Abstract
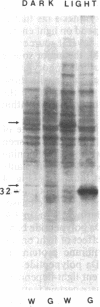
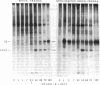
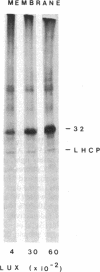
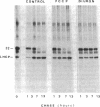
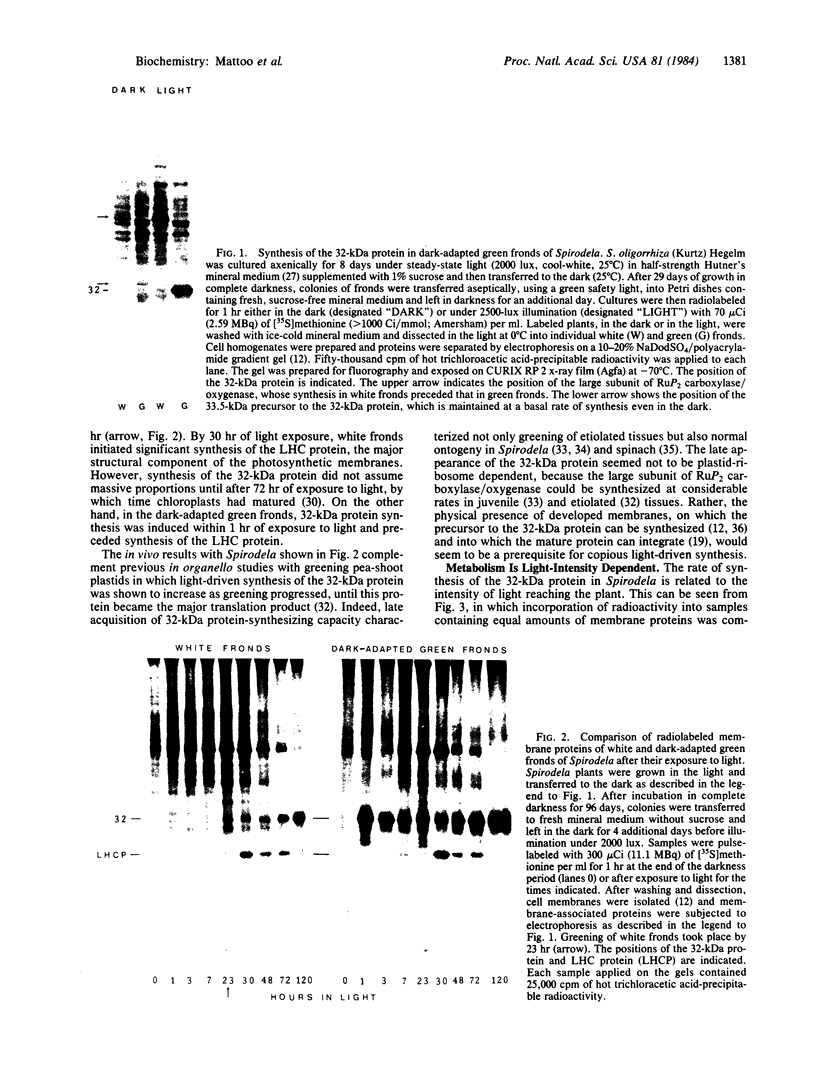
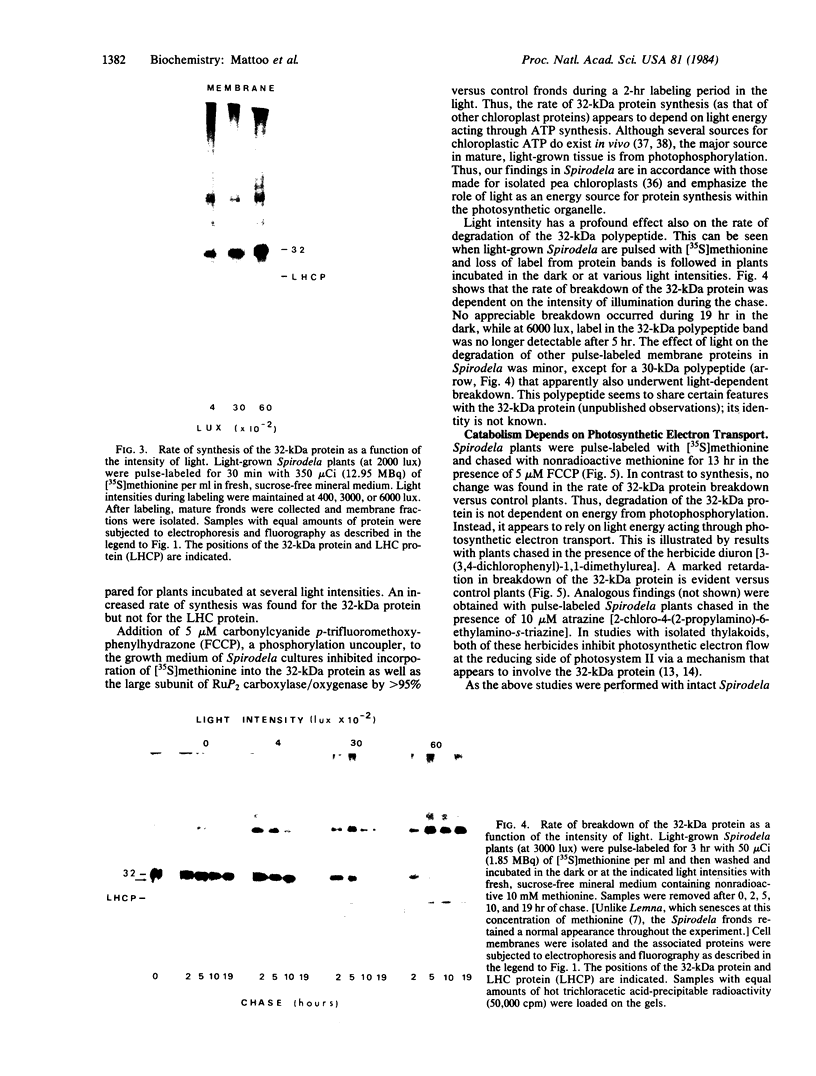
In Spirodela oligorrhiza, mature chloroplasts copiously synthesize and degrade a 32-kilodalton membrane protein. The rates of synthesis and degradation are controlled by light intensity, the protein being unstable in the light and stable in the dark. Light-driven synthesis, but not degradation, is dependent on ATP. Degradation is blocked by herbicides inhibiting photosystem II electron transport, such as diuron and atrazine. Thus, both anabolism and catabolism of the 32-kilodalton protein are photoregulated, with degradation coupled to electron transport rather than phosphorylation.
Keywords: Spirodela oligorrhiza, light intensity, protein turnover, diuron, atrazine
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Nyunt T., Boardman N. K. Light-induced redox changes of cytochrome b-559. Arch Biochem Biophys. 1973 Apr;155(2):436–444. doi: 10.1016/0003-9861(73)90134-3. [DOI] [PubMed] [Google Scholar]
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Speirs J., Bohnert H. J. Spinach chloroplast mRNA for a 32 000 dalton polypeptide: size and localization on the physical map of the chloroplast DNA. Biochim Biophys Acta. 1980 Dec 11;610(2):297–310. doi: 10.1016/0005-2787(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Steinback K. E., Bogorad L. Comparison of the Molecular Weights of Proteins Synthesized by Isolated Chloroplasts with Those Which Appear during Greening in Zea mays. Plant Physiol. 1979 Mar;63(3):436–439. doi: 10.1104/pp.63.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Levi C., Gibbs M. Starch degradation in isolated spinach chloroplasts. Plant Physiol. 1976 Jun;57(6):933–935. doi: 10.1104/pp.57.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Radosevich S. R., Arntzen C. J. Modification of Herbicide Binding to Photosystem II in Two Biotypes of Senecio vulgaris L. Plant Physiol. 1979 Dec;64(6):995–999. doi: 10.1104/pp.64.