Abstract
Objective
Everolimus, an inhibitor of the mammalian target of rapamycin, has recently demonstrated efficacy and safety in a Phase III, double-blind, randomized trial (RADIANT-3) in 410 patients with low- or intermediate-grade advanced pancreatic neuroendocrine tumours. Everolimus 10 mg/day provided a 2.4-fold improvement compared with placebo in progression-free survival, representing a 65% risk reduction for progression. The purpose of this analysis was to investigate the efficacy and safety of everolimus in the Japanese subgroup enrolled in the RADIANT-3 study.
Methods
Subgroup analysis of the Japanese patients was performed comparing efficacy and safety between everolimus 10 mg/day orally (n = 23) and matching placebo (n = 17). The primary endpoint was progression-free survival. Safety was evaluated on the basis of the incidence of adverse drug reactions.
Results
Progression-free survival was significantly prolonged with everolimus compared with placebo. The median progression-free survival was 19.45 months (95% confidence interval, 8.31–not available) with everolimus vs 2.83 months (95% confidence interval, 2.46–8.34) with placebo, resulting in an 81% risk reduction in progression (hazard ratio, 0.19; 95% confidence interval, 0.08–0.48; P< 0.001). Adverse drug reactions occurred in all 23 (100%) Japanese patients receiving everolimus and in 13 (77%) patients receiving placebo; most were grade 1/2 in severity. The most common adverse drug reactions in the everolimus group were rash (n = 20; 87%), stomatitis (n = 17; 74%), infections (n = 15; 65%), nail disorders (n = 12; 52%), epistaxis (n = 10; 44%) and pneumonitis (n = 10; 44%).
Conclusions
These results support the use of everolimus as a valuable treatment option for Japanese patients with advanced pancreatic neuroendocrine tumours.
Keywords: pancreatic neuroendocrine tumours, mTOR inhibitors, everolimus, Japanese population
INTRODUCTION
Pancreatic neuroendocrine tumours (pNET) are neuroendocrine neoplasms originating from islets of Langerhans cells in the pancreas (1). According to US population-based estimates [Surveillance, Epidemiology and End Results (SEER) program], pNET account for ∼1.3% of pancreatic neoplasms in incidence; however, the prevalence represents 10% of pancreatic neoplasms (1). The incidence of pNET has increased significantly in the past three decades, reaching 0.32 per 100 000 patients in the SEER registry during 2000–04 (2). Because these tumours are often not recognized until the advanced stages of the disease progress, the prognosis for patients is poor (2). Most patients with pNET (∼64%) present with advanced (metastatic) disease (2). The estimated median survival time for patients with distant metastatic pNET is 24 months (2).
The epidemiology of pNET in Japan is of increasing interest, but data are limited. According to a national survey conducted in 2005, the overall incidence of pNET in Japan was 1.01 per 100 000 people (approximately three times higher than US estimates), and the overall prevalence was 2.23 per 100 000 people (3). The geographical difference in epidemiological data suggests that ethnic differences underlie the disease, but ethnic differences in efficacy and safety of drug therapies have not yet been investigated.
While surgery is the mainstay of treatment for patients with limited disease burden confined to the primary site and regional lymph nodes (4,5) and for patients with resectable liver metastases based on National Comprehensive Cancer Network guidelines (6), it is not an option for patients with advanced or unresectable metastatic disease (6). Systemic chemotherapy with streptozocin (Zanosar®; Keocyt in the EU and Teva Parenteral Medicines, Inc. in the USA) is the approved treatment option for advanced pNET in the USA (6), France, Canada and Israel, but it is not available in Japan. The role of chemotherapy in the treatment of pNET remains inconclusive. Response rates differ among studies (5,7,8), and their rigorousness for response evaluation (e.g. clinical evaluation vs exclusive use of radiologic evaluation) has been questioned (5,7). Modest response rates, coupled with concerning toxicity profiles often associated with the use of chemotherapeutic agents (5,9,10), underscore the continuing unmet need for effective, approved treatment options for advanced pNET.
Liver metastases of NET have high hepatic artery blood flow, and liver-directed therapies are used in the treatment of NET patients despite the lack of randomized controlled trials (11). Transarterial embolization and transarterial chemoembolization are, thus, useful treatments for hepatic metastases of NET, including downstaging for those patients with high tumour burden and controlling the symptoms of hormonal hypersecretion (11–13). It has also been reported that radiofrequency ablation may be useful in conjunction with surgical resection or as an alternative to resection in patients with unresectable tumours and limited numbers of hepatic metastases (14–17). However, this procedure is limited to tumours that measure ≤5 cm in diameter and are not near vital structures (11). Reports on hepatic intra-arterial chemotherapy as monotherapy for hepatic metastases are few. Therefore, National Comprehensive Cancer Network (NCCN) guidelines (6) and European Neuroendocrine Tumor Society (ENETS) guidelines (11) do not recommend it. Among the major limitations of liver-directed therapies are that no randomized controlled trial has been conducted and that the complexity and high risk of morbidity make it necessary that the patient use an experienced centre (11).
