaviors (e.g., motor stereotypies) are diagnostic for autism spectrum disorders and commonly observed in a variety of other neurodevelopmental disorders such as Rett, Fragile X, and Prader-Willi syndromes [1,2]. Such behaviors are also associated with other conditions including congenital blindness [3] and early social impoverishment [4]. Various forms of repetitive behavior have also been reported in a significant percentage of individuals without cognitive impairment or neurological or psychiatric disorder [5–7]. There is also ample documentation that repetitive behavior is common in normative development as well (e.g., [8,9]).
Currently, relatively little is known about the specific neurobiological mechanisms that underlie repetitive behavior. Clinical studies have provided only very limited findings based on a small number of neuroimaging and genetic studies that have been conducted (reviewed in [2]). In addition, there is little evidence for the efficacy of pharmacotherapy for repetitive behaviors in individuals with neurodevelopmental disorders (e.g., [10]).
Animal models with the requisite validity could aid substantially in identifying pathophysiological factors associated with aberrant repetitive behavior and aid in the development of effective treatments. Most animal models relevant to repetitive behavior in neurodevelopmental disorders generally fall into three categories (reviewed in [11]). The first includes repetitive behavior associated with developmental, typically genetic, insults to the central nervous system (CNS) such as the Hoxb8 homozygous mutant mouse [12] and the Sapap3 knockout mouse [13]. Much of what we know about the neurobiology of repetitive behavior comes from studies of repetitive behavior induced by pharmacological agents such as the induction of motor stereotypies by psychostimulants including amphetamine and cocaine (e.g., [14]). The third category encompasses repetitive behavior observed in a wide range of species that is induced by rearing in restricted or impoverished environments [15].
Although reared in the restricted confines of standardized laboratory rodent housing, differences in both the form and level of aberrant repetitive behavior have been reported in specific inbred mouse strains. For example, Nevison et al. [16] examined cage stereotypies across multiple inbred mouse strains nightly for four weeks. C57BL/6J mice were observed to engage in stereotypy only 0.5% of intervals scored, the least of any of the inbred strains studied whereas CBA/Cas mice showed the most stereotypies (9.7% of intervals observed). Several groups have examined repetitive behavior in the BTBR inbred strain as this mouse has been shown to exhibit a number of autistic-like traits [17]. Pearson et al. [18] have replicated previous reports (e.g., [17]) of elevated levels of self-grooming in BTBR mice as well as shown higher levels of cage bar biting in this strain compared to C57BL/6 mice. Deacon et al. [19] anecdotally reported repetitive hindlimb jumping in C57BL/10 mice compared to C57BL/6 mice (a difference that we have quantified and replicated; unpublished observations). Repetitive hindlimb jumping was also observed by Moy et al. [20] in the C58 inbred strain in the context of standard behavioral tests (i.e., a social approach test and habituation to a T-maze). Persistent back-flipping was observed in the home cage environment in older C58 mice. In a subsequent publication [21], this same research group reported repetitive back-flipping in C58 dams as well as higher levels of self-grooming compared to C57BL/6 dams. Repetitive jumping emerged in C58 offspring by PND 16–17. Repetitive jumping was observed in 69% to 88% of adolescent and young adult C58 mice during tests of olfactory discrimination, social approach, and social transmission of food preference.
The C58 inbred strain model is promising as the repetitive behaviors appear to occur spontaneously (i.e., do not require a specific stimulus for elicitation), early in development, and persist over time. Moreover, these behaviors (vertical hindlimb jumping and backward somersaulting) are qualitatively or topographically atypical for this species as well as quantitatively excessive. Finally, because these repetitive behaviors are expressed in an inbred mouse strain, they become amenable to molecular genetic analysis. Moreover, comparisons can be made to the closely-related C57BL/6 strain, which exhibits little aberrant repetitive behavior. Thus, in the present study, we sought to expand characterization of the repetitive behavior of C58 mice using C57BL/6 mice for comparison purposes. This expanded characterization included an automated quantitative measurement of both the frequency and temporal organization of the repetitive behavior over the full dark cycle of the animal. In addition, we sought to evaluate further reported strain differences in self-grooming [21]. We also assessed the behavior of C58 mice in an exploratory task (holeboard) as well as in the marble burying test. We also examined C58 mice longitudinally in order to plot the developmental trajectory of repetitive behavior in the post-weaning period. Finally, we tested the hypothesis that rearing C58 mice in a larger, more complex environment would significantly attenuate the development of repetitive motor behavior.