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath D., Shaul Y. B. Growth, greening, and phytochrome in etiolated spirodela (lemnaceae). Plant Physiol. 1973 Mar;51(3):474–477. doi: 10.1104/pp.51.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld A., Mattoo A. K., Edelman M. Processing of a chloroplast-translated membrane protein in vivo. Analysis of the rapidly synthesized 32 000-dalton shield protein and its precursor in Spirodela oligorrhiza. Eur J Biochem. 1982 May;124(1):125–129. doi: 10.1111/j.1432-1033.1982.tb05914.x. [DOI] [PubMed] [Google Scholar]
- Renger G. Studies on the structural and functional organization of system II of photosynthesis. The use of trypsin as a structurally selective inhibitor at the outer surface of the thylakoid membrane. Biochim Biophys Acta. 1976 Aug 13;440(2):287–300. doi: 10.1016/0005-2728(76)90063-3. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Ellis R. J. Protein synthesis in chloroplasts. Characteristics and products of protein synthesis in vitro in etioplasts and developing chloroplasts from pea leaves. Biochem J. 1975 Mar;146(3):675–685. doi: 10.1042/bj1460675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Ellis R. J. Protein synthesis in chloroplasts. VIII. Differential synthesis of chloroplast proteins during spinach leaf development. Biochim Biophys Acta. 1980 Apr 30;607(2):319–330. doi: 10.1016/0005-2787(80)90084-2. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Ellis R. J. Light-stimulated accumulation of transcripts of nuclear and chloroplast genes for ribulosebisphosphate carboxylase. J Mol Appl Genet. 1981;1(2):127–137. [PubMed] [Google Scholar]
- Tobin E. M. Light regulation of specific mRNA species in Lemna gibba L. G-3. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4749–4753. doi: 10.1073/pnas.75.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S. A., Gressel J., Reisfeld A., Edelman M. Characterization of the 32,000 Dalton Chloroplast Membrane Protein: III. Probing Its Biological Function in Spirodela. Plant Physiol. 1979 Nov;64(5):828–832. doi: 10.1104/pp.64.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]