The molecular pathogenesis of pNET continues to be explored. Current data suggest that constitutional activation of the mammalian target of rapamycin (mTOR) signalling pathway may be a key underlying factor involved in pNET proliferation. mTOR, a cytoplasmic serine/threonine kinase, is a central modulator of the cell cycle (18). Aberrant upstream activation of mTOR by phosphatidylinositol-3-kinase (P13K)/Akt3 (due to various mechanisms causing signalling defects, such as mutations) results in dysregulated downstream signalling, leading to uninhibited cell growth and proliferation, cellular metabolism and angiogenesis (19). This mechanism has been implicated in the development and progression of many cancers (20), including pNET (21). Inhibition of this pathway is, thus, an important therapeutic target in oncology.
Everolimus (Afinitor®; RAD001; Novartis Pharmaceuticals), an orally bioavailable inhibitor of mTOR, was recently approved in the USA and Europe for the treatment of patients with progressive pNET that are unresectable, locally advanced or metastatic. Everolimus has shown promising anti-tumour activity in Phase II studies of patients with pNET (22,23) and in a recently published Phase III trial (24). The RADIANT-3 trial evaluated the efficacy and safety of oral everolimus, 10 mg/day vs placebo, in 410 patients with low- or intermediate-grade advanced pNET (24). In this trial, everolimus demonstrated a statistically and clinically significant 2.4-fold improvement in progression-free survival (PFS). The median PFS was 11.0 months in the everolimus group vs 4.6 months with placebo, representing a 65% risk reduction for progression compared with placebo [hazard ratio (HR), 0.35; P < 0.001] (24). Most common adverse drug reactions (ADRs) occurring in at least 30% of patients receiving everolimus were stomatitis (64%), rash (49%), diarrhoea (34%) and fatigue (31%) (24). The subgroup analysis of Japanese patients who participated in the RADIANT-3 trial is presented herein to investigate whether the efficacy and safety of everolimus were comparable in the Japanese population and the overall study population.
PATIENTS AND METHODS
Patients
The RADIANT-3 inclusion and exclusion criteria have been described in detail (24). Briefly, eligibility criteria included age ≥18 years, low- or intermediate-grade advanced (unresectable or metastatic) pNET and radiologic documentation of disease progression within 12 months before randomization.
The additional inclusion criteria were presence of measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0; World Health Organization (WHO) performance status (PS) ≤2; adequate bone marrow, renal and hepatic function and adequately controlled lipid and glucose levels. The exclusion criteria included hepatic artery embolization within 6 months before enrolment (within 1 month in the presence of other sites of measurable disease), cryoablation or radiofrequency ablation of hepatic metastasis 2 months before enrolment, presence of severe or uncontrolled medical condition, previous therapy with an mTOR inhibitor or long-term treatment with corticosteroids or other immunosuppressants.
Study Oversight
The study was approved by the institutional review board or ethics committee at each participating centre and was conducted in accordance with Good Clinical Practice principles and applicable local regulations. Written informed consent was obtained from all patients.
Study Design and Treatment
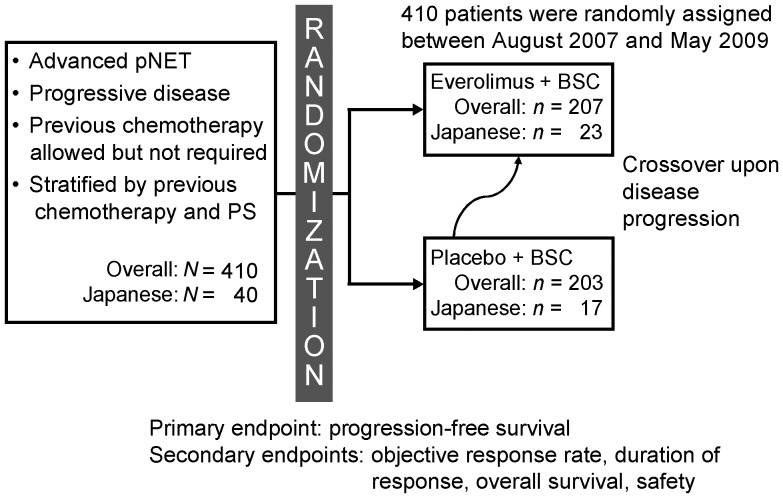
RADIANT-3 was a double-blind, placebo-controlled, randomized, multicentre, Phase III study (NCT00510068) conducted in 82 centres within 18 countries worldwide (24). Patients were randomly assigned to receive either oral everolimus 10 mg/day (n = 207) or placebo (n = 203), both in conjunction with best supportive care (Fig. 1). Forty Japanese patients from three different centres participated; 23 received everolimus and 17 received placebo. Patients were stratified on the basis of whether they had received chemotherapy and on baseline WHO PS (0 vs 1–2). Treatment was continued until the occurrence of disease progression, development of an unacceptable ADR (treatment-related adverse event), interruption of treatment for ≥3 weeks or withdrawal of patient consent. Investigators were unaware of study group assignments; however, disclosure was allowed if an investigator determined disease progression (per RECIST criteria), in which case a patient initially assigned to placebo could switch to open-label everolimus. Dose reductions or delays were permitted in the event of clinically significant ADRs (24).