2. MATERIAL AND METHODS
2.1 Animals
Two cohorts of mice were used for these studies. Cohort 1 was ordered from Jackson Laboratory and arrived at approximately 6 weeks post-weaning. Forty C58 mice (20 males, 20 females) and 40 C57BL/6 mice (20 mice, 20 females) were used for repetitive behavior testing (at approximately 8 weeks post-weaning). Of these mice, 16 mice (8 males, 8 females) of each strain were tested in the holeboard exploration test and 24 mice (12 male, 12 female) of each strain were tested in the marble burying paradigm.
The C58 mice in cohort 2 were from our breeding colony, which was established from mice acquired from the University of North Carolina. Developmental trajectories and temporal organization of repetitive behavior were tested in 14 C58 mice (9 males, 5 females) and compared to 12 C57BL/6 mice (Jackson Laboratories; 6 males, 6 females). Analysis of grooming duration was completed with these mice at 6 weeks post-weaning. The environmental enrichment study used only C58 mice from cohort 2 (9 males, 2 females in standard cages; 6 males, 7 females in environmental enrichment).
2.2 Housing conditions for the environmental enrichment study
2.2.1 Standard cage
Animals randomly assigned to standard cage conditions following weaning were placed with one or two same-sex mice into mouse cages (29 × 18 × 13 cm) with room temperature maintained within a range of 70–75F, and a 12:12 light:dark cycle, with lights off at 8 PM. Food and water were available ad lib, and birdseed (1 oz.) was placed in one corner of the cage each week on the same schedule as the enriched cages. Two Nestlet squares were added for nest construction.
2.2.2 Environmental enrichment
The environmental enrichment protocol used in this experiment was similar to our previous work [22–26]. Animals assigned to environmental enrichment were placed with five other same-sex weanlings in customized large dog kennels (121.9 × 81.3 × 88.9 cm), each with a similar design consisting of two extra levels constructed from galvanized wire mesh and connected by ramps of the same material to create three interconnected levels. Bedding, four Nestlets, a running wheel, a shelter (inverted baking pan or similar opaque, concave object), and various other objects (Habitrail tubes, plastic toys, mesh structures for climbing) were placed in each kennel prior to introducing the mice. Throughout the 42 day experimental phase, the shelter, the habitrails, and running wheel remained undisturbed in the kennel, but other objects (except those in which mice were hiding) were removed and replaced with novel objects on a weekly basis. Food and water were available ad lib, and a small quantity (approximately 1 oz.) of bird seed was scattered throughout the kennel each week to encourage foraging behaviors.
All animals for the enrichment study were housed within the same room with maintenance and transportation performed during the light cycle to minimize stress and human contact. All animals within a particular enclosure were tested at the same time to minimize the effects of changes in social density and the stress of handling.
2.3 Housing conditions for all other studies
Up to 8 same sex mice were housed in cages (29 × 18 × 13 cm) with room conditions the same as described above. Food and water were available ad lib, and two Nestlet squares were provided for nest construction.
2.4 Measurement of stereotypy
The stereotypy observed in C58 inbred mice consists largely of two response topographies: jumping and backward somersaulting [20,21]. The former topography involves the animal rearing against the cage wall and engaging in vertical hindlimb jumping. The second topography (backward somersaulting) involves the animal rotating its body such that it starts with all four paws on the cage floor, inverts its ventral surface to the cage top, and returns to the cage floor, upright and on all four paws. As these behaviors involve vertical activity, they were quantified using photobeam arrays which, when interrupted, recorded a count. These photobeams are located high enough to avoid recording counts for rearing, drinking, eating, or any other behavior that does not include all four paws leaving the ground. We routinely video-recorded test sessions in order to identify the topography of stereotypy, insure accuracy of the automated counters, and measure the occurrence of non-stereotyped behavior.
Measurement of stereotypy was done in six standardized test cages using the automated apparatus described. The automated data collection method captured the real time value associated with each photobeam interruption (i.e., jump or backward somersault) which allowed characterization of the temporal distribution of individual repetitive behaviors as described in Tanimura et al. [27]. We also employed a video surveillance system that allows digital recording of each automated test cage during the entire 12 hour dark cycle. The system makes use of a DVR capture card (GeoVision) and acquires images at 240 frames per second which provided a high resolution per individual cage (ca. 40 fps). This permitted precise determination of the individual behavior of the animals and precise estimates of the reliability of the automated apparatus. The testing protocol involved removing mice from their home cages and placing them singly in standard testing cages (22 × 15 × 28 cm) one hour prior to the beginning of the dark cycle. Food and water were provided, and the mice were left undisturbed to allow for habituation. Each animal was assessed for the 12 hours of the dark cycle over the subsequent test day.
2.5 Measurement of grooming
As described previously, a video surveillance system that allows digital recording of each automated test cage during the entire 12 hour dark cycle was used. The videos were analyzed for grooming of 14 C58 (5 females, 9 males) mice and 12 C57BL/6 (6 females, 6 males) mice at two selected time intervals: a five minute time sample at one hour and six hours after the start of the dark cycle. The presence of grooming was scored for each one minute interval to measure bout frequency. The bout duration of the first six episodes of grooming that followed these times were analyzed. Grooming was defined as cleaning the coat or paws with either the paws or the tongue. If the behavior the animal was engaging in was not obvious, because its body was turned away from the camera or the motion was not clear enough, the subsequent episode of grooming was analyzed.
2.6 Exploration Task (Holeboard)
To assess strain differences in exploration, a 35cm × 41cm × 41cm Plexiglas box chamber was used with a plastic base and Plexiglas sides. A metal floorboard with 16 equidistant holes (4 rows with 4 holes per row) was placed in the box over a hard plastic base that contained 16 indented spaces that were vertically aligned with the holes of the metal piece. The holes were all 3 cm deep. A camera, placed over the holeboard, detected and video-tracked the mouse placed in the chamber using Ethovision 2.3 (Noldus). Holes were numbered from top left hole to bottom right hole, indicating four corner holes (1, 4, 13, 16), eight wall holes (2, 3, 5, 8, 9, 12), and four center holes (6, 7, 10, 11).
Holeboard testing was conducted 2–3 hours into the light cycle for both inbred strains (n=16/strain, 8 males, 8 females) over four consecutive days. On each day, eight mice were tested (two males, two females of each strain) for 30 min. each. The holeboard was cleaned thoroughly with ethanol and allowed to dry between test sessions.
2.7 Marble-burying
For the marble burying test a separate cohort of 23 C58 (12 females, 11 males) and 24 C57BL/6 (12 females, 12 males) were used. Animals were tested in Plexiglas cages (21cm × 25cm × 29cm) filled 1 inch deep with Sanichips bedding. Food and water were not available. Twenty blue glass marbles of 1.4 cm diameter were placed equidistantly in each Plexiglas testing cage with 5 rows of 4 marbles. Four mice were tested at a time with four testing cages, each containing 1 mouse of the same strain and sex, placed touching each other in a 2 × 2 form. A video camera was placed on a tripod, over the 4 cages, and pointed down to record the animal’s behavior.
The marble burying test was conducted several hours into the light cycle in a separate testing room. Thirty minutes before testing, the animals were moved into the testing room from the colony room for habituation. After the 30-minute habituation period, 1 mouse was placed in each of the experimental cages and was video-recorded for 30 minutes. After 30 minutes, the animals were returned to their home cages. The number of marbles buried in the bedding was recorded using the criterion of at least two-thirds of the marble had to be covered by bedding in order for it to be counted as buried. Counts were agreed upon by two experimenters. Once data were collected, the marbles were retrieved from the cages and cleaned using dish soap and water. The marbles were dried before re-using. From the video taped testing sessions, the duration of repetitive behavior (jumping, backward somersaulting, and upright scrabbling) performed during the 30 minute test was scored.