Figure 1.
Study design. BSC, best supportive care; pNET, pancreatic neuroendocrine tumours; PS, performance status.
Efficacy and Safety Assessments
The primary endpoint was PFS evaluated by local investigator assessment according to the RECIST criteria, version 1.0. The secondary endpoints included confirmed objective response rate (complete response or partial response), duration of response, overall survival (OS) and safety. All randomly assigned patients were assessed for efficacy (i.e. intention-to-treat analysis). Tumour measurements were performed at baseline and repeated every 12 weeks. PFS by central review was a secondary endpoint in the trial; when needed, adjudication was performed by an independent central adjudication committee composed of a board-certified radiologist and an oncologist, both of whom were unaware of study group assignments (24). To detect radiologic lung changes suggestive of pneumonitis, a central radiology review of chest computed tomography (CT) scans and chest X-rays was performed.
All the patients who received at least one dose of the study drug and had at least one follow-up assessment were included in the safety analysis. Safety assessments included ADRs, laboratory evaluations and physical examinations (24).
Statistical Analysis
Kaplan–Meier methods were used to assess PFS and OS, as reported earlier (24). Statistical comparisons between study groups were made using a one-sided log-rank test. A Cox proportional hazards model was used to assess HR.
RESULTS
Patients and Treatment
As seen in Table 1 (24), baseline demographic characteristics of and previous therapies for the Japanese patients were well balanced between the treatment groups. The median age of the Japanese subgroup (45 years in the everolimus group, 53 years in the placebo group) was similar to that of the overall study population (24), as was the number of disease sites. Disease severity was generally lower in the Japanese subgroup than in the overall population; a higher percentage had a WHO PS of 0, and a slightly higher percentage had a well-differentiated histologic grade.
Table 1.
Baseline characteristics and previous therapies
| Japanese subgroup |
Overall populationa |
|||
|---|---|---|---|---|
| Everolimus (n = 23) | Placebo (n = 17) | Everolimus (n = 207) | Placebo (n = 203) | |
| Median age, years (range) | 45 (33–85) | 53 (38–77) | 58 (23–87) | 57 (20–82) |
| Male/female, % | 57:44 | 47:53 | 53:47 | 58:42 |
| WHO PS 0/1/2, % | 87/13/0 | 88/12/0 | 67/30/3 | 66/32/3 |
| No. disease sites 1/2/≥3, % | 30/35/35 | 29/29/41 | 25/41/34 | 31/32/38 |
| Histologic grade, % | ||||
| Well-differentiated | 100 | 94 | 82 | 84 |
| Moderately differentiated | 0 | 6 | 17 | 15 |
| Unknown | 0 | 0 | 1 | 1 |
| Previous therapies, % | ||||
| Chemotherapy | 61 | 53 | 50 | 50 |
| Radiotherapy | 13 | 12 | 23 | 20 |
| Targeted therapy | 0 | 0 | 5 | 7 |
| Immunotherapy | 0 | 0 | 3 | 4 |
| Hormone therapy | 0 | 0 | 1 | 1 |
| Other | 4 | 0 | 10 | 13 |
| Somatostatin analogues | 22 | 35 | 49 | 50 |
WHO PS, World Health Organization performance status.
aData previously presented by Yao JC et al. N Engl J Med. 2011;364: see also 514–23 (24).
The percentage of patients receiving previous chemotherapy was similar between the treatment groups for the overall population (50% in the everolimus and 50% in the placebo groups) and slightly higher in the everolimus arms (61%) compared with the placebo arm (53%) in the Japanese subgroup. In addition, a higher percentage of patients in the overall population received previous somatostatin analogue treatment (49% in the everolimus group and 50% in the placebo group) compared with the Japanese subgroup (22 and 35%, respectively). None of the Japanese patients in the RADIANT-3 trial received previous targeted therapy, immunotherapy or hormone therapy.
Patient disposition is shown in Table 2 (24). At a median follow-up of 16.1 months for the Japanese subgroup and 17.0 months for the overall population (24), median durations of exposure were 60 weeks (range, 4–103) and 38 weeks (range, 1–118), respectively, in the everolimus groups and 12 weeks (range, 4–70) and 16 weeks (range, 0.4–132), respectively, in the placebo groups. Higher percentages of patients in the placebo groups discontinued treatment (82% in Japanese subgroup and 87% in the overall population) compared with the everolimus groups (48% in the Japanese subgroup and 68% in the overall population).