2.8 Statistical Analyses
Frequency of repetitive behavior, frequency and duration of grooming bouts, holeboard exploration, and marble burying were analyzed by two-way (sex x strain) analysis of variance (ANOVA). The association between marble burying and repetitive behavior was analyzed by Pearson correlation, and developmental trajectory of repetitive behavior was analyzed by repeated measures ANOVA (RM-ANOVA) and Bonferroni post-tests. Temporal organization of repetitive behaviors (as revealed by y-scores) was analyzed as described in Tanimura et al. [27]. Y-score changes across time were analyzed by one-way RM-ANOVA. Differences in repetitive behavior and y-scores between mice reared in enriched or standard cages was analyzed by two-tailed t-tests because of uneven representation of each sex in the different housing conditions. Effects were considered significant when p<0.05.
3. RESULTS
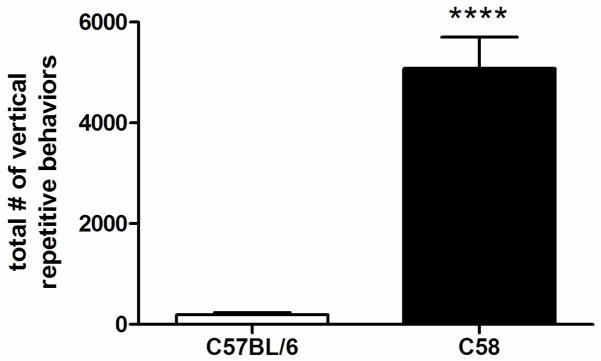
At 8 weeks postweaning, C58 mice exhibited substantial frequencies of repetitive behavior involving, on average, about 5,000 individual stereotypic responses, largely vertical hindlimb jumping during the 12 hour testing session (Fig. 1). Significant differences in levels of repetitive behavior were found between C58 and C57BL/6 mice (F(1,28)=53.05, p<0.0001). There was no significant main effect for sex.
Figure 1.
Total repetitive behavior counts taken over 12 hours at 8 weeks post-weaning. C58 mice show significantly higher rates of repetitive behavior compared to C57BL/6 mice. Values are group means + standard error of the mean (SEM). **** represents statistical significance at p<0.0001.
Performance in the holeboard apparatus is displayed in Fig. 2. C58 mice demonstrated significantly more exploration, measured by number of nosepokes, compared to C57BL/6 mice (F(1,28)=11.69, p<0.01; Fig. 2a). In addition, females exhibited more nosepokes than males (F(1,28)=5.09, p<0.05), though there was no significant strain x sex interaction. This effect was mediated by the higher rates of center hole visits by the C58 mice (F(1,28)=13.89, p<0.001; Fig. 2b). There was no significant effect of sex or stain x sex interaction. Also, there was no significant strain difference in percent of nosepokes into the surround holes (Fig. 2c), yet a significant effect of sex was found (F(1,28)=12.91, p<0.01), whereby females visited the surround holes more often than did the males. There was no significant strain x sex interaction effect. C58 mice showed a significantly lower percentage of nosepokes into holes in the corner of the apparatus (F(1,28) = 15.23, p<0.001; Fig. 2d). There was also a significant effect of sex on corner hole visits (F(1,28)=8.393, p<0.01) where males visited more often than the females. There was no significant interaction effect, however.
Figure 2.
Behavior in the holeboard exploration task. C58 mice exhibit significantly more nosepokes in the holeboard apparatus when compared to C57BL/6 mice (A). When analyzed by holeboard apparatus region, C58 mice display more nosepokes in the center holes (B), equal percentage of nosepokes in the surround holes (C), and fewer nosepokes in the corner holes (D). Values are group means + SEM. ** and *** represent statistical significance at p<0.01 and p<0.001, respectively.
Behavior during the marble burying test is exhibited in Fig. 3. There were no significant differences in the number of marbles buried between C58 and C57BL/6 mice (Fig. 3a). There was also no significant effect of sex. However, the number of marbles buried by C58 mice was significantly negatively correlated with the amount of repetitive behavior displayed during the marble burying task (r = −0.67, p<0.001; Fig. 3b).
Figure 3.
Behavior in the marble burying test. There were no significant differences in the number of marbles buried between C58 and C57BL/6 mice (A), though the amount of repetitive behavior during the marble burying test was negatively associated with the number of marbles buried in C58 mice (B). Values in A are group means + SEM.
The development of repetitive behavior in C58 mice, in comparison to C57BL/6 mice, is displayed in Fig. 4a. Data include repetitive behaviors exhibited during the entire 12 hour test session. An examination of the development of repetitive behavior in C58 mice revealed significant main effects of strain (F(1,120) = 33.33, p<0.0001) and time (F(5,120) = 13.63, p<0.0001), as well as a significant strain x time interaction (F(5,120) = 9.11, p<0.0001), when compared to C57BL/6 mice. Temporal organization analysis of C58 repetitive behavior revealed decreasing y-scores across early development, which corresponds to increasing temporal organization of behavior (Fig. 4b). A one-way RM-ANOVA revealed a significant change over time (F(5,60) = 10.738, p<0.0001). This effect was mediated by a strong decrease in y-scores after week 1. A one-way RM-ANOVA on the y-score data from weeks two through six was not significant. There were no discernible sex differences in these measures of repetitive behavior development.
Figure 4.
Development and temporal organization of repetitive behavior. Repetitive behavior significantly increases in C58 mice, relative to C57BL/6 mice, throughout early post-weaning development (A). Tests for repetitive behavior lasted 12 hours. Temporal organization of the repetitive behavior in C58 mice becomes more patterned, as evidenced by lower y-scores, during the early post-weaning period (B). All values are group means ± SEM. ** and **** represent statistical significance at p<0.01 and p<0.0001, respectively.
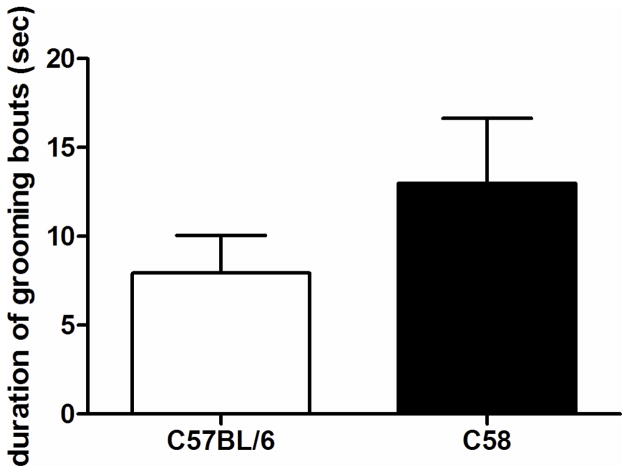
Grooming bout duration in C58 and C57BL/6 mice is represented in Fig. 5. There were no significant differences in grooming bout duration in either strain at 6 weeks post-weaning. The number of one minute intervals in which grooming was exhibited was higher in C58 compared to C57BL/6 mice (average of 4.4 versus 2.9 intervals out of 10 total), though this was not statistically significant (data not shown). There was also no significant effect of sex on these grooming measures.
Figure 5.
Duration of grooming bouts in C58 and C57BL/6 mice. There were no significant differences in grooming bout length between C58 and C57BL/6 mice when tested at 6 weeks post-weaning.
The effect of environmental enrichment on repetitive behavior expression in C58 mice is depicted in Fig. 6. Environmental enrichment significantly attenuated the expression of repetitive behavior in C58 mice, when compared to frequencies of repetitive behavior in litter-matched controls raised in standard laboratory caging (t(22) = 5.62, p < 0.0001). Data include repetitive behaviors exhibited during the entire 12 hour test session. Furthermore, the temporal organization of the repetitive behavior was also altered by environmental enrichment. The repetitive behavior of the C58 mice that were reared in enriched environments was significantly less organized, resulting in higher y-scores, than the behavior of the C58 mice reared in standard laboratory cages (t(22) = 5.111, p<0.0001).
Figure 6.
Repetitive behavior in C58 mice raised in standard cages or in environmental enrichment. Environmental enrichment significantly lessened the expression of repetitive behavior in C58 mice (A). Tests for repetitive behavior lasted 12 hours. Environment enrichment also altered the temporal organization of the repetitive behavior (B). Values are group means + SEM. **** represents statistical significance at p<0.0001.