Table 2.
Patient disposition
| Japanese subgroup, n (%) |
Overall population,a
n (%) |
|||
|---|---|---|---|---|
| Everolimus (n = 23) | Placebo (n= 17) | Everolimus (n = 207) | Placebo (n = 203) | |
| Treatment ongoing | 12 (52) | 3 (18) | 66 (32) | 26 (13) |
| Patient discontinuation | 11 (48) | 14 (82) | 141 (68) | 177 (87) |
| Disease progression | 6 (26) | 14 (82) | 92 (44) | 163 (80) |
| Adverse events | 4 (17) | 0 | 36 (17) | 7 (3) |
| Death | 1 (4) | 0 | 4 (2) | 3 (1) |
| Withdrawal of consent | 0 | 0 | 4 (2) | 4 (2) |
| Other reasons | 0 | 0 | 5 (2) | 0 |
| Duration of exposure, weeks, median (range) | 60 (4–103) | 12 (4–70) | 38 (1–118) | 16 (0.4–132) |
aData previously presented by Yao JC et al. N Engl J Med. 2011;364:514–23 (24).
For both the Japanese subgroup and the overall population, disease progression was the primary reason for treatment discontinuation and occurred in substantially more patients in the placebo groups (82% in the Japanese subgroup and 80% in the overall population) than in the everolimus groups (26% in the Japanese subgroup and 44% in the overall population). In the Japanese subgroup population, one patient in the everolimus group died of acute respiratory distress syndrome caused by sepsis that was considered to be treatment related; no patient in the placebo group died during the study. In the overall population, 12 patients (6%) in the everolimus group and 4 patients (2%) in the placebo group died while receiving the study drug; five deaths in the everolimus group and three deaths in the placebo group were attributed to the underlying cancer or disease progression, and seven deaths in the everolimus group and one death in the placebo group were attributed to adverse events. One death in the everolimus group that was related to the study drug was the Japanese patient described earlier (24).
Efficacy
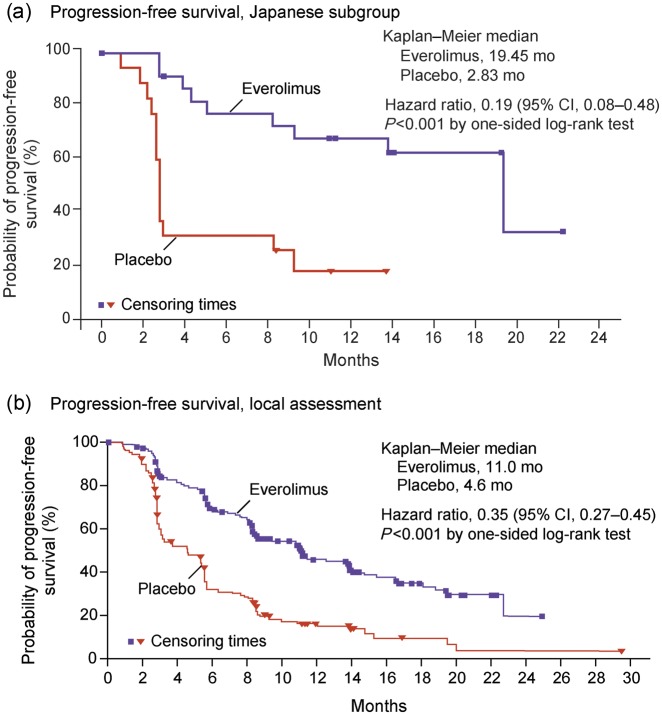
In the Japanese subgroup, PFS was significantly prolonged in patients treated with everolimus compared with placebo [HR, 0.19; 95% confidence interval (CI), 0.08–0.48; log rank, P< 0.001] (Fig. 2a) (24). PFS was achieved for a median of 19.45 months (95% CI, 8.31-not available) in the everolimus group compared with 2.83 months (95% CI, 2.46–8.34) in the placebo group.
Figure 2.
Kaplan–Meier plot of progression-free survival (PFS) for (a) Japanese subgroup and (b) local assessment of the overall population (24). Hazard ratios were obtained from a Cox model. CI, confidence interval; HR, hazard ratio. Reprinted from Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–23 (24). Copyright© 2011 Massachusetts Medical Society.
These PFS results represent a 16.62 month prolongation in median PFS and an 81% risk reduction in disease progression in the everolimus group. They are consistent with those demonstrated for the overall population (24), wherein median PFS was 11.0 months (95% CI, 8.4–13.9) in the everolimus group compared with 4.6 months (95% CI, 3.1–5.4) in the placebo group, correlating with a 65% risk reduction in progression in the everolimus group (HR, 0.35; 95% CI, 0.27–0.45; P < 0.001) (Fig. 2b) (24).