4. DISCUSSION
In the present study, we extended previous work on the characterization of the repetitive behavior of C58 mice using C57BL/6 mice as controls. As previously reported, we observed that C58 mice engaged in spontaneous bouts of repetitive hindlimb jumping and backward somersaulting as well as upright scrabbling [20,21]. A quantitative measure of the frequencies of jumping and somersaulting showed a monotonic increase to adult levels of repetitive behavior by five weeks post-weaning. By this point in development, the average frequency of repetitive behavior exceeded 10,000 individual responses over the 12 hour dark cycle (Fig. 4a). In addition to frequency, we also provided a quantitative measure of the temporal organization of these repetitive behaviors. When assessed across development, this measure exhibited a more rapid onset to adult levels reaching asymptotic levels by two weeks post-weaning. The development of repetitive behavior in C58 mice was also very much dependent on environmental factors as rearing these mice for the first six weeks post-weaning in a larger, more complex environment dramatically attenuated the development of repetitive behavior. Finally, we found elevated levels of self-grooming in C58 mice compared to C57BL/6 controls although this difference failed to reach statistical significance.
The two major topographies of repetitive motor behavior observed in the C58 strain are identical to the two major topographies we have reported in deer mice (Peromyscus maniculatus) (e.g., [27]). Consistent with Ryan et al. [21] we also observed what they termed “upright scrabbling” in which the mouse appears to be running or climbing in place against the side of the test cage. We have only rarely observed this behavior in deer mice. Interestingly, in a comprehensive comparison of C57BL/10 and C57BL/6 mice on a wide variety of behavioral tasks, Deacon et al. [19] reported vertical hindlimb jumping in C57BL/10 but not C57BL/6 mice. We have replicated this observation although our quantitative assessments indicated that while identical in form to C58 mice, the repetitive behavior in C57BL/10 mice occurred at only about half the frequency.
The developmental trajectory of stereotypy in the C58 was strikingly similar to what we have observed in deer mice [27]. We initiated our quantitative evaluation of stereotypy at the end of the first week of the post-weaning period (PND 28). Ryan et al. [21] reported observing hindlimb jumping although at very low levels as early as PND 16/17 in the context of an open field test. Future studies with this strain should include quantitative assessment of repetitive behavior prior to PND 28, including measurement of these behaviors prior to weaning. Moreover, an analysis of the variability in developmental trajectories of repetitive behavior should be undertaken using group based trajectory modeling methods [27].
We did not observe a statistically significant strain difference in self-grooming (bout presence or duration) at 6 weeks post-weaning. Similarly, Ryan et al. [21] reported no difference in grooming duration between C58 and C57BL/6 mice either in the presence of the dam or alone in an open field at 20–21 days of age. They did observe, however, a significantly higher number of self-grooming bouts in C58 dams. When we restricted our analysis and tested for strain differences in self-grooming only in females, we found longer bout duration in C58 females, though this did not reach statistical significance. A comparison of C57BL/10, which exhibit motor stereotypies, with C57BL/6 mice also failed to show a significant difference in self-grooming [19].
Although we had hypothesized that environmental enrichment would attenuate the development, expression, and organization of stereotyped behavior in C58 mice, the magnitude of this effect was surprising. We have reported substantial enrichment effects on stereotyped behavior in deer mice (e.g., [22–26]) and other groups have demonstrated the efficacy of enrichment for stereotypy in other species including zoo and farm animals [15]. In these cases, the stereotyped behavior was inferred to be a consequence of environmental restriction. Although standard laboratory caging of C58 mice clearly constitutes environmental restriction, the repetitive behavior phenotype of the C58 strain appears to be far more robust in form and frequency than observed in most other inbred mouse strains. Thus, there appears to be a genotype by environment interaction mediating the repetitive behavior of the C58 mice.
In addition to characterizing repetitive motor behavior, we also assessed related forms of inflexible behavior that reflect restricted and perseverative responding. Contrary to our hypothesis, C58 mice did not exhibit increased marble burying nor did they display reduced exploratory behavior in the holeboard task. Marble-burying behavior has been suggested to be a reflection of the tendency for repetitive digging behavior that is perseverative and highly resistant to change [28]. Thus, it may serve as a good test for “higher order” restricted, repetitive behavior. Our results did not support the hypothesized increase in marble burying in C58 mice. Indeed, we observed that this strain did not differ from C57BL/6 mice in the number of marbles buried, and the smaller number of marbles buried by C58 mice was likely due to interference by engagement in motor stereotypies. The significant inverse correlation between stereotypy and marble burying supports this conclusion. Interestingly, in a comparison of C57BL/10 mice which exhibit motor stereotypies and C57BL/6 mice, Deacon et al. [19] reported no strain difference in marble burying. It may be that marble burying reflects a more compulsive or perseverative phenotype which may be independent of repetitive motor behavior in these animal models. Alternatively, C58 mice dig less than C57BL/6 mice do [21], which may confound the testing of the marble burying task. Given the potential of decreased proclivity for digging and repetitive behavior interference in the C58 mice, the marble burying task may not be a valid test in this model.
Moy et al. [29] suggested that a holeboard task may be useful in characterizing inbred mouse strains in terms of repetitive, restricted behaviors. Compared to C57BL/6 mice, we did not find any evidence for restricted or inflexible exploration in the holeboard test. Indeed, C58 mice explored a significantly greater number of holes than control mice. This effect was mediated by more visits to center holes by C58 mice, although they also made fewer visits to corner holes, compared to C57BL/6 mice. The higher number of nosepokes is likely due to the significantly higher mean velocity and total distance traveled in the holeboard that C58 mice exhibited compared to the C57BL/6 strain. This is consistent with previous reports of less resting behavior [30] and greater distance traveled [21] in C58 mice in an open field test, compared to C57BL/6 mice [30]. Meandering or the change in direction of movement of the mouse relative to the distance it moves also had non-significant results for strain differences suggesting no difference in the inflexibility in the pattern of exploration (data not shown). The lack of significant differences for these measures implies that motor stereotypy does not translate to increased levels of restricted or invariant patterns of exploration. Thus, neither the number of holes explored nor meandering were significantly related to the motor stereotypy scores calculated for the C58 mice.
In order to evaluate whether C58 mice exhibited resistance to change, we attempted to compare them to C57BL/6 mice in a reversal learning test using a water T-maze. C58 mice exhibited significant difficulty in swimming in a coordinated fashion and frequently submerged themselves. Thus, we did not pursue this measure. Interestingly, Moy et al. [20] reported that C58 mice had markedly poor swimming ability in the Morris water maze. In addition, in assessing reversal learning in an appetitive T-maze task, this group found that C58 mice had poor acquisition and those few mice that met criterion for learning failed to meet criterion for reversal learning. Related to the difficulties in swimming, we have observed lack of coordination and balance in C58 mice during some rearing episodes after 16 weeks post-weaning. Interestingly, Deacon et al. [19] reported that C57BL/10 mice were impaired on tests that required coordination and balance compared to C57BL/6 mice. It is unclear, for either C58 or C57BL/10 mice, whether difficulties in coordination and balance are related to stereotyped motor movements.
5. CONCLUSIONS
The C58 inbred mouse strain appears to be a very useful model particularly for the repetitive motor behavior characteristic of autism spectrum and related neurodevelopmental disorders. It is less clear whether this model will have additional value as an animal model of the “higher order” repetitive behaviors characterized by resistance to change or insistence on sameness. Additional studies will be needed to evaluate this component of the model. As an inbred mouse strain, studies with the C58 model can take full advantage of the complete tool kit of modern genetics and molecular neuroscience. This technical advantage makes it a compelling choice for use in studies designed to elucidate the genetic etiology of aberrant repetitive behavior as well as the pathophysiology of repetitive behavior. Finally, our work mapping the development of repetitive behavior and its marked attenuation by environmental enrichment suggests that the C58 strain may be a useful model for examination of gene by environment interactions in the mediation of the development and expression of repetitive behavior including the role of epigenetic mechanisms. It should be mentioned, however, that C58 mice develop lymphomas and leukemia as they mature. Thus, to avoid potential confounds, use of younger adult animals would be prudent.
Highlights.