In the Japanese subgroup, 13 patients crossed over from the placebo arm to receive everolimus; 148 patients crossed over in the overall population. Median OS was not reached at the time of this analysis, and no significant difference between treatment groups was observed in the Japanese subgroup (HR, 0.90; 95% CI, 0.20–4.05; P= 0.45) or in the overall population (HR, 0.89; 95% CI, 0.64–1.23) (24).
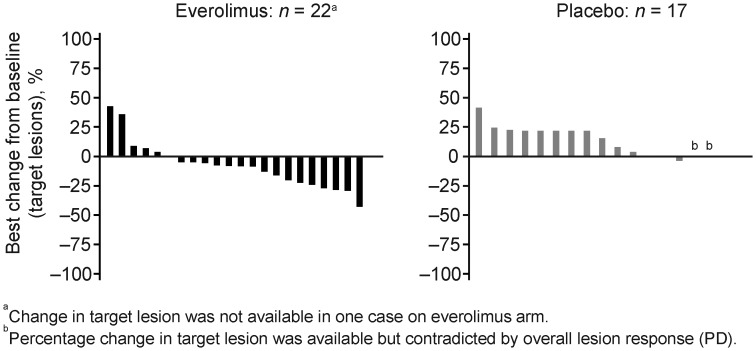
No patient in either treatment group achieved complete response; however, significantly more patients in the everolimus group than in the placebo group achieved stable disease [83 vs 29%, respectively; Table 3(24)], and fewer patients had progressive disease (9 vs 65%, respectively). Graphical analysis of the best percentage change from baseline in the Japanese subgroup (Fig. 3) illustrates that more patients experienced reduction in target lesion size in the everolimus group than in the placebo group. All these findings were consistent with those determined for the overall population (Table 3) (24).
Table 3.
Best overall response
| Response | Japanese subgroup, n (%) |
Overall population,a
n (%) |
||
|---|---|---|---|---|
| Everolimus (n = 23) | Placebo (n = 17) | Everolimus (n = 207) | Placebo (n = 203) | |
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 1 (4) | 1 (6) | 10 (5) | 4 (2) |
| Stable disease | 19 (83) | 5 (29) | 151 (73) | 103 (51) |
| Progressive disease | 2 (9) | 11 (65) | 29 (14) | 85 (42) |
| Unknown | 1 (4) | 0 | 17 (8) | 11 (5) |
| Objective response rate (CR or PR) [95% CI] | 1 (4) [0.1–21.9] | 1 (6) [0.1–28.7] | 10 (5) [2.3–8.7] | 4 (2) [0.5–5.0] |
CI, confidence interval; CR, complete response; PR, partial response.
aData previously presented by Yao JC et al. N Engl J Med. 2011;364:514–23 (24).
Figure 3.
Best percentage change from baseline in Japanese subgroup. PD, progressive disease.
Safety
As shown in Table 4 (24), in the Japanese subgroup, ADRs occurred in all the 23 (100%) patients treated with everolimus and in the 13 (77%) patients who received placebo; most ADRs were grade 1 or 2 in severity. The most common ADRs in the everolimus group were rash (n = 20; 87%), stomatitis (n = 17; 74%), infections (n = 15; 65%), nail disorders (n = 12; 52%), epistaxis (n = 10; 44%) and pneumonitis (n = 10; 44%), all of which occurred at higher rates than in the overall population (24). Grade 3 ADRs occurred in 15 (65%) patients in the everolimus group and in 5 (29%) patients in the placebo group. One grade 4 infection occurred in the everolimus group (4%); none was reported in the placebo group. The most common grade 3/4 ADRs occurring in Japanese patients in the everolimus group were neutropenia (n = 4; 17%), anaemia (n = 2; 9%), pneumonitis (n = 2; 9%), leucopoenia (n = 2; 9%), infections (n = 2; 9%) and abnormal hepatic function (n = 2; 9%). In the overall population, the most common ADRs affecting ≥30% of patients treated with everolimus were stomatitis (n = 131; 64%), rash (n = 99; 49%), diarrhoea (n = 69; 34%) and fatigue (n = 64; 31%) (24). Most ADRs were grade 1 or 2. Common grade 3 or 4 ADRs in the everolimus group were stomatitis (n = 14; 7%), anaemia (n = 12; 6%) hyperglycaemia (n = 11; 5%), thrombocytopenia (n = 8; 4%) and diarrhoea (n = 7; 3%) (24).
Table 4.
Adverse drug reactions
| Japanese subgroup, n (%) |
Overall populationa, n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Everolimus (n = 23) |
Placebo (n =17) |
Everolimus (n = 204) |
Placebo (n = 203) |
|||||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Any ADR | 23 (100) | 16 (70) | 13 (77) | 5 (29) | 195 (96) | 92 (45) | 151 (74) | 28 (14) |
| Rash | 20 (87) | 0 | 2 (12) | 0 | 99 (49) | 1 (<1) | 21 (10) | 0 |
| Stomatitisb | 17 (74) | 0 | 4 (24) | 0 | 131 (64) | 14 (7) | 34 (17) | 0 |
| Infectionsc | 15 (65) | 2 (9) | 3 (18) | 0 | 46 (23) | 5 (2) | 12 (6) | 1 (<1) |
| Nail disorders | 12 (52) | 0 | 0 | 0 | 24 (12) | 1 (<1) | 2 (1) | 0 |
| Epistaxis | 10 (44) | 0 | 0 | 0 | 35 (17) | 0 | 0 | 0 |
| Pneumonitisd | 10 (44) | 2 (9) | 0 | 0 | 35 (17) | 5 (2) | 0 | 0 |
| Dysgeusia | 8 (35) | 0 | 2 (12) | 0 | 35 (17) | 0 | 8 (4) | 0 |
| Fatigue | 8 (35) | 0 | 2 (12) | 0 | 64 (31) | 5 (2) | 29 (14) | 1 (<1) |
| Anaemia | 7 (30) | 2 (9) | 0 | 0 | 35 (17) | 12 (6) | 6 (3) | 0 |
| Glossitis | 7 (30) | 0 | 0 | 0 | 7 (3) | 0 | 0 | 0 |
| Headache | 7 (30) | 0 | 3 (18) | 0 | 39 (19) | 0 | 13 (6) | 0 |
| Hyperlipidemia | 7 (30) | 0 | 1 (6) | 0 | 9 (4) | 0 | 2 (1) | 0 |
| Neutropenia | 7 (30) | 4 (17) | 3 (18) | 3 (18) | 13 (6) | 6 (3) | 4 (2) | 4 (2) |
| Vomiting | 7 (30) | 0 | 0 | 0 | 31 (15) | 0 | 13 (6) | 0 |
| Decreased appetite | 6 (26) | 0 | 1 (6) | 0 | 40 (20) | 0 | 14 (7) | 2 (1) |
| Diarrhoea | 6 (26) | 0 | 2 (12) | 0 | 69 (34) | 7 (3) | 20 (10) | 0 |
| Hyperglycaemia | 6 (26) | 1 (4) | 1 (6) | 0 | 27 (13) | 11 (5) | 9 (4) | 4 (2) |
| Hypertension | 6 (26) | 0 | 1 (6) | 1 (6) | 10 (5) | 1 (<1) | 4 (2) | 1 (<1) |
| Leucopenia | 6 (26) | 2 (9) | 2 (12) | 0 | 12 (6) | 2 (1) | 4 (2) | 1 (<1) |
| Pyrexia | 6 (26) | 0 | 0 | 0 | 22 (11) | 0 | 0 | 0 |
| Cheilitis | 5 (22) | 0 | 1 (6) | 0 | 8 (4) | 0 | 2 (1) | 0 |
| Diabetes mellitus | 5 (22) | 1 (4) | 0 | 0 | 17 (8) | 5 (2) | 0 | 0 |
| Gingivitis | 5 (22) | 0 | 0 | 0 | 7 (3) | 0 | 1 (<1) | 0 |
| Nausea | 5 (22) | 0 | 1 (6) | 0 | 41 (20) | 5 (2) | 37 (18) | 0 |
Other grade 3/4 ADRs in the everolimus group: abnormal hepatic function, liver abscess, abdominal pain, acute respiratory distress syndrome, aplasia pure red cell, decreased blood phosphorus, cellulitis, decreased haemoglobin, hypophosphatemia, thrombocytopenia, lymphopenia, ileus and staphylococcal sepsis.
ADR, adverse drug reaction.
aData previously presented by Yao JC et al. N Engl J Med. 2011;364:514–23 (24).
bIncluding aphthous stomatitis, mouth ulceration and tongue ulceration.
cIncluding all types of infection.
dIncluding interstitial lung disease, lung infiltration, pulmonary fibrosis and restrictive lung disease.
In the overall population, 68 (33.3%) patients treated with everolimus had post-baseline findings suggestive of pneumonitis based on the central review of chest X-ray or chest CT scan. In the Japanese subgroup, seven (30.4%) patients treated with everolimus had these post-baseline findings based on the central review, indicating that in this treatment arm Japanese patients did not differ from the overall population in the rate of lung changes. However, only 27 (13.2%) patients treated with everolimus in the overall population were reported by the investigator to have pneumonitis as an adverse event compared with seven (30.4%) patients in the Japanese subgroup (Table 5), indicating that Japanese investigators reported the lung changes more frequently.
Table 5.
Central radiology assessment by combined X rays and CT scans on radiologic lung changes suggestive of pneumonitis
| Japanese subgroup, n (%) |
Overall population, n (%) |
|||
|---|---|---|---|---|
| Everolimus (n = 23) | Placebo (n = 17) | Everolimus (n = 204) | Placebo (n = 203) | |
| Patients with radiologic lung changes suggestive of pneumonitis | ||||
| Baseline | 1 (4.3) | 0 | 15 (7.4) | 10 (4.9) |
| Post-baselinea | 7 (30.4) | 0 | 68 (33.3) | 27 (13.3) |
| Newly occurring or worsenedb | 7 (30.4) | 0 | 62 (30.4) | 23 (11.3) |
| Without review | 0 | 0 | 1 (0.5) | 2 (1.0) |
| Pulmonary adverse events in patients with newly occurring or worsened lung changes suggestive of pneumonitis | ||||
| Total | 7 (30.4) | 0 | 27 (13.2) | 0 |
| Pneumonitis | 5 (21.7) | 0 | 19 (9.3) | 0 |
| Interstitial lung disease | 2 (8.7) | 0 | 4 (2.0) | 0 |
| Lung infiltration | 0 | 0 | 5 (2.5) | 0 |
| Pulmonary fibrosis | 0 | 0 | 1 (0.5) | 0 |
CT, computed tomography.
aNumber of patients with any evidence of pneumonitis at any post-baseline assessment regardless of the baseline status.
bNumber of patients with newly occurring or worsened pneumonitis compared with baseline.
DISCUSSION
The RADIANT-3 trial represents the largest placebo-controlled Phase III clinical trial in patients with advanced pNET (24). As was seen with the overall population, the Japanese subgroup demonstrated large and significant increases in PFS [81% (P< 0.001) vs 65% (P< 0.001) for the overall population] (24). In addition, median PFS in the Japanese subgroup increased nearly 7-fold in the everolimus group compared with the placebo group as against an ∼2-fold increase in the overall population (24). Because patients whose disease progressed on placebo were allowed to cross over to open-label everolimus, this study was not designed to analyse differences in OS. RECORD-1, the Phase III trial of everolimus in patients with metastatic renal cell carcinoma, also showed longer PFS in the Japanese subgroup than in the overall population (25,26). However, in the RECORD-1 trial, the ratio of PFS in the everolimus and placebo arms was only 1.6-fold (5.75 months vs 3.61 months, respectively; HR, 0.19; 95% CI, 0.05–0.83) (25,26).
Reasons for the disparity in the PFS ratio in the patients with NET remain inconclusive. The number of disease sites was similar between the everolimus groups for the two populations; however, the Japanese subgroup had a lower percentage of patients with a WHO PS of 1 or 2 than the overall population (13 vs 33%, respectively), a higher percentage of patients with well-differentiated tumours (100 vs 82%, respectively) and a lower percentage of patients who previously received somatostatin analogue therapy (22 vs 49%, respectively) (24). The pharmacokinetics of everolimus in Japanese patients was previously shown to be similar to those in Caucasians (27), who accounted for 79% (n = 322) of the patients in the overall population (24). Though the Japanese patients were smaller than the overall population enrolled in RADIANT-3, the similar pharmacokinetics of everolimus in Japanese and Caucasian patients suggest that exposure of everolimus should not have accounted for its increased efficacy in this subgroup. As previously noted, the incidence of pNET is nearly three times higher in Japanese than in Caucasians (3). Ethnic differences that might have been responsible for the different incidence might also have contributed to the different response to everolimus in these groups and continue to be investigated.
Patients with pNET who have mutations in tuberous sclerosis 2 (TSC2) and phosphatase and tensin homolog (PTEN)—tumour suppressor genes of the P13K/Akt pathway—tend to have more aggressive tumours and subsequent quicker times to disease progression (21). Decreased protein expression of these negative regulators (TSC2 and PTEN) leads to overstimulation of the mTOR pathway by constitutive activation of PI3K/Akt, thus resulting in uninhibited tumour growth (19). Notably, tumours exhibiting PTEN mutations have been shown to be particularly sensitive to treatment with mTOR inhibitors (19).
Safety evaluations found everolimus to be generally well tolerated in this Japanese subgroup; no new safety concerns were observed. Select ADRs including epistaxis (44 vs 17%), pneumonitis (44 vs 17%), dysgeusia (35 vs 17%), anaemia (30 vs 17%) and headache (30 vs 19%) tended to occur with greater frequency in the everolimus group of the Japanese subgroup than in the overall population, respectively (24). The higher incidence of ADRs in Japanese patients is attributable to careful monitoring and conservative assessments by Japanese investigators. Incidence of grade 3/4 ADRs in the Japanese subgroup was comparable to that in the overall population, respectively, for select events, including anaemia (9 vs 6%), hyperglycaemia (4 vs 5%) and diabetes mellitus (4 vs 2%) (24). Stomatitis, which affected 7% of patients, was the most commonly occurring grade 3/4 ADR in the everolimus group in the overall population; none of the cases of stomatitis in the Japanese subgroup was rated as grade 3/4. Neutropenia, which affected 17% of patients in the everolimus group compared with 3% of patients in the overall population, was the most commonly occurring grade 3/4 ADR in the Japanese subgroup.
The frequency of interstitial lung disease (ILD)-type events reported as ‘adverse events’ in the investigator's opinion appeared to be higher in Japanese patients than in the overall population. However, a central radiology review found similar proportions of radiologic findings compatible with pneumonitis in Japanese and non-Japanese patients. Moreover, the presence of the characteristic symptoms associated with ILD, such as cough (4.3% in Japanese patients vs 23.8% in non-Japanese patients) and dyspnoea (13.0% in Japanese patients vs 17.1% in non-Japanese patients), was less common in Japanese than in non-Japanese patients. The higher report in Japanese patients may be explained by Japanese investigators’ greater awareness of the disease , which could lead to more frequent radiologic assessments and diagnoses.
In conclusion, in this subgroup analysis of Japanese patients, everolimus demonstrated a clinically meaningful improvement in PFS over placebo and was generally well tolerated, as demonstrated in the larger RADIANT-3 trial (24). These results support the use of everolimus as a valuable treatment option for Japanese patients with advanced pNET.
Funding
This work was supported by Novartis Oncology. Financial support for medical assistance was provided by Novartis Pharma K.K.
Conflict of interest statement
Takeshi Tajima, Akio Kasuga and Yoshie Fujita are employed by Novartis Pharma K.K. Junji Furuse received consulting fees from Bayer, Chugai, Eisai, Eli Lilly, Taiho, Pfizer and Novartis and received honoraria from Bayer, Chugai, Eisai, Eli Lilly, Taiho, Pfizer and Novartis; Takuji Okusaka received honoraria from Novartis; Chigusa Morizane received honoraria from Novartis; Masafumi Ikeda received honoraria from Bayer Yakuhin Ltd. and Dainippon Sumitomo Pharma; Tetsuhide Ito, Hisato Igarashi and Kohei Nakachi declare that they have no conflict of interest.
Acknowledgements
We thank all the investigators for their cooperation. We also thank Michael Yount, Tara Beers Gibson, David Gibson and Denise Myers (ApotheCom) for their medical editorial assistance with the preparation of the manuscript.
References
- 1.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–43. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 4.Capurso G, Fazio N, Festa S, Panzuto F, de Braud F, Delle Fave G. Molecular target therapy for gastroenteropancreatic endocrine tumours: biological rationale and clinical perspectives. Crit Rev Oncol Hematol. 2009;72:110–24. doi: 10.1016/j.critrevonc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Basu B, Sirohi B, Corrie P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr Relat Cancer. 2010;17:R75–90. doi: 10.1677/ERC-09-0108. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. National Comprehensive Cancer Network Web site; NCCN Clinical Practice Guidelines in Oncology™ Neuroendocrine Tumors. http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. (11 April 2012, date last accessed) [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–23. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 8.Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944–8. [PubMed] [Google Scholar]
- 9.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–71. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Kulke MH. Gastrointestinal neuroendocrine tumors: a role for targeted therapies? Endocr Relat Cancer. 2007;14:207–19. doi: 10.1677/ERC-06-0061. [DOI] [PubMed] [Google Scholar]
- 11.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 12.Dudeck O, Ricke J. Advances in regional chemotherapy of the liver. Expert Opin Drug Deliv. 2011;8:1057–69. doi: 10.1517/17425247.2011.574125. [DOI] [PubMed] [Google Scholar]
- 13.Kress O, Wagner HJ, Wied M, Klose KJ, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors—a retrospective single-center analysis. Digestion. 2003;68:94–101. doi: 10.1159/000074522. [DOI] [PubMed] [Google Scholar]
- 14.Berber E, Flesher N, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985–90. doi: 10.1007/s00268-002-6629-5. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson J, Stalberg P, Nilsson A, et al. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg. 2008;32:930–8. doi: 10.1007/s00268-008-9510-3. [DOI] [PubMed] [Google Scholar]
- 16.Guenette JP, Dupuy DE. Radiofrequency ablation of colorectal hepatic metastases. J Surg Oncol. 2010;102:978–87. doi: 10.1002/jso.21658. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–9. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Chan JA, Kulke MH. New treatment options for patients with advanced neuroendocrine tumors. Curr Treat Options Oncol. 2011;12:136–48. doi: 10.1007/s11864-011-0148-2. [DOI] [PubMed] [Google Scholar]
- 19.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 20.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245–55. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II trial. J Clin Oncol. 2008;26:4311–8. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukamoto T, Shinohara N, Tsuchiya N, et al. Phase III trial of everolimus in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from RECORD-1. Jpn J Clin Oncol. 2011;41:17–24. doi: 10.1093/jjco/hyq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto I, Doi T, Ohtsu A, et al. Phase I clinical and pharmacokinetic study of RAD001 (everolimus) administered daily to Japanese patients with advanced solid tumors. Jpn J Clin Oncol. 2010;40:17–23. doi: 10.1093/jjco/hyp120. [DOI] [PMC free article] [PubMed] [Google Scholar]