C58 mice provide a useful model for repetitive motor behavior
The frequency of repetitive behavior increases during early post-weaning development
The organization of the repetitive behavior becomes fixed at 2 weeks post-weaning
Environmental enrichment significantly reduces repetitive behavior in C58 mice
Acknowledgments
This project was funded by Autism Speaks. We also thank Dr. Sheryl Moy and Dr. James Bodfish for their contributions to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Ment Retard Dev Disabil Res Rev. 1998;4:80–89. [Google Scholar]
- 2.Lewis MH, Kim SJ. The pathophysiology of restricted repetitive behavior. J Neurodev Disord. 2009;1:114–32. doi: 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fazzi E, Lanners J, Danova S, Ferrarri-Ginevra O, Gheza C, Luparia A, et al. Stereotyped behaviours in blind children. Brain Dev. 1999;21:522–28. doi: 10.1016/s0387-7604(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 4.Bos KJ, Zeanah CH, Smyke AT, Jr, Fox NA, Nelson CA., 3rd Stereotypies in children with a history of early institutional care. Arch Pediat Adol Med. 2010;164:406–11. doi: 10.1001/archpediatrics.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellanos FX, Ritchie GF, Marsh WL, Rapoport JL. DSM-IV stereotypic movement disorder: Persistence of stereotypies of infancy in intellectually normal adolescents and adults. J Clin Psychiat. 1996;57:116–22. [PubMed] [Google Scholar]
- 6.Rafaeli-Mor N, Foster L, Berkson G. Self-reported body-rocking and other habits in college students. Am J Ment Retard. 1999;104:1–10. doi: 10.1352/0895-8017(1999)104<0001:SBAOHI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Singher HS. Motor stereotypies. Semin Pediatr Neurol. 2009;16:77–81. doi: 10.1016/j.spen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Evans DW, Leckman JF, Carter A, Reznick JS, Henshaw D, King RA, Pauls D. Ritual, habit, and perfectionism: the prevalence and development of compulsive-like behavior in normal young children. Child Dev. 1997;68:58–68. [PubMed] [Google Scholar]
- 9.Thelen E. Determinants of amounts of stereotyped behavior in normal human infants. Ethol Sociobiol. 1980;1:141–50. [Google Scholar]
- 10.King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–90. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- 13.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper SJ, Dourish CT, editors. Neurobiology of Stereotyped Behavior. New York: Oxford University Press; 1990. [Google Scholar]
- 15.Mason G, Rushen J, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. 2. Cambridge, MA: CABI Publishing; 2006. [Google Scholar]
- 16.Nevison C, Hurst J, Barnard C. Strain specific effects of cage enrichment in male laboratory mice (Mus musculus) Anim Welfare. 1999;8:361–79. [Google Scholar]
- 17.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 18.Pearson B, Pobbe R, Defensor E, Oasay L, Bolivar V, Blanchard D, et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deacon R, Thomas C, Rawlins J, Morley B. A comparison of the behavior of C57BL/6 and C57BL/10 mice. Behav Brain Res. 2007;179:239–47. doi: 10.1016/j.bbr.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Moy S, Nadler J, Young N, Nonneman R, Segall S, Andrade G, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–29. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–88. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner CA, Yang MC, Lewis MH. Environmental enrichment: Effects of stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- 23.Turner CA, Lewis MH, King MA. Environmental enrichment: Effects of stereotyped behavior and dendritic morphology. Dev Psychobiol. 2003;43:20–7. doi: 10.1002/dev.10116. [DOI] [PubMed] [Google Scholar]
- 24.Turner CA, Lewis MH. Environmental enrichment: effects on stereotyped behavior and neurotrophin levels. Physiol Behav. 2003;80:259–66. doi: 10.1016/j.physbeh.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Hadley C, Hadley B, Ephraim S, Yang M, Lewis MH. Spontaneous stereotypy and environmental enrichment in deer mice (Peromyscus maniculants): Reversibility of experience. Appl Anim Behav Sci. 2006;97:312–22. [Google Scholar]
- 26.Tanimura Y, Vaziri S, Lewis MH. Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav Brain Res. 2010;210:116–22. doi: 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanimura Y, Yang MC, Ottens AK, Lewis MH. Development and temporal organization of repetitive behavior in an animal model. Dev Psychobiol. 2010;52:813–24. doi: 10.1002/dev.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacol. 2009;204:361–73. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moy S, Nadler J, Poe N, Nonneman R, Young N, Koller B, et al. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav Brain Res. 2008;188:178–94. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey JS, Grabowski-Boase L, Steffy BM, Wiltshire T, Churchill GA, Tarantino LM. Identification of quantitative trait loci for lcoomotor activation and anxiety using closely related inbred strains. Genes Brain Behav. 2008;7:761–769. doi: 10.1111/j.1601-183x.2008.